Bone morphogenetic proteins (BMPs) play a key role during vertebrate embryogenesis and organogenesis, and have also been described to function in self-renewing tissues in adults. Several reports have demonstrated that distinct BMPs are involved in the control of the proliferation of different hematopoietic cell types. In this study, we provide evidence that murine peripheral CD4+ T cells express the three type I BMP receptors (BMPRIA, BMPRIB, ActRIA), and the proportions of BMP-expressing T cells increases notably after both anti-CD3 and anti-CD3/CD28 stimulation. The BMP signalling pathway is functional in peripheral CD4+ T lymphocytes since the culture of CD4+ T cells in the presence of BMP4 increases the levels of phosphorylated Smad1. In addition, our results show that the addition of BMP2 and BMP4 during anti-CD3/CD28 stimulation differentially modulates the proliferation of CD4+ T cells. Altogether, the data indicate a role for the signalling pathway of BMP in T cell responses.
Las Proteínas Morfogenéticas Óseas (BMPs) desempeñan un papel clave durante la embriogénesis y organogénesis de vertebrados, y también se ha descrito que llevan a cabo diversas funciones en tejidos adultos con capacidad auto-renovadora. Varios trabajos han demostrado que distintas BMPs están implicadas en el control de la proliferación de diferentes tipos celulares hematopoyéticos. En este estudio nosotros evidenciamos que las células T CD4+ periféricas de ratón expresan los tres tipos de receptores tipo I de BMP (BMPRIA, BMPRIB, ActRIA), y que la proporción de células T que expresan los receptores para BMPs incrementa notablemente tras estimulación con anticuerpos anti-CD3 y anti-CD3 más anti-CD28. La vía de señalización BMP es funcional en estas células T CD4+ periféricas puesto que al cultivarlas en presencia de BMP4 se incrementan los niveles de Smad1 fosforilada. Además, nuestros resultados demuestran que la adición de BMP2 y BMP4 durante la estimulación con anti-CD3/CD28 modula de manera diferencial la proliferación de las células T CD4+. En conjunto, los resultados indican que la vía de señalización BMP juega un papel en las respuestas de células T.
Bone morphogenetic proteins (BMPs) are secreted signalling proteins, which form a subgroup of the TGF-β superfamily(1). BMPs initiate signalling by binding to a heteromeric complex constituted by type I and type II serine/threonine kinase receptors. Type II receptors are constitutively active kinases, which transphosphorylate type I receptors upon ligand binding. Type I receptors activate intracellular substrates by phosphorylation, and thus determine the specificity of intracellular signals. The main BMP receptors are constituted by the combination of type II BMP receptor (BMPRII) and the following type I receptors: type IA BMP receptor (BMPRIA)/ALK- 3, type IB BMP receptor (BMPRIB)/ALK-6, and type IA Activin receptor (ActRIA)/ALK-2(2–6). BMP signal transduction to the nucleus is mediated by proteins of the Smad family.
Specifically, Smad1, Smad5 and Smad8 (also called BMP receptor-regulated Smads or BR-Smads) are phosphorylated and activated by type I BMP receptors. BR-Smads then form complexes with Smad-4, which translocate into the nucleus where they regulate gene expression(5–7). In addition to this canonical Smad-dependent signalling pathway, BMP receptors may activate a noncanonical Smad-independent pathway leading to phosphorylation of p38(8).
BMPs were originally identified by their ability to induce ectopic cartilage and bone formation(9). Nevertheless, it has been shown that BMPs also regulate cell proliferation, differentiation and apoptosis during embryogenesis(10), and also in adult self-renewing tissues, such as the hematopoietic and immune systems. Different BMPs have been demonstrated to participate in the control of the expansion and differentiation of hematopoietic precursor cells, in synergy with several cytokines(11–13). BMP6 inhibits the growth of human B cell progenitors and mature B lymphocytes(14,15), and several BMPs have been shown to have anti-proliferative and pro-apoptotic effects in multiple myeloma cells(16,17). We and others have demonstrated the expression of BMP2 and BMP4 in the thymus, where these BMP ligands are involved in regulating proliferation, survival and differentiation during early T-cell development(18–20).
The expression of BMP receptors has been reported in lymphoblastoid cell lines as well as in the Jurkat TAg cell line, whose proliferation is inhibited in the presence of BMP6(21,22). We describe in this study the expression of functional BMP receptors in peripheral murine CD4+ T lymphocytes and the modulation of T cell proliferation by BMP2 and BMP4.
MATERIALS AND METHODSMiceYoung adult BALB/c mice (4–6 weeks old) were purchased from Harlan Ibérica (Barcelona, Spain) and maintained under pathogen-free conditions. Experiments were performed according to institutional guidelines and were approved by the Complutense University Ethical Committee for Animal Experimentation.
Flow cytometryThe following mAb conjugated with FITC, PE, Cychrome or APC were used for flow cytometric analysis: CD4 (RM4- 5), CD25 (PC61), and CD69 (H1.2F3) from BD Biosciences (San José, CA). The extracellular domains of BMP receptors were detected with PE-conjugated anti-BMPRIA (E-16) and anti-BMPRIB (N-17) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA), FITC-conjugated anti-BMPRIA antibodies (R&D Systems, Minneapolis, MN), and unconjugated anti-ActRIA antibodies (R&D Systems), followed by fluorochromeconjugated, multiadsorbed F(ab')2 fragments of donkey anti- goat IgG (Jackson ImmunoResearch Laboratories; West Grove, PA). Two- and three-color immunofluorescence stainings were performed by incubating the cells in PBS containing 1% FCS and 0.1% NaN3 in the presence of saturating amounts of fluorochrome-conjugated antibodies for 30 min at 4°C. For the intracellular stainings of phosphorylated Smad1, and according to the manufacturer's instructions, cells were treated with Cytofix/Cytoperm solution (BD Biosciences) for 20 min at 4°C, washed with Perm/Wash buffer (BD Biosciences), and stained with antiphospho-Smad1 (Ser463/Ser465) (Santa Cruz Biotechnology) followed by fluorochrome-conjugated, multiadsorbed F(ab')2 fragments of donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories), all diluted in Perm/Wash buffer. Analyses were conducted in a FACSCalibur flow cytometer (Centro de Microscopía y Citometría, Complutense University of Madrid).
Proliferation assaysNaive CD4+ T cells were isolated from lymph nodes by magnetic sorting using a negative selection protocol (Miltenyi Biotech, Bergisch, Germany). Isolated CD4+ T cells (1–2 x105) were cultured in 96-well flat-bottom culture plates in AIM-V serum-free medium (Invitrogen, Grand Island, NY) and stimulated with immobilized anti-CD3 (3 μg/ml) with or without anti-CD28 (1 μg/ml) mAb (BD Biosciences). In some cultures different doses (1-300 ng/ml) of rhBMP4 (R&D Systems) and rhBMP2 (RDI Research Diagnostics, Flanders, NJ) were added. At different time points, cultures were pulsed for 12 h with 10 pM 5-bromo-2′-deoxyuridine (BrdU). A specific kit from Roche Diagnostics (Barcelona, Spain), BrdU Labeling and Detection Kit III, was used to measure BrdU incorporation into newly synthesized DNA. Briefly, the labeling medium was removed, and cells were dried (2 h at 60 °C), fixed in ethanol in HCl (0.5 M) for 30 min at −20°C, treated with nucleases (30 min at 37°C), and then incubated with peroxidase-conjugated Fab fragments of mouse anti-BrdU (30 min at 37°C). The peroxidase reaction was developed with ABTS substrate, and the sample absorbance was measured using an ELISA reader (ELX800MB, Bio-Teck Instruments, VT) at 405 nm with a reference wavelength at 492 nm.
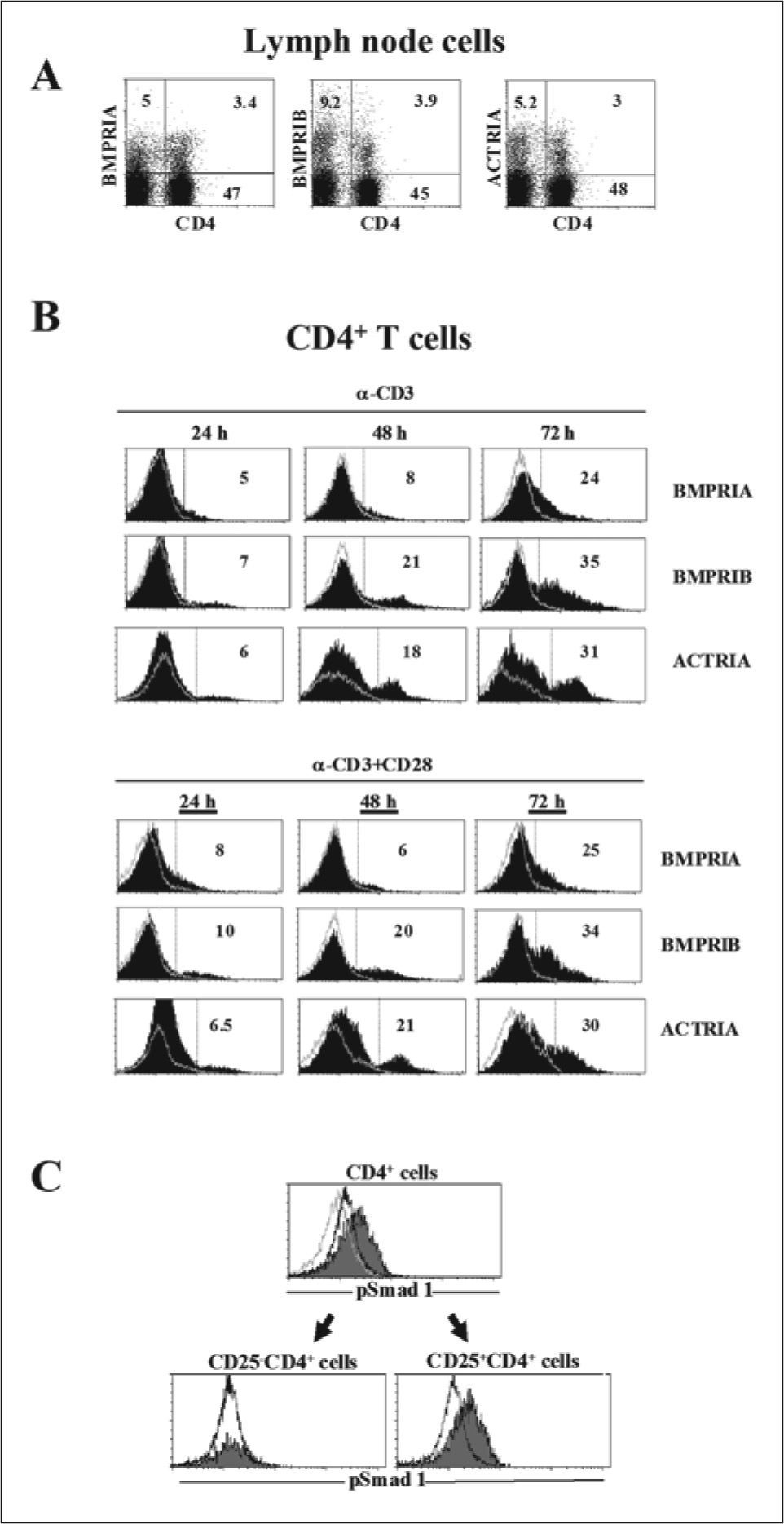
RESULTSExpression of a functional BMP signalling pathway in peripheral CD4+ T cellsTo determine whether murine CD4+ T cells are targets for BMP, the cell surface expression of BMP receptors was analyzed by flow cytometry. As shown in Figure 1A, about 6-8% of naive resting CD4+ T cells expressed the three type I BMP receptors, BMPRIA, BMPRIB and ActRIA. Next, naive CD4+ T cells were isolated from lymph nodes, stimulated with immobilized anti-CD3 mAb with or without anti-CD28 mAb, and the BMP receptor expression was analyzed at different time points. Figure 1B shows that both anti-CD3 and anti-CD3/CD28 stimulations induced a similar upregulation of BMP receptor expression. Activated CD4+ T cells up-regulated BMPRIA expression at 72 h, whereas BMPRIB and ActRIA expression was clearly up-regulated at 48h, increasing the proportion of positive cells at 72 h (Figure 1B). After 48 h of stimulation, BMP receptor-bearing T cells mostly express CD69, and about 60% express CD25 (data not shown).
Peripheral CD4+ T cells express functional BMP receptors. A) Dot plots show the expression of CD4 vs type I BMP receptors (BMPRIA, BMPRIB and ActRIA) on murine lymph node cells. B) CD4+ T cells were isolated from lymph nodes, and stimulated with anti-CD3 or anti-CD3 plus CD28 mAb for 24, 48 and 72 h. Black histograms show the expression of BMPRIA, BMPRIB and ActRIA on activated T cells. Grey lines indicate background staining. The percentages of positive cells are shown in each histogram. C) Isolated CD4+ T cells were stimulated with CD3/CD28 mAb for 48 h and after cultured in the absence (black lines) or presence (solid histograms) of BMP4 (100 ng/ml) for two more hours. The expression of CD4, CD25 and phosphorylated Smad1 was then analyzed. Grey lines indicate background staining.
To demonstrate that the BMP signalling pathway is active in CD4+ T cells, we studied the effect of BMP addition on Smad1 phosphorylation. Smad1 is a receptor regulated Smad, directly phosphorylated and functionally activated by the BMP receptor upon ligand binding(23). The levels of phosphorylated Smad1 notably increased when CD4+ T cells were cultured in the presence of BMP4 after stimulation with anti-CD3/CD28 mAb for 48 h (Figure 1C). In addition, we showed that the expression of phosphorylated Smad1 was associated with activated CD4+ CD25+ T cells `
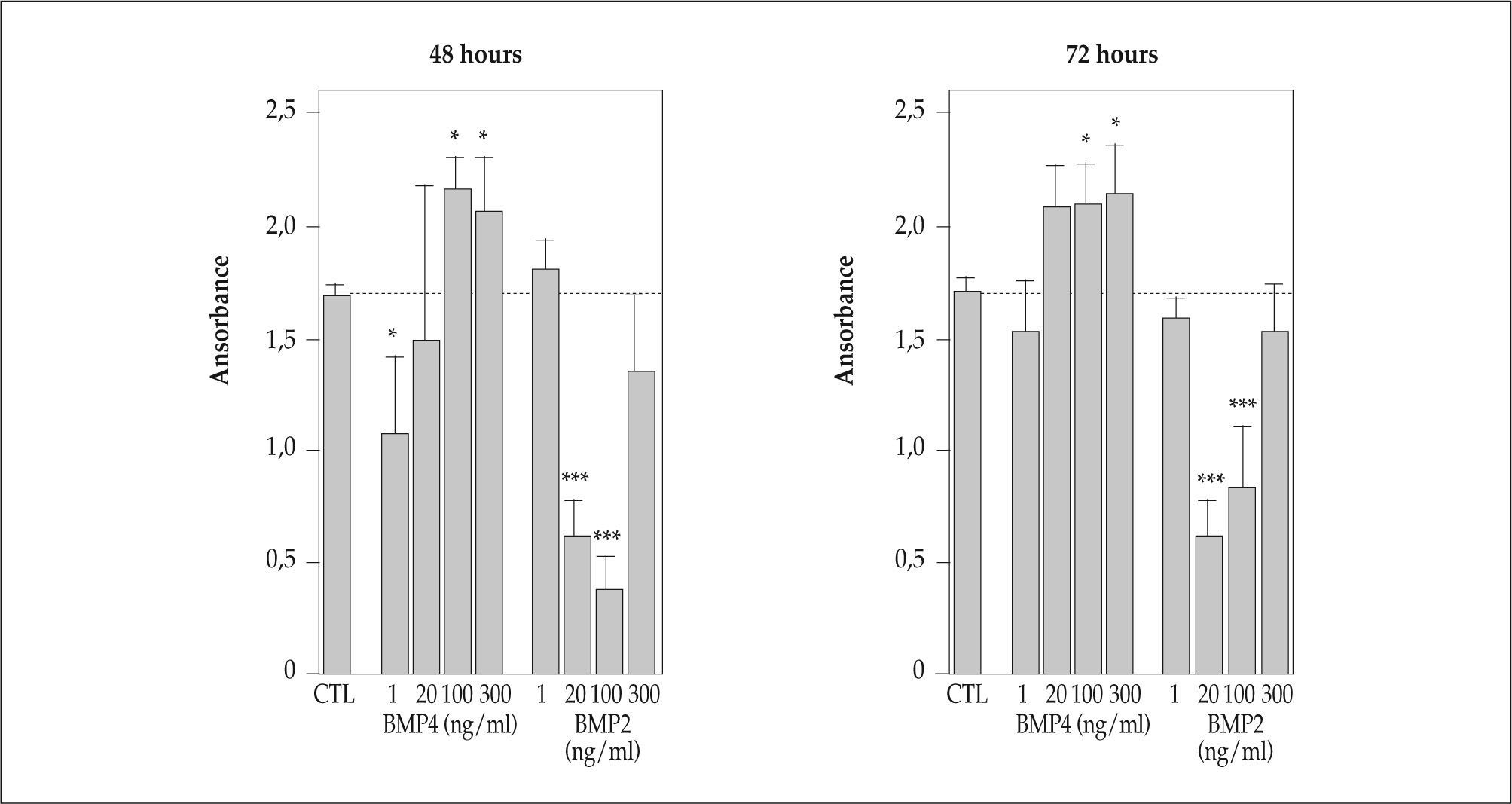
BMP influences CD4+ T cell proliferationGiven that the BMP signalling pathway is functionally active in peripheral CD4+ T cells and has been also involved in regulating the proliferation of several cell types(11,12,14–17,22), we investigated whether BMP signalling modulates CD4+ T cell proliferation. Naive peripheral CD4+ T cells were isolated and stimulated with anti-CD3/CD28 mAb for 48 and 72 h in the presence of different doses of BMP4 and BMP2, which we have previously shown that play an important role in T-cell differentiation in the murine thymus(20). Interestingly, the effects of the addition of BMP4 and BMP2 on CD4+ T cell proliferation were different. Low doses of BMP4 decreased the proliferative rate of CD4+ T lymphocytes, whereas the addition of medium to high doses of BMP4 significantly stimulated the proliferation over control values (Figure 2). On the contrary, the presence of medium BMP2 doses drastically inhibited the proliferative response of CD4+ T cells, whereas the addition of low and high doses of BMP2 hardly affected T-cell proliferation (Figure 2).
BMP2 and BMP4 influences CD4+ T cell proliferation. Isolated CD4+ T lymphocytes were stimulated with anti-CD3/CD28 in the absence or presence of different doses (1–300 ng/ml) of BMP2 and BMP4. After 48 and 72 h, T cell proliferation was analyzed by measuring BrdU incorporation into newly synthesized DNA, as described in Materials and Methods. Bars represent the mean (± SD) of three to four independent experiments (*, p ≤ 0.05; ***, p ≤ 0.001, by t test).
In this study we report the expression of a functionally active BMP signalling pathway in murine peripheral CD4+ T cells. Low numbers of naive CD4+ T cells express BMP receptors but the proportions of BMP receptor-expressing T cells increase notably after anti-CD3 and anti-CD3/CD28 stimulation. The increase in the levels of phosphorylated Smad1 after short-term culture with BMP4 demonstrates that the BMP pathway is functional in peripheral CD4+ T lymphocytes. In agreement with our results, de la Peña et al.(21) reported the expression of functional BMP receptors in lymphoblastoid cell lines established from human peripheral blood mononuclear cells. Likewise, Sivertsen et al.(22) showed that human Jurkat TAg cells as well as human peripheral blood CD4+ T cells, mostly with a CD45RO+ memory phenotype, express BMP receptors, and BMP6 stimulation of these cells led to Smad1/5/8 phosphorylation. However, the composition of the active BMP receptor complex expressed by T cells remains controversial. Our data indicate that the three type I BMP receptors are expressed similarly in both naive and activated CD4+ T lymphocytes, whereas BMPRIA is the main type I BMP receptor expressed by lymphoblastoid cell lines(21) and ActRIA is largely expressed in Jurkat TAg cells and human peripheral blood CD4+ T cells(22). A differential BMP receptor expression in distinct T cell subpopulations and species-specific differences could account for the discrepancy between these results.
Our results indicate that the BMP signalling pathway plays a role in peripheral T lymphocytes mainly after activation via CD3/TCR, influencing, at least, T cell proliferation. Different reports have demonstrated that BMP ligands have the ability to modulate the proliferation of several hematopoietic cell types such as thymocytes(18–20), B lymphocytes(14,15), hematopoietic stem cells(11–13) and Jurkat TAg cells(22).
An interesting finding derived from this study is that BMP2 and BMP4 ligands differentially modulate T cell proliferation. BMP2 and BMP4 normally induce similar cellular responses(24,25), but differential effects of different doses of BMP4 compared with BMP2 have been also reported on human hematopoietic stem cells(11). Bathia and collaborators explained those results according to the possible existence of different cell subpopulations expressing different BMP receptors and/or regulatory intracellular molecules(11). Since both canonical (Smad mediated) and non-canonical (p38 MAPK mediated) BMP signalling pathways have been described to function in T cells(21,22), an alternative explanation is that BMP2 and BMP4 could differentially trigger the Smad and non-Smad-dependent signalling pathways leading to distinct cell responses, as reported in other cellular systems(26,27).
In summary, we show that the BMP pathway is functional in peripheral murine CD4+ T cells. Furthermore, the expression of BMP receptors mainly in activated T cells and the BMP modulation of T cell proliferation suggest that BMP signalling is a physiological component of CD4+ T cell responses.
DISCLOSURESThe authors declare no financial conflict of interest.
ACKNOWLEDGMENTSThis work was supported by grants BFU2006-00651 (Ministerio de Educación y Ciencia), RD06/0010/0003 (Instituto de Salud Carlos III), GR74/07-910552 (Universidad Complutense / Comunidad Autónoma de Madrid), and PR34/07-15867 (Universidad Complutense / Grupo Santander).