The aim of this study was to evaluate the effects of passive immunization with polyclonal anti-swine early pregnancy factor (EPF) antibody in pregnant rats during the pre-implantation period, and to analyse the regional distribution of EPF in placenta of rats. The immunodetection of EPF in rat placenta, as well as fertilization, development and immunological parameters, was evaluated. The average number of embryos and the embryo/corpora lutea ratio of rats treated with anti-EPF antibody were significantly lower than control groups. Also, as expected, the embryos showed a delay in their development. Furthermore, while IL-10 and INF-γ levels increased, serum and placental TGF-β decreased significantly in the group treated with anti-EPF. EPF was present in giant and decidual cells, as well as in blood vessels and trophoblastic lacuna cells, as demonstrated by positive immunostaining. This study provides new results on the function and location of EPF, and has demonstrated the usefulness of polyclonal anti-swine EPF antibody.
El objetivo fue evaluar los efectos de la inmunización pasiva con anticuerpos policlonales anti-factor precoz de preñez (EPF) porcino en ratas preñadas durante el periodo preimplantacional, y analizar la distribución del EPF en la placenta de estas ratas. Se evaluaron los parámetros de fertilización, de desarrollo e inmunológicos. El número de embriones y la relación embriones/cuerpos lúteos de las ratas tratadas con el anticuerpo anti-EPF disminuyeron significativamente y los embriones presentaron un retraso en el desarrollo. Hubo un aumento significativo de IL-10 e INF-γ, y una disminución significativa de TGF-β en el grupo tratado con anti-EPF tanto en el suero como en la placenta. Se encontró una inmunotinción positiva en células gigantes y deciduales, en los vasos sanguíneos y en las lagunas trofoblásticas. Este estudio ha proporcionado nuevos resultados sobre la funcionalidad y la ubicación del EPF, y ha revelado la utilidad del anticuerpo policlonal anti-EPF porcino para ese propósito.
It is well-known that a successful interrelationship between maternal and embryonic tissues during the pre-implantation period activates mechanisms which prepare pregnancy implantation and development. It has been suggested that both the conceptus and immunosuppressive factors derived from the mother help to protect the foetus from immune rejection.1
Morton et al.2 reported that in rats, the rosette formation between lymphocytes and erythrocytes was inhibited when lymphocytes from male or non-pregnant females were pre-incubated with serum from pregnant rats. This inhibitory effect was caused by a pregnancy associated substance, called EPF (early pregnancy factor). Under normal physiological conditions, EPF is detected only during pregnancy. EPF is released in response to the presence of the embryo after 4h post-fertilization.3
EPF is composed of two subunits called EPF-A and EPF-B, which have different synthesis sites. EPF-A is synthesized within the oviduct during oestrus and pregnancy, while EPF-B is exclusively associated to pregnancy and is produced by ovaries through the pre-implantation period, and by the embryo during peri- and post-implantation period.4 The EPF is an important factor in establishing a successful pregnancy, and it may act both as a growth factor and as an immunosuppressive agent.5,6 Its role as a growth factor has been detected mainly during the first stage of embryonic development. The presence of EPF in tumour cell cultures is associated with cell division, suggesting a role of EPF as autocrine/paracrine growth factor. This hypothesis was confirmed by observing that the application of anti-EPF monoclonal antibodies decreased tumour cell growth and viability.7 The immunosuppressive function may be mediated by the induction of two soluble suppressor factors identified in mice and humans, EPF-S1 (EPF-induced suppressor factor-1) and EPF-S2 (EPF-induced suppressor factor-2).8,9 It is known that products of activated leukocytes from humans and mice, such us lymphokines and monokines (INF-γ, CSF, TNF-β, IL-10), produce developmental delay and embryonic death.10–13
It has been shown that EPF decreases clinical signs of autoimmune encephalomyelitis in rats and mice, suppressing the inflammatory response, the expression of adhesion molecules and the lymphocyte infiltration that induces the demyelination of the central nervous system.14–16 In the same model the Hsp 10/EPF exerts a protective role as a survival factor for oligodendrocytes.17
There are few reports about the localization of EPF. Cruz et al.18 carried out an immunohistochemical analysis of ovaries from pregnant and non-pregnant marsupials (Sminthopsis macroura) using an affinity-purified rabbit antibody against either recombinant EPF, which recognizes both EPF and Cpn-10. Sadacharan et al.,19 in a location study of EPF in tissues from rats by electron microscopy, found EPF extramitochondrial compartments using polyclonal antibodies anti-human Cpn-10. Also, an EPF localization study on tissue from human colon carcinoma showed diffuse punctate cytoplasmic staining.20 However, to our knowledge, there is no information about EPF in rat placental tissues.
Thus, the aim of this study was to evaluate the effect of passive immunization with polyclonal anti-swine EPF antibody, on embryonic developmental and immunological parameters during the pre-implantation period in rats, and to analyse the location and distribution of EPF in rat placenta. Also, the present investigation was undertaken to confirm a specific association between EPF and pregnancy, and to support the histological evidence of the presence and distribution of the EPF in the pregnant rat placenta. Considering the role suggested for EPF, it is hypothesized that passive immunization of pregnant rats with polyclonal anti-EPF antibodies during the pre-implantation period, will modify the normal embryonic development. Our study attempts to provide relevant knowledge for understanding the leading role of EPF in the normal course of pregnancy.
Materials and methodsPolyclonal anti-early pregnancy factor antibodiesProductionIn this study the polyclonal antibody used was developed against the N-terminal synthetic peptide. The N-terminal extreme of EPF, obtained from serum of a 7 day pregnant sow, was determined by Merkis,21 with an approximate molecular weight of 29kDa. Rabbit anti-swine EPF polyclonal IgG and nonspecific rabbit polyclonal IgG were produced and purified by the method described by Grosso et al.22
Antibody specificity assessmentWestern blotThe specificity of rabbit anti-EPF polyclonal IgG against swine and rat EPF was analysed through western blot. Antibody was tested for detection of EPF in rat and sow sera. Sera from non-pregnant and 10 days pregnant rats from the assay described below (Passive Immunization of pregnant rats with anti-swine EPF antibody) were used. Also, sera from both pregnant sows at different stages (10, 30, 60, and 90 days) and non-pregnant sows were evaluated (sera obtained from Escuela Agrotécnica Salesiana Ambrosio Olmos, Córdoba, Argentina). The sera were analysed on a 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE [Xcell SureLock™ Mini Cell/PowerEase® 500, Power Supply-Invitrogen Life Technologies]). The proteins were transferred to polyvinylidene fluoride membrane (PVDF Immobilon-P™ [Millipore, Bedford, MA, USA]) with a semi-dry blot device (Blot Module-Invitrogen™ Life Technologies). The membrane was incubated overnight at 4°C with rabbit polyclonal antibody anti-swine EPF diluted at 1:100 in blotto [Tris Saline Buffer with 0.1% Tween 20 (Merck, Art 8.22184) and 10% skim milk (Nestle, Vevey, Switzerland)]. Then washed and incubated 30min at 37°C with anti-rabbit IgG horseradish peroxidase conjugated (A9046 Sigma®) diluted 1:1000 in blotto. The membranes were stained with DAB (3-3′ diaminobenzidine [DAB Fast™ Tablets, Sigma® D4418]).
Passive immunization of pregnant rats with anti-swine early pregnancy factor antibodyAnimalsWistar rats (n=18) of approximately 180–200g were housed in individual cages (250cm2 and 8cm high) at standard bioterio conditions: positive air pressure, temperature between 20°C and 25°C and relative humidity between 50 and 60%, with an artificial light–dark system (12h:12h). Food and water were provided ad libitum.
Ethical aspectsRats were kept in the Bioterio of Facultad de Ciencias Exactas, Físico-Químico y Naturales of Universidad Nacional de Río Cuarto. Ethics protocol was approved by Universidad Nacional de Río Cuarto Ethics Committee. In order to guarantee a safe, correct and careful use and handling of experimental animals, the investigators proceeded according to specifications of the Canadian Council Animal Care Guide.23
Mating protocolThe oestrus cycle was studied through the evaluation of vaginal cells by optic microscopy for a month until the onset of regular cycles. Virgin female rats were caged overnight with one male (1:1). The mating was confirmed the next morning by the presence of a copulatory plug and/or sperm in the vaginal saline lavage. The pregnant rats were placed in individual cages again.
Animal groups and passive immunizationThe female rats were inoculated intra-peritoneally at 8, 16, 32 and 40h post-mating. Rats were inoculated with 100μl saline physiological solution (saline physiological control group, n=8), 500μg of nonspecific rabbit polyclonal IgG diluted in 100μl sterile physiological solution (nonspecific IgG control group, n=8) and 500μg of rabbit anti-swine EPF polyclonal IgG diluted in 100μl sterile physiological solution (anti-EPF IgG group, n=12).
Surgical procedureTen-day pregnant rats were sacrificed by decapitation. The reproductive tract was completely removed (ovaries, oviduct, uterine horns and cervix), washed thoroughly with sterile saline, and placed in sterile Petri dishes. Corpora lutea were counted using a binocular magnifying glass. Uterine horns were incised through the anti-mesometrial side; the total fetoplacental units were counted, and finally they were carefully removed. Before sacrifice, serum samples were taken for cytokines quantification (IL-10, INF-γ and INF-β) by ELISA and to evaluate the specificity of anti-EPF antibody by western blot.
Fertilization and developmental parametersOnce removed, the fetoplacental units were weighed on an analytical balance and measured in their antero-posterior axis with a scale ruler. The following data were recorded from each pregnant rat:
Fertilization parameters: total numbers of embryos and corpora lutea, and embryo/corpora lutea ratio.
Development parameters: weight and length of fetoplacental units.
The fetoplacental unit samples were fixed with buffered formalin at pH 7.4 for 72h, then dehydrated and embedded in paraffin. Thin sectioning was performed using a Leica microtome (3–4μm). The paraffin sections were rehydrated and then stained with haematoxylin–eosin (H/E) through routine protocols. A Carl Zeiss Axiostar Plus optic microscope was used, with a Canon PowerShot G6 7.1 Mega Pixel digital camera.
Placenta homogenatesApproximately 5g of feto-maternal interface was homogenized (Heidolph Diax 900) in saline sterile solution 1:3. The homogenates were centrifuged twice at 400×g for 5min to obtain the supernatant and stored at −20°C.
Immunological parametersCommercial enzyme-linked immunosorbant assay (ELISA) kits were used to determine maternal rat serum and placenta (homogenates) levels of the cytokines INF-γ, IL-10 (Endogen® ERIFNG/ERIL10 Pierce Biotechnology, Inc.) and TGF-β (Quantikine®, R&D Systems). In all cases procedures were conducted according to manufacturer's instructions.
Immunohistochemistry of early pregnancy factor in rat placentaTissue samples were fixed with buffered formalin at pH 7.4 for 72h and embedded in paraffin. Cuts of 5μm were made out of paraffin blocks and mounted on a slide treated with Vectabon™ (Vector Laboratories, CA). The samples were deparaffinized with xylene, and dehydrated with ethanol at increasing graduation. Endogen peroxidase was inhibited with 3% H2O2. The blocking solution, made of normal serum of goat diluted in PBS (1:66), was used for the first incubation. The samples were incubated with the primary antibody (rabbit anti-swine EPF IgG) diluted 1:10 in moist chamber at room temperature for 30min. After washing, the samples were incubated with the secondary antibody (biotinylated goat anti-rabbit IgG) diluted 1:1000 in moist chamber at room temperature for 30min. Later they were incubated for 30min with ABC reactant. Then the samples were stained with DAB (Sigma® D4418) at room temperature for 2–10min and a counterstaining was performed with Harris haematoxilin (15–40s). Sections were dehydrated through successive passages of alcohol at increasing graduation and xylene.
Samples of the same series of sections which were immunostained with rabbit nonspecific IgG and secondary antibody were used as negative controls. A Carl Zeiss Axiostar Plus optic microscope was used, with a Canon PowerShot G6 7.1 Mega Pixel digital camera.
Data analysisAll values were expressed as mean±SEM and data were evaluated through one-way ANOVA by LSD test using the software STATISTICA 6.0 (Stafsoft Inc., Tulsa, Oklahoma, USA) provided by UNRC (Serial No. ABA11113362827d60). To ensure validity, every experiment was carried out in triplicate. The correlation analysis was used to identify associations between variables including serum IL-10 and INF-γ, and serum and placenta cytokines. The correlation analysis was conducted with 95% confidence interval and P<0.05.
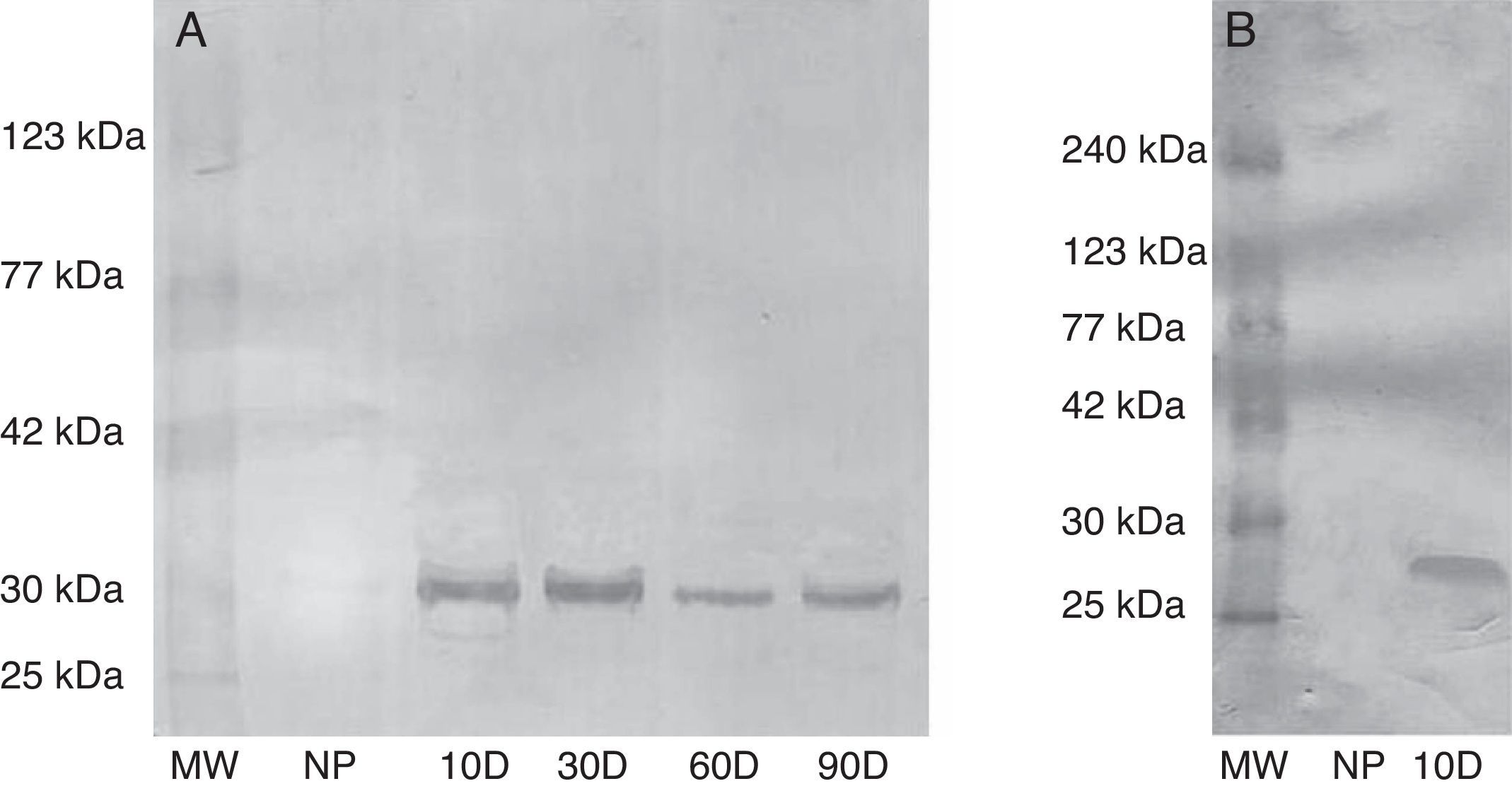
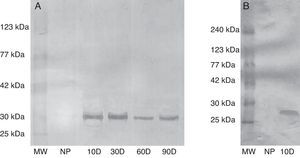
ResultsSpecificity of anti-swine early pregnancy factor antibodyTo investigate the ability of rabbit anti-swine EPF polyclonal antibody to detect EPF in swine and rat sera, western blots were conducted. The antibody showed specificity against both swine and rats EPF. This fact was evidenced by detection of a band of approximately 29kDa in sera from sows at different stage of pregnancy (Fig. 1a). Also, in pregnant rats sera a band of approximately 28kDa appears (Fig. 1b). These bands could correspond to EPF from swine and rat respectively. These bands do not appear in sera from non-pregnant rats neither sows.
Evaluation of the specificity of rabbit anti-swine EPF polyclonal IgG by western blot. (a) Serum from sow tested for EPF. (b) Serum from rats tested for EPF. MW: molecular weight, NP: not pregnant, 10D: 10 day pregnant, 30D: 30 day pregnant, 60D: 60 day pregnant, and 90D: 90 day pregnant. Results from three independent experiments carried out.
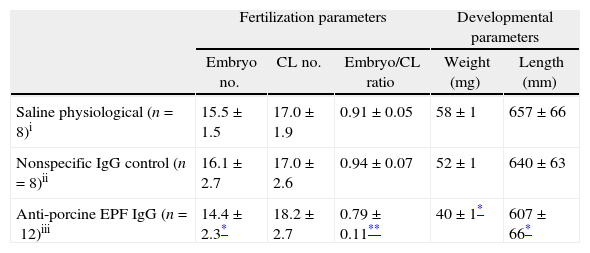
To evaluate the effect of EPF neutralization on embryonic development, pregnant rats were passively immunized with rabbit anti-swine EPF polyclonal antibody. The treatment of rats during the pre-implantation period with rabbit anti-swine EPF polyclonal IgG significantly decreased the average number of embryos per mother compared with control groups. Despite this decrease in number of embryos, all mothers continued their pregnancy, at least, until day 10. There was a significant decrease (P<0.05) in the embryo/corpora lutea ratio in the anti-EPF IgG group as compared with the control groups. This was not due to a natural loss of embryos from ovulation, because the average number of corpora lutea in the three groups was not significantly different (Table 1).
Fertilization and developmental parameters of pregnant rats and embryos.
| Fertilization parameters | Developmental parameters | ||||
| Embryo no. | CL no. | Embryo/CL ratio | Weight (mg) | Length (mm) | |
| Saline physiological (n=8)i | 15.5±1.5 | 17.0±1.9 | 0.91±0.05 | 58±1 | 657±66 |
| Nonspecific IgG control (n=8)ii | 16.1±2.7 | 17.0±2.6 | 0.94±0.07 | 52±1 | 640±63 |
| Anti-porcine EPF IgG (n=12)iii | 14.4±2.3* | 18.2±2.7 | 0.79±0.11** | 40±1* | 607±66* |
CL, corpora lutea; values were expressed as a mean±SEM.
The developmental parameters were also affected after the treatment with the rabbit anti-swine EPF IgG. This antibody inhibited the normal growth of the embryos. The treated group showed a significantly decreased (P<0.05) weights and lengths of fetoplacental units compared with those observed in the control groups (Table 1).
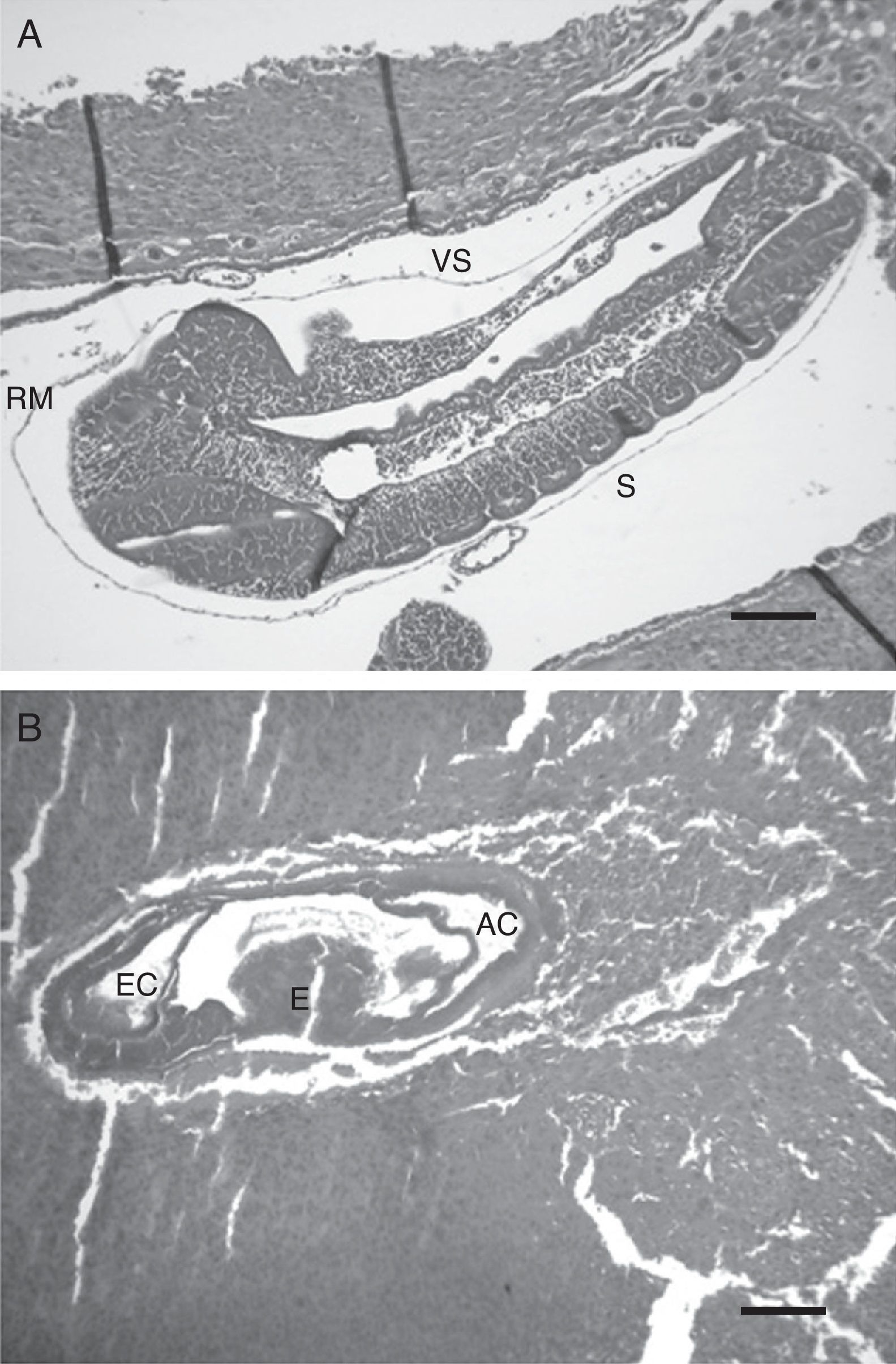
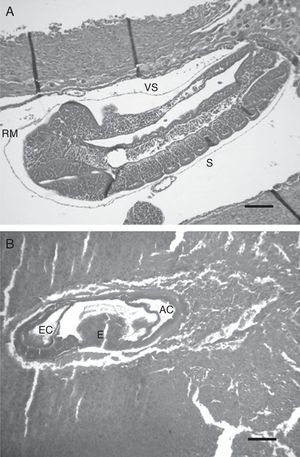
Microscopic studyThe inhibition of embryo growth caused by anti-swine EPF antibody was accompanied by retardation in the embryo development. The embryos in the anti-EPF IgG group showed a delay of 1–1.5 days compared with the control groups. These embryos belonged to 8.5–9th day of pregnancy; at this stage no somite formation was observed, and the intestine was developed but the allantoids could not be distinguished (Fig. 2b). On the other hand, embryos of control groups showed a normal development condition which belonged to the 10th day of pregnancy. An embryo of the nonspecific IgG control group is shown in Fig. 2a. Embryos were deemed normal of 10 day of pregnancy due to the presence of occipital, cervical and thoracic somites, the development of intestines and the presence of Reichert's membrane and vitalline sac.
Microscopic appearance of embryos after the passive immunization in pregnant rats. Photomicrographs illustrating the developmental stage of embryos of 10 days: (a) nonspecific IgG control group – H/E, 200×. Scale bar=248μm, (b) anti-EPF IgG group – H/E, 100×. Scale bar=234μm. RM: Reichert's membrane, S: somites, EC: ecto-placental cavity, E: exo-coelom, AC: amniotic cavity, and VS: vitelline sac. Representative embryos are shown.
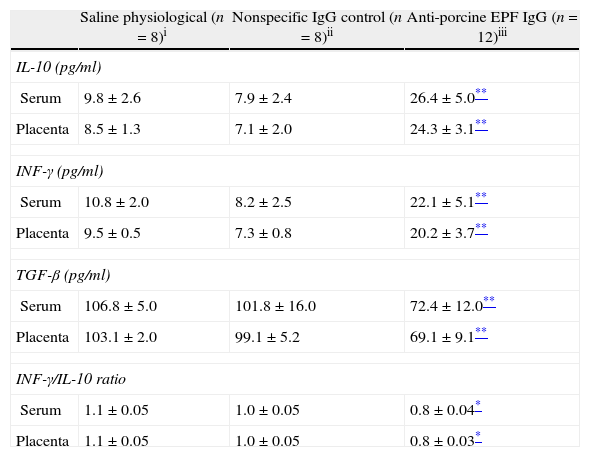
To determine the effect of neutralization of EPF on the immunologic mechanism in pregnancy, levels of type Th1 (INF-γ), Th2 (IL-10) and Th3 (TGF-β) cytokines were evaluated by ELISA. IL-10 and INF-γ concentrations in sera and placenta of the anti-EPF IgG group were significantly higher compared with the control groups. In contrast, the TGF-β was significantly lower in sera and placenta of the anti-EPF IgG group (P<0.01) (Table 2).
Immunological parameters in serum and placenta of pregnant rats.
| Saline physiological (n=8)i | Nonspecific IgG control (n=8)ii | Anti-porcine EPF IgG (n=12)iii | |
| IL-10 (pg/ml) | |||
| Serum | 9.8±2.6 | 7.9±2.4 | 26.4±5.0** |
| Placenta | 8.5±1.3 | 7.1±2.0 | 24.3±3.1** |
| INF-γ (pg/ml) | |||
| Serum | 10.8±2.0 | 8.2±2.5 | 22.1±5.1** |
| Placenta | 9.5±0.5 | 7.3±0.8 | 20.2±3.7** |
| TGF-β (pg/ml) | |||
| Serum | 106.8±5.0 | 101.8±16.0 | 72.4±12.0** |
| Placenta | 103.1±2.0 | 99.1±5.2 | 69.1±9.1** |
| INF-γ/IL-10 ratio | |||
| Serum | 1.1±0.05 | 1.0±0.05 | 0.8±0.04* |
| Placenta | 1.1±0.05 | 1.0±0.05 | 0.8±0.03* |
Values were expressed as a mean±SEM.
The group treated with the anti-EPF IgG showed a significantly lower INF-γ/IL-10 ratio (P<0.05) in serum and placenta compared with the control groups (Table 2). Moreover, there were positive correlation between serum concentration of IL-10 and INF-γ (r=0.84, P<0.05), and between concentrations of cytokines in serum and placenta (r=0.89, P<0.05).
Immunohistochemistry of early pregnancy factor in rat placentaWe have no found studies describing the distribution of EPF in rat placenta. So we have decided to carry out an immunohistochemical method to describe the localization and distribution of EPF in rat placental tissues.
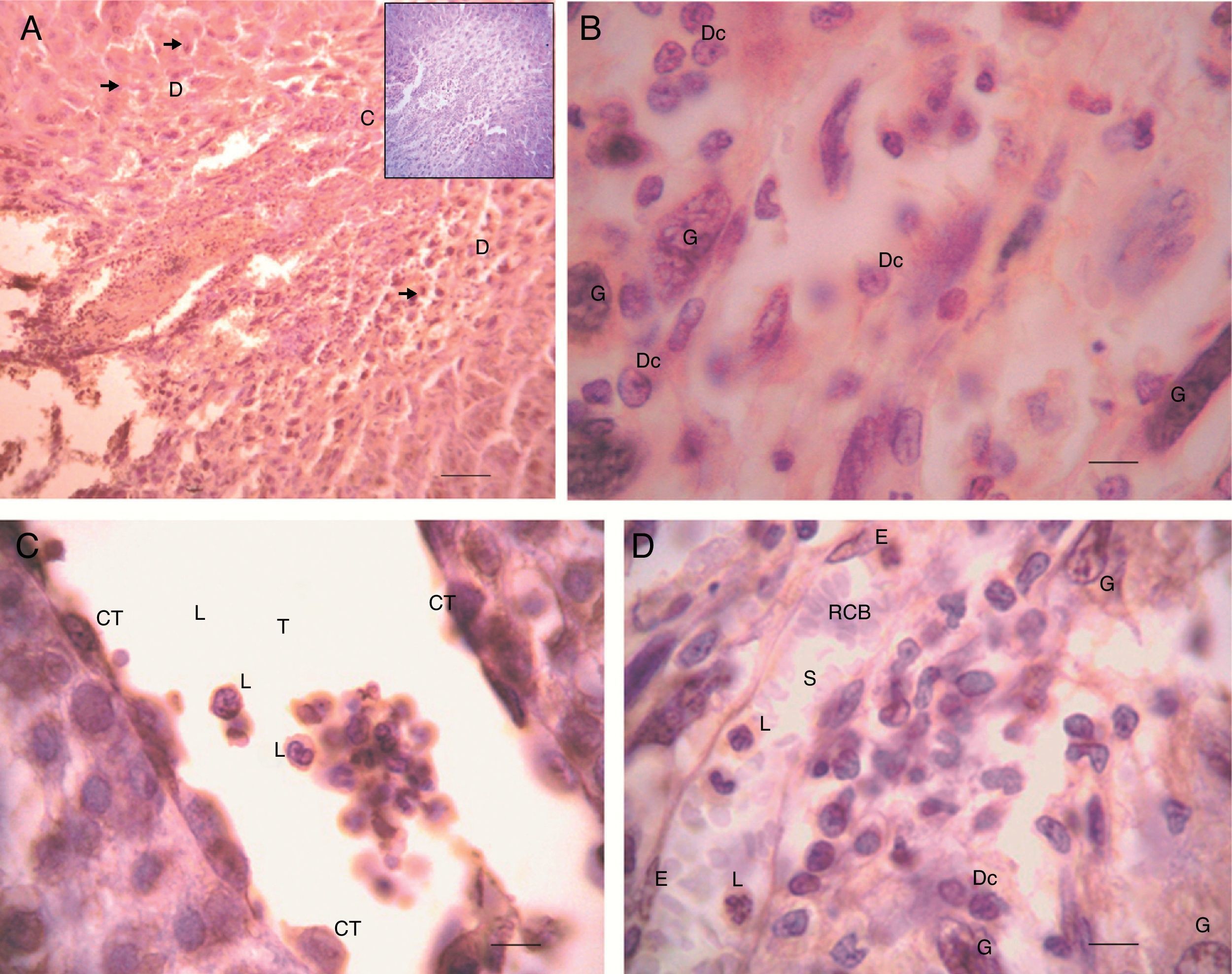
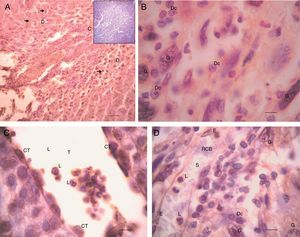
No differences in EPF staining intensity were found among the placentas from the three groups studied. A substantial immunostaining of EPF was found throughout the deciduous placenta (Fig. 3a). Intracytoplasmatic immunostaining was particularly found in giant and decidual cells (Fig. 3b). Thus, trophoblastic lacunas (Fig. 3c), particularly trophoblastic cuboid cells and leukocytes, showed EPF positive immunostaining, as well as maternal sinusoids, mainly the cytoplasm of epithelial cells (Fig. 3d).
Detection of EPF through rabbit anti-swine EPF polyclonal IgG by IHQ. Placenta from 10 days pregnant rat from non-specific IgG control group (a) 100×. D: decidua and C: ecto-placental cone. The arrows indicate giant trophoblastic cells. Scale bar=4.5μm. Negative controls are shown as inserts. (b) 1000×. G: giant trophoblastic cells and Dc: decidual cells. Scale bar=15μm. (c) H/E, 1000×. T: trophoblastic lacuna, L: leukocyte and CT: cuboidal trophoblastic cells surrounding the blood. Scale bar=15μm. (d) 1000×. S: maternal sinusoid, L: leukocyte, RBC: red blood cells, E: endothelial cell (flat) light lining the sinusoid, G: gigant trophoblastic cells, and D: decidual cells. Scale bar=15μm. Technique: Avidin–Biotin–Peroxidase with Harris haematoxylin counterstain, representative microphotographs of the studied group.
This study showed that passive immunization with anti-swine EPF antibody had an adverse effect on early embryonic development in rats. This result reveals that EPF of both swine and rat shares some epitopes. While there is no homology at rat24 and swine21 EPF N-terminal sequence the fact that the anti-swine EPF antibody binds to rat EPF (sera and placenta) leads us to believe that there is cross-reaction between these proteins. Probably the antibody developed against the N-terminal of the swine EPF detects any other internal amino acid sequence of the rat EPF.
In this study, the inoculation of anti-EPF IgG decreased the total number of rat embryos, as observed by Athanasas-Platsis et al.25 who carried out the experiment in mice. Unlike these results, in our study every rat treated with anti-EPF antibody maintained their pregnancy until day 10. However, the blocking of EPF through antibodies caused a significant decrease in the embryo/corpora lutea ratio compared with the values of the control groups.
Due to the fact that the ovulation degree (estimated by the average number of corpora lutea) was similar in the three groups, the decrease on the embryo/corpora lutea ratio indicates a lower presence of viable embryos in the treated group after 10 days of gestation.
A successful implantation requires a good synchronization between embryonic and endometrial development. Therefore, an implantation failure could occur due to the delay in growth and development of embryos caused by the anti-EPF treatment. In addition, the length and weight of embryo sacs were significantly lower after the blocking of EPF, suggesting a major role for EPF as a growth factor in the first period of pregnancy. Moreover, the microscopic examination showed that the decrease in weight and length corresponds to a remarkable delay in embryonic growth rate and, therefore in its developmental stages.
Embryological characteristics of the embryos treated with anti-swine EPF antibodies resemble those of an embryo from 8.5 to 9 days of gestation, without the formation of somites or neural plate development. On the other hand, embryos that were inoculated with control solutions showed greater lengths and other development conditions, with typical characteristics of 10 days embryos, such as presence of occipital, cervical and thoracic somites, and development of anterior, medium and posterior intestine. Studies by Quinn et al.7 revealed that EPF has a role as an autocrine growth factor in tumour cells, suggesting that EPF can act as a growth factor in cells with high growth rate. It is known that embryos experience a series of mitotic divisions during the early hours of gestation, causing a great increase in the number of cells. This exponential growth could be the reason why EPF is required for both, growth and development of normal embryos.
The decrease detected in the development of embryos, after treatment with anti-EPF antibodies, suggests that its application can modulate, at least partly, the role of EPF as a growth factor. Other researchers have also studied the importance of EPF in mice during pre- and post-implantation periods, using anti-EPF during these stages, observing embryonic loss and delayed embryonic development.25–27 EPF may be acting indirectly in early stages of pregnancy promoting important embryonic development pathways.
It is well known that EPF also has immunosuppressive activity.28 It has been suggested that the immunosuppressive action of EPF may have a direct influence on lymphocytes through high-affinity receptors, which may be provided by CD4+, CD8+, NK cells and monocytes. This is due to the fact that these are effector cells of the cell-mediated immune response and are involved in establishing a successful pregnancy. The direct influence of EPF on these cells could be the reason for its importance in a successful implantation and normal embryonic development.15,16,29
EPF also induces the expression of suppressor factors (EPF-S1 and EPF-S2). These factors act on the cells causing the release of cytokines responsible for immunomodulatory functions. These cytokines can alter some maternal reactions to the fertilized eggs at implantation time, when the blastocyst is in contact with the maternal circulation.8,9 One of the cytokines, INF-γ, is a proinflammatory cytokine involved in immune defense against intracellular organisms which determine an adaptive immune response with a Th1 pattern. Another cytokine, IL-10, is an anti-inflammatory cytokine which determines an immune response with a Th2 pattern. In this study these two cytokines increased, in sera and placenta, as a result of the treatment with anti-porcine EPF antibody. A substantial increase was observed in concentrations of INF-γ and IL-10 from treated rats, both in sera and placenta, more than twofold for both cytokines compared with those found in the control group. In our study, there was a positive correlation between these two cytokines regardless of the treatment. The decrease in number, length/weight and embryo development, as well as the embryo/corpora lutea ratio of the group treated with anti-EPF antibody, may be due to a failure in the balance of Th1/Th2 cytokines. The decrease in the INF-γ/IL-10 ratio in the treated group reflects this cytokine (Th1/Th2) imbalance. This could be due to a greater increase of IL-10 than that observed for INF-γ. The cytokine (Th1/Th2) imbalance has been associated with multiple failures in pregnancy and intrauterine growth delay.30,31 In normal pregnancies of mice, IFN-γ plays critical roles that include initiation of endometrial vasculature remodelling, angiogenesis at implantation sites, and maintenance of the decidual (maternal) component of the placenta.32 The third TGF-β is Th3 cytokine type, which may have both protective and regulatory effects promoting tolerance throughout the rat pregnancy period.33,34 For this cytokine, a significant decrease in the sera and placenta levels could be observed in the group treated with anti-EPF antibody compared with the control group. There was, also, a correlation between the concentration of cytokines in sera and placenta. The cytokine levels in sera are higher than that in placenta due to multiple sources of cytokines synthesis in pregnant animals, including trophoblasts, maternal endothelial cells and circulating leukocytes. Several authors reported the importance of different cytokines on normal embryonic development.35,36 Athanasas-Platsis et al.37 studied the importance of suppressor factors induced by EPF in pre- and peri-implantation stages in mice, the application of anti-EPF-S1 caused both, developmental delay and embryonic loss.
The results of our immunohistochemical study are, to the best of our knowledge, the first describing the localization of EPF in rat placental tissues. These results revealed a substantial staining, although there were no differences in the staining degree on the treated group compared with the control groups. A significant intracytoplasmatic staining was found in both, giant and decidual cells, as well as in cells which form the trophoblastic lacuna. The presence of EPF in placenta and serum at day 10 of gestation, regardless of treatment, shows that the effect caused by the application of the antibody is point in time. Once the EPF neutralization finishes, its prescence return to normal. The presence of EPF in placenta also suggests the key role as a growth factor, considering that placental tissue has a significant proliferative activity throughout the entire pregnancy. EPF may be playing an autocrine/paracrine role, has been advantageous for embryonic development, regulating maternal anti-fetal responses and stimulating development and trophoblast invasion.27
The presence of EPF was also found in the maternal sinusoids, mainly in endothelial cells. Probably EPF in conjunction with Epidermal Growth Factor (EGF) played an important role in the epithelialization of neoformed blood vessels. Therefore EPF would probably be acting as a growth factor to complete the process of angiogenesis. EGF and EPF have a high homology, indicating that both factors could share biological functions.38
We conclude that the blocking of EPF through antibodies may cause a delay in embryonic development and a decrease in development parameters, which ultimately leads to embryonic loss. EPF appears to be an important regulatory factor in cytokine balance, ensuring a proper immune environment to carry out pregnancy. Therefore, EPF may be considered to be an important molecule involved, either directly or indirectly, in the complex interaction that leads to successful pregnancy. The location and distribution of EPF in rat placenta constitutes an histochemical evidence of its autocrine-paracrine function in normal cells. This study has provided new results on the function and location of EPF, and has revealed the usefulness of polyclonal anti-swine EPF antibody.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Conflict of interestThe authors declare no conflict of interest.
This work was supported by FONCYT, SECYT-UNRC, CONICET (Argentina) and the project of bilateral cooperation in Science and Technology Argentina-Germany (SECYT-BMBF). The authors are grateful to Technician Graciela Sagripanti realizing the histological sections. The authors thank Professors Ileana A. Martínez and Verónica Muñoz for language assistance.