The efficacy of heat-killed Mycobacterium tuberculosis (HKMtb) and its culture filtrate proteins (CFP) to activate blood mononuclear cells (MC) and polymorphonuclear neutrophils (PMN) from tuberculosis patients was investigated. Respiratory burst, NO-derived metabolites, IL-2, IL-12 and TNF-α production of stimulated cells from 16 HIV- tuberculosis patients and 12 healthy controls were analyzed. Increased amounts of TNF-α in supernatants from baseline and stimulated polymorphonuclear and mononuclear cells of tuberculosis patients were detected when compared with controls, except for CFP stimulated neutrophils. Augmented IL-2 and IL-12 levels were observed in supernatants of both stimulated MC and PMN from TBP while no differences were found in control supernatants. The patients had a lower respiratory burst response than the controls, for both cell types, regardless of the stimulus employed. Higher nitrite concentrations were found in HKMtb- and CFP-stimulated mononuclear supernatants from patients, compared with controls.
The obtained data of the stimulated cellular responses provides us information about the protective immunity against Mycobacterium tuberculosis and some resources to obtain a more efficient anti-tuberculous therapy.
En este estudio se investigó la eficacia de M. tuberculosis muerto por calor (Mtbi) y las Proteínas del Filtrado del Cultivo (PFC) en la activación de las células mononucleares (MC) y polimorfonucleares neutrófilos (PMN) de sangre periférica de pacientes tuberculosos. Se evaluó en 16 pacientes tuberculosos, HIV- y 12 controles sanos el Estallido Respiratorio, los metabolitos derivados del NO y la producción de IL-2, IL-12 y TNF-α por las células estimuladas. Se detectó un incremento en la concentración de TNF- α en el sobrenadante de cultivo (s.c.) de PMN al comparar con los valores basales y en la evaluada en s.c. de MC y PMN estimulados, al ser comparadas con las del grupo control, excepto para los neutrófilos estimulados con PFC. Se mostraron niveles aumentados de IL-12 e IL-2 en s.c. de ambas células, MC y PMN estimuladas por en PTB, mientras que no se hallaron diferencias en los s.c. de los controles. Los valores basales de Estallido Respiratorio (RB) detectada en MC y PMN de pacientes no difirieron significantivamente de los correspondientes al grupo control. La expresión del Estallido Respiratorio en ambos tipos celulares fue menor en los pacientes que en los controles, independientemente del estímulo empleado. Se determinaron concentraciones de nitritos más elevadas en los sobrenadantes de las MC estimuladas con Mtbi y PFC provenientes de pacientes, comparadas con las de los controles. Los datos obtenidos relacionados al estímulo de la respuesta celular, nos proporcionan información sobre la inmunidad protectiva contra el M. tuberculosis y, a la vez, aportan algunos recursos útiles para una terapia anti-tuberculosa más eficiente.
Tuberculosis (TB) is still one of the major public health problems in the world and the control of this disease, essential for the preservation of people's health, will need the development of a safe and highly efficacious vaccine, or the discovery of a more efficient anti-tuberculous therapy(1-3).
Different mycobacterial components have been found to induce either a protecting or detrimental immune response. Consequently, we thought that increasing the information on the mechanisms that induce a protective immune response in the host would provide some resources to obtain a more efficient anti-tuberculous therapy.
Cell-mediated immunity, leading to granuloma formation to constrain Mycobacterium tuberculosis (Mtb), is the main component of the host defense against tuberculosis, and is regulated by the balance of cytokines secreted mostly by mononuclear phagocytes and lymphocytes. Thus, the bacteriostactic and bactericidal function of cytokine-activated macrophages mediate to a great extent the immune response against Mtb(4). Previous investigations by our group in Tuberculosis Patients (TBP) with different degree of lung involvement demonstrated that the in vitro synthesis of NO, TGF-β and TNF-α was increased in cases with progressive disease, whereas a more adequate production of IFN-γ predominated in patients with mild forms of the disease(5).
TNF-α, mainly produced by macrophages, is a crucial mediator that up-regulates the microbicidal activity against mycobacteria in human monocytes or monocyte-derived macrophages(6). After cellular recognition of Mtb through Toll Like Receptors (TLRs), a stimulatory effect with production of IL-12, a strong proinflammatory cytokine, is also induced(7).
IL-2 produced by activated T lymphocytes performs a central function in the regulation of cell mediated immune response to Mtb, being responsible for the activation and expansion of T cells. Protective immunity against Mtb is mediated by IL-2 and IFN-γ, which stimulate the expansion and functional capacity of NK cells, as well as the pool of antigen-specific lymphocytes. As an important mediator for eradicating intracellular Mtb, IL-2 limits the bacillus' replication through the activation of macrophages by an IFN-γ-mediated process. IL-2 also acts directly through the development of cytotoxic T lymphocytes, which recognize Mtb antigens on infected cells, further contributing to their destruction(8).
Phagocytosis is a strong signal for IL-12 production by Mtb-infected monocytes, which in turn modulates IFN- γ production(9). It is accepted that IL-12 actively participates in the initiation and regulation of Th1 responses, and that it is rapidly induced after Mtb infection. In previous studies by our group, it was shown that abnormalities in the cellular immune response in pulmonary tuberculosis are related to the amount of lung involvement, the administration of anti-tuberculosis therapy, and the regulatory influences of cytokines and NO produced by immunocompetent cells(10-14).
In addition, a relationship between the oxidative capacity of PMN and MC from TBP with disease severity and treatment efficacy was also reported(5).
On the other hand, numerous findings indicate that biosynthesis of nitrate occurs in mammalian cells and that macrophages are a major source of mammalian nitrate synthesis(15,16). Studies in mice have demonstrated that the mycobacteriostatic activity involves the generation of nitric oxide and its derivatives (NO-2, NO-3). Several reports demonstrated an important role of NO in mycobacterial killing, particularly during the initial phase of the infection(17-19).
As neutrophils and mononuclear cells play an essential role in the initiation and direction of adaptive T-cell immunity, an effective stimulus is needed to activate these cellular mechanisms to elicit effective host defenses against Mtb. Accordingly, this study was designed to find out the efficacy of heat killed Mtb (HKMtb) and its culture filtrate proteins (CFP) to activate peripheral blood mononuclear cells (MC) and polymorphonuclear neutrophils (PMN) from pulmonary TBP. Our studies analyzed their oxidative capacity (Respiratory Burst, RB), as well as the production of NO-derived metabolites and several cytokines like IL-2, IL-12 and TNF-α.
MATERIALS AND METHODSStudy design and patientsIn this study we investigated the activation of PMN and MC when exposed to HKMtb and Mtb CFP. The sample population comprised 16 TBP and 12 healthy controls (HCo). The patients had a newly diagnosed pulmonary TB and HIV negative serology with a mean age of 36.5±16.8 years (mean ± SD). On presentation the patients were given a full clinical examination, a chest x-ray was performed, and sputum samples were taken for smear and culture for tubercle bacilli. Twelve age- and sex-matched HCo (mean overall age, 37.7±15.9 years), PPD negative, were also included. Informed consent was obtained from each subject. The studies were approved by the Ethical Committee of the Carrasco Hospital of Rosario and the Ethical Committee of the Medical Sciences Faculty, National University of Rosario (on March 30th, 2005).
MethodsMycobacterium tuberculosis H37Rv cultureMycobacterium tuberculosis H37Rv (ATCC 27294) strain was provided by Malbrán Institute, Buenos Aires, Argentine. The bacilli were cultured in Middlebroock 7H9, during 23 weeks; they were then inoculated in Sauton´s medium, and incubated during 3-4 weeks at 37°C with gentle agitation until a concentration of 6×108 bacteria/ml was obtained. Two drops of the sample were inoculated onto 5% blood agar to test for contamination by other bacteria, and plates were incubated for 24 h at 37°C. As no colonies were observed in the blood agar plates, the bacteria were dissolved 1:1 in 80% glycerol and the batch was frozen at −20ºC.
Heat inactivationTwo milliliters of culture medium containing 6x108 bacteria/ml were loaded in each centrifuge tube. Heat treatment at 80ºC for 30 minutes, with gentle agitation effectively killed bacteria. Viability was controlled in each tube by culturing its content in Middlebrook 7H10 medium for 6 to 8 weeks at 37ºC.
CFP obtentionMtb was obtained from a frozen stock. Culture flasks containing 50 ml of preheated (37ºC) modified Sauton's medium were inoculated with 6x108 bacteria/ml and they were cultured during 10 to 14 days at 37ºC with gentle agitation. Then, 50 ml of Sauton's medium were inoculated with 1-1.5×108 bacteria/ml of the starter culture and incubated a 37ºC with agitation for 7-8 days. 100 μl from each flask was taken under sterile conditions and inoculated onto 5% blood agar plates which were incubated for 24 h at 37ºC, to test for contamination by other bacteria. Bacteria were allowed to settle, the culture was filtered with 0.2 μm filters, and the absence of mycobacteria in the filtrate was verified by Ziehl-Nielsen staining and Middlebrook 7H10 culture during 6-8 weeks at 37ºC. The filtrate was stored at −20ºC.
Subsequently, the filtrate was concentrated by dialysis with PEG 10000, the concentrate volume was measured, and ammonium sulfate at 80% saturation at 4ºC was added. Next, it was centrifuged at 13000 xg, the precipitate dissolved in PBS, pH 7.4, dialyzed against PBS, filtered through 0.2 μm filters, and stored at −70ºC.
Analysis of CFPTo analyze CFP protein concentration, the Standard Bradford Assay and the SDS-PAGE profile were applied according to Laemmli(20).
Protein concentration was determined by the Bradford method, in which Comassie Brilliant Blue G-250 bound to the protein, changing the color absorption from 465 to 595 nm, detected at 595 nm absorbance. A standard curve with BSA was done and samples were processed in duplicate.
The electroforesis was done on slab gels as described by Laemmli. The resolving gel was 12% acrylamide in Tris-HCl (pH 8.8) and the stacking gel was 5% acrylamide in Tris-HCl (pH 6.8). The CFP batches (30-45 μg/lane) were diluted 1:2 in sample loading buffer (Tris-HCl 62.2 mM, glycerol 25%, SDS 2%, bromophenol blue 0.01%, BioRad, dithiothreitol 350 mM), and was heated to 100ºC for 5 minutes. Electrophoresis was carried out by applying a constant current of 80V per slab until the proteins were stacked and then applying 150V per slab until the bromophenol blue migrated to the end of the gel. The gels were stained with Coomassie blue.
The prestained molecular weight markers used in the gel were obtained from BioRad, containing phosphorylase B, 116 Kd; bovine serum albumin, 80 Kd; ovalbumin, 52 Kd; carbonic anhydrase, 34.9 Kd; soybean trypsin inhibitor, 29.9 Kd; and lysozyme, 21.8 Kd.
Isolation and culture of MC and PMNFor isolation of MC and PMN, heparinized blood obtained by venipuncture from TBP and HCo was diluted 1:1 in phosphate buffer saline (PBS) in a polypropylene tube, layered over a Ficoll-Hypaque gradient (Density 1.077) and centrifuged at 400 xg for 20 min at room temperature (19- 22ºC). The following three fractions were obtained after centrifugation: fraction 1: plasma; fraction 2, at plasma-Ficoll interface: peripheral blood mononuclear cells; fraction 3 (pellet): granulocytes and red blood cells (RBC). MC were recovered from the plasma-Ficoll interface, and PMN from the upper part of the pellet. MC and PMN were washed three times with PBS.
PMN were added into a 50 ml tube with lysis reactive 1× (150 mM NH4Cl, 10 mM NaHCO3, 1.28 mM EDTA) which was incubated at room temperature for 10 minutes, centrifuged and washed with PBS. The monocyte preparations were >95% viable and contained >95% mononuclear cells on cytocentrifuge specimens (Shandon Southern Cytospin; Shandon, Pittsburgh, PA) stained with a modified Wright- Giemsa stain (Diff-Quick; American Scientific Products, McGaw Park, IL). More than 95% expressed CD14 as determined by immunofluorescence flow cytometry. The purity of PMN was determined by using flow cytometric analysis; the preparations were >95% viable and contained >98% polymorphonuclear cells.
Following washing, MC and PMN were resuspended in RPMI 1640 (Sigma) containing standard concentrations of L-glutamine. A cell count was performed; the cell concentrations were adjusted with RPMI 1640 to 5×106 cells/ml, and 250 μl of the cell suspensions were distributed into polypropylene 6 ml tubes. Triplicate tubes were either left unstimulated (baseline, resuspended in 5 μl of RPMI), HKMtb stimulated [with the addition of 5 μl of a HKMtb suspension at 37ºC (1.8×108/μl, in accordance to the Mc Farland Scale concentration)], or CFP-stimulated [with the addition of 5 μl of CFP (40 μg/ml)]. The cell culture tubes were incubated for 20 h at 37ºC, as described(4,15). Following incubation, culture supernatants (c.s.) were collected and stored at −70º C for further assessment of nitrite, TNF-α, IL- 12, and IL-2 concentration.
Measurement of TNF-α, IL-12 and IL-2 in c.s. of MC and PMNThe levels of TNF-α, IL-12 and IL-2 in the c.s. of MC and PMN were determined by Enzyme linked immunoabsorbent assay (ELISA, R&D Systems, Minneapolis, MN, USA), as described by the manufacturer. The samples were assayed in duplicate and results expressed as the average of two readings in an ELISA reader at 450 nm (corrected to 540 nm). The cytokines were quantified with reference to standard curves generated using a human recombinant cytokine. The sensitivity of the assays for TNF-α, IL-12 and IL-2 was 4.4, 5.0, and 7.5 pg/ml, respectively.
Nitrite assayThe NO produced by MC and PMN was measured as nitrite by the Griess reaction. The Griess reagent was prepared by mixing equal volumes of 1% sulfanilamide in 2.5% H3PO4, and 0.1% naphthylethylene diamine dihydrochloride in 2.5% H3PO4. One hundred μl of Griess reagent and 100 μl of basal or stimulated MC c.s. were incubated for 5 minutes at room temperature in the dark in 96 well plates (NUNCLON, NUNC Brand Products), and absorbance was measured at 540 nm (corrected to 650 nm) in an ELISA Reader (Microwell System Reader, Organon Teknika). Nitrite concentration was determined using various NaNO2 concentrations in culture medium as standard, and data were expressed as μM. The assessments were done in duplicate(21-23).
Respiratory burstFor evaluation of the RB (21), 100 μl of a cell suspension containing 106 cells from HKMtb, CFP-stimulated or unstimulated MC and PMN, were added to 5 ml polystyrene tubes to be further diluted with 0.9 ml of PBS. To each tube, 25 μl of dihydro-rhodamine-123 (50 μg/ml) were added and the tubes were incubated at 37ºC in a water bath for 15 min. Then, 10 μl of phorbol myristate acetate (10 μl/ml) were added to each tube, which were incubated again for 15 min in the 37μC water bath. After incubation, the samples were centrifuged at 400 xg for 5 minutes and the supernatants were discarded. The cells were resuspended in 500 μl, and 104 cells were taken for fluorescence activated flow cytometry in a FACS-Calibur flow cytometer with computer-assisted evaluation of data (Becton-Dickinson/Cell Quest software). For each sample, 10,000 events were acquired with gates drawn around neutrophils for the PMN preparations or for mononuclear cells for the MC preparations. Results were expressed as the percentage of fluorescent cells, and as the oxidative index (R). This was calculated by dividing the mean fluorescence intensity (MFI) of stimulated cells by the MFI of unstimulated cells. The MFI is proportional to the amount of reactive oxygen intermediates generated.
Statistical analysisSubject groups were the independent variables and the functional studies the dependent ones. Comparisons were performed by means of non parametric tests: Kruskall Wallis analysis of variance, Mann-Whittney U test, and Wilcoxon signed test.
RESULTSAnalysis of CFPThe obtained CFP content was separated by SDS-PAGE, showing a prominent 80 kD band of high concentration in the 4 CFP seeded lanes. The presence of another band may be attributed to another CFP protein or to BSA remaining from Midllebroock 7H9 medium, or both. The CFP profile is shown in Figure 1. Approximately 45 μg or 30 μg of CFP were seeded on lanes 2 and 3, and on lanes 5 and 6, respectively. On lanes 1 and 4, molecular weight standards could be observed. No protein was detected by staining any of the gels seeded with different supernatants obtained after the ammonium sulfate precipitation (data not shown).
SDS-PAGE separation of culture filtrate proteins of M. tuberculosis. Lanes 1 and 4 are molecular weight controls. Lanes 2,3,5, and 6 show culture filtrate proteins (CFP) bands (45 μg and 30 μg respectively) obtained from a Middlebrook initial culture. CFP lanes are duplicates from the same samples with different concentration.
The total protein concentration, evaluated by Bradford assay, was 4 mg/ml. Subsequently, CFP were diluted to 40 μg/ml, and this concentration was used for the cell stimulation experiments.
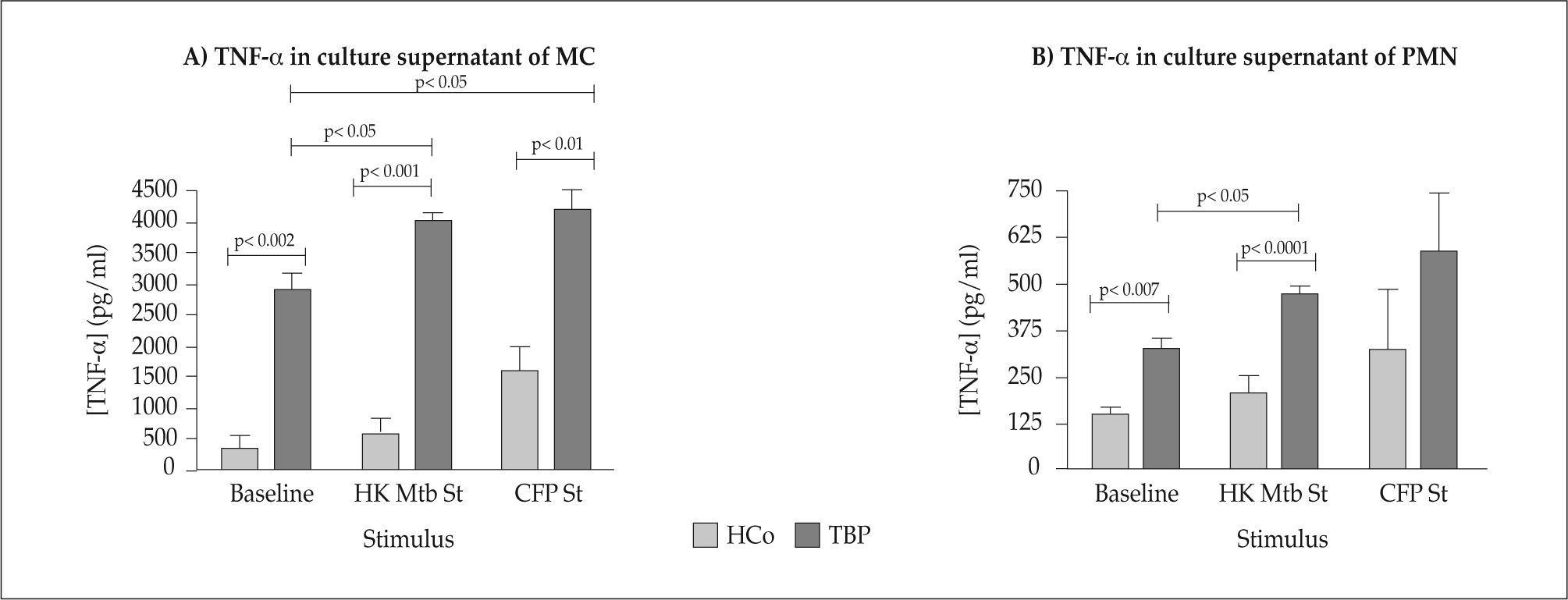
Cytokine levels in MC and PMN culture supernantants TNF-αThe measurements of TNF-α concentrations in MC and PMN c.s. from TBP and HCo are depicted in Figure 2 (Panels A and B). The baseline concentration of TNF-α in MC c.s. of TBP was higher than the values detected in HCo. Further analysis indicated that supernatants from HKMtb and CFP- stimulated MC of TBP, contained markedly increased amounts of TNF-α if compared with their baseline values (p<0.05) or levels seen in their HCo counterparts (baseline: p<0.002, HKMtb: p<0.001, CFP: p<0.01).
The baseline concentration of TNF-α in PMN supernatants from TBP was higher than the values detected in HCo (p< 0.007).
The supernatants from HKMtb- and CFP-stimulated PMN of TBP contained significantly increased levels of TNF-α, as compared to unstimulated PMN (overall difference p<0.002). The same was true when comparing supernatants from HKMtb-stimulated PMN of TBP with the HCo counterpart (p<0.0001). Supernatants of HKMtb-stimulated PMN of TBP contained increased levels of TNF-α compared with baseline concentrations (p<0.05).
CFP-induced stimulation of TNF-α production by PMN from TBP and HCo showed no significant differences.
IL-12Results of IL-12 concentrations in MC and PMN c.s. from TBP and HCo are shown in Figure 3, Panels A and B. Stimulation of MC with HKMtb and CFP resulted in significantly increased amounts of IL-12 in the culture supernatant from TBP, even more so when CFP was added (p< 0.05, p<0.05 respectively). No differences were found when analyzing c.s. from HCo.
As regards to PMN, greater amounts of IL-12 were found in the culture supernatant of HKMtb- and CFP- stimulated PMN from TBP compared to unstimulated cells (p<0.05, p<0.05 respectively). The baseline levels in supernatants from MC and PMN of TBP were higher than values seen in HCo (p<0.05 and p<0.03 respectively). Inter-group comparison in antigen-stimulated PMN, revealed that HKMtb-stimulated cells from TBP had significantly higher IL-12 concentrations than HCo (p<0.02), this not being the case when analyzing c.s. of CFP-stimulated PMN (ns).
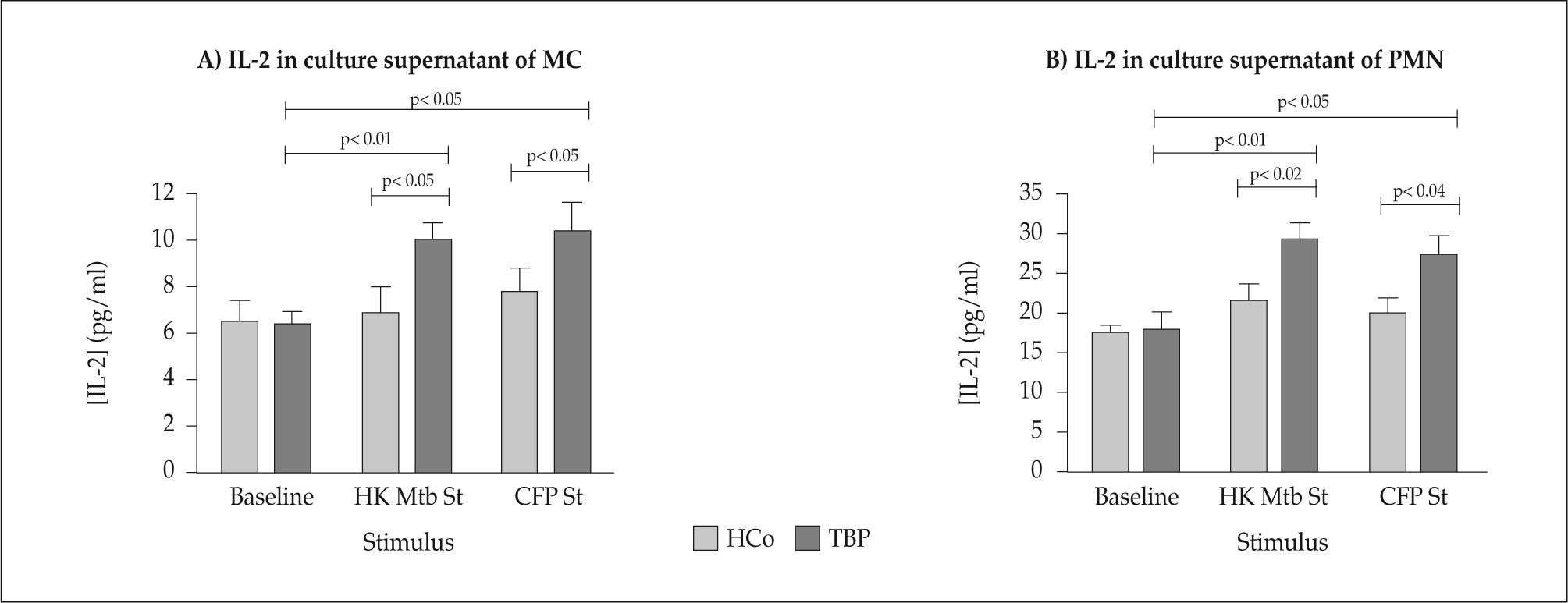
IL-2Data on IL-2 studies are shown in Figure 4 (Panels A and B). There were no differences in IL-2 contents from baseline c.s. of TBP and HCo. Augmented IL-2 concentrations were seen in supernatants of HKMtb- and CFP-stimulated MC from TBP compared to their baseline values (overall difference p<0.001, HKMtb vs Baseline: p<0.01, CFP vs Baseline: p<0.05). Stimulation of MC from HCo with HKMtb and CFP did not result in significantly enhanced IL-2 amounts. Increased IL-2 levels of HKMtb and CFP stimulated MC were detected if compared with HCo (p<0.05 and p<0.05 respectively).
C.s. from HKMtb- and CFP-stimulated PMN from TBP contained increased IL-2 concentrations in relation to baseline levels (overall difference p<0.008, HKMtb vs Baseline: p<0.01, CFP vs Baseline: p< 0.05). Significant differences in IL-2 levels were found when comparing c.s. from HKMtb and CFP-stimulated PMN from HCo and TBP (p<0.02 and p<0.04, respectively).
Nitrite ProductionResults from nitrite measurements are depicted in Figure 5 (both panels). Raised nitrite concentrations were detected in c.s. of HKMtb- and CFP-stimulated MC from TBP in relation to their baseline levels (p<0.05 and p<0.01 respectively, Panel A). C.s. from HKMtb- and CFP-stimulated MC from TBP had increased nitrite levels compared to their HCo counterparts but the trend was only significant for the latter stimulus (p<0.003).
While comparisons between-TBP and HCo showed no differences in baseline nitrite concentrations, levels in supernatants from HKMtb- and CFP-stimulated PMN of TBP were significantly higher than in their HCo counterparts (p<0.05 and p<0.02, respectively). Nitrite baseline contents in PMN c.s. of TBP differed if compared with increased levels found in CFP-stimulated cultures (p<0.001).
Respiratory burstEvidence for a significant activation of the respiratory burst in the interaction of MC and PMN with HKMtb and CFP stimulus was obtained. The baseline respiratory burst in HKMtb and CFP-stimulated MC and PMN, expressed as R index (R1 for HKMtb stimulated cells and R2 for CFP- stimulated cells) is represented in Figure 6 (Panels A and B). The data indicate that in TB patients both cell types increase in a significant manner their oxidative capacity when stimulated compared to their unstimulated counterparts (MNc: p<0.003 and p<0.005, respectively; PMN: p<0.05 and p<0.02, respectively). Differences also reached statistical significance when R indexes of stimulated MC and PMN from TB patients were compared with the values yielded by HCo (Figure 6). After a 20 h culture, HCo cells did not show a very significant change compared to unstimulated cultures.
Production of ROI during Respiratory burst by MC (Panel A) and PMN (Panel B) cells previously separated by a Fycoll-Hypaque gradient and stimulated or not with HKMtb and CFP from HCo (n=12) and TB Patients (n=16). Results were expressed as the percentage of fluorescent cells, and oxidative index (R). R= mean fluorescence intensity of stimulated cells/ mean fluorescence intensity of unstimulated cells. R1 and R2 were calculated by dividing the mean fluorescence value for HKMtb and CFP respectively stimulated cells by the mean fluorescence value for unstimulated cells. The mean fluorescence intensity is proportional to the amount of reactive oxygen intermediates generated. Data are means ± sem. Values between brackets indicate the number of patients.
This work focuses on the measurement of cytokine and nitrite levels as well as the RB in MC and PMN of TBP after triggering with Mtb antigens, as an appraisal of the immune response developed by these cell types. Both cell types, when properly stimulated, modified to some extent the production of Reactive Oxigen Intermediates (ROI), induced the synthesis of considerable amounts of Reactive Nitrogen Intermediates (RNI), and relevant cytokines in the immune response against tuberculosis like TNF-α, IL-12 and IL-2. These results extend our previous demonstration that HKMtb stimulation of MC and PMN cells from TBP, led to a higher TNFRI and CXCR2 expression and cytokine amounts in c.s. and a slight increase of the respiratory burst(4,5).
The outcome after infection with Mtb may be partly explained by the efficiency of innate host defense mechanisms. The interindividual differences in the effects induced by HKMtb and CFP stimulation of MC and PMN may be due to several influences working together, such as complement components, collectins, and pattern-recognition receptors on responding cells. In this study, we were able to demonstrate that mycobacterial stimulation of MC and PMN induces various changes in the basic biology of these subsets of leukocytes.
The essential protective effects of TNF-α in the generation of immunity against pathogens have been shown in many systems(24). TNF-α is a prototype proinflammatory cytokine, playing a key role in granuloma formation and macrophage activation, although it may also account for some unwanted side effects. Results from antigenic stimulation with HKMtb and CFP in vitro revealed an increased TNF-α production, which bears some similarities with the inflammatory process observed in TBP, as TNF-α is a cytokine closely connected to the killing of mycobacteria by macrophages(24-26).
The mainly beneficial role of TNF-α in tuberculosis involves the recruitment of the immune cells necessary for sealing up infectious foci inside granulomas in mice(27).
IL-12 is a relevant cytokine in the immune response against Mtb. It connects the innate and adaptive host response to mycobacteria(27,28) and exerts its protecting effects mainly through the induction of IFN-γ Immunity to Mtb requires a Th1 response, and genetic defects in the IL-12 or IFN-γ pathways lead to increased disease susceptibility(11,29-31). However, Mtb or its antigens rapidly evoke a potent Th1 response, even in hosts prone to develop the disease.
Several reports indicate that NO is one of the effector molecules of mononuclear cells capable of producing a mycobactericidal effect, although other studies suggest an important participation of NO in tissue damage during the late phase of the disease(16,17).
In previous reports, PMN and MC specific activation has been described. The significant increase in cytokine and chemokine levels of activated PMN in other studies confirmed their role in amplifying a protective cell mediated immune response to effectively curtail the infection(32-34).
It was also shown that although MC and PMN from TB patients had diminished IL-8 and TNF-α receptors, their expression raised after antigen stimulation. Culture supernatants from MC and PMN of TB patients contained increased amounts of several cytokines like IL-8 and TNF- α(5). Aleman et al. have demonstrated that circulating PMN from patients with active TB have enhanced parameters of activation, such as generation of superoxide anion and high production of TNF-α and IL-1β, and high TNF-R55 expression upon stimulation with Mtb(35,36).
On the other hand, S. Korbel et al. suggest that a robust innate immune stimulation mediated by a diverse group of activated cell types and mechanisms, including monocytes/ macrophages, natural killer (NK) cells, neutrophils, dendritic cells (DCs) eosinophils and basophils, and by the elaboration of proinflammatory cytokines, type I interferons (IFNs) and other soluble factors in response to Toll-like receptor (TLR) interaction with tubercle bacilli, would be a crucial and an essential mechanism responsible for protective immunity(37).
Several analysis of the production of nitrite levels showed that HKMtb, and particularly the stimulus with CFP, efficiently activated MC and PMN of TBP. Besides, several results were found controversial(4,38-40). Lecoanet-Henchoz et al. documented this type of production of RNI by MC cells. Several reports point out to an important role of NO in mycobacterial killing, particularly during the early phase of the infection(39), although Ratnikov et al. demonstrated decreased levels of final NO metabolites in all biological fluids in TBP, compared with healthy individuals, which correlated with the severity and extent of a specific pulmonary process(40). Decreased NO production following a potent antigenic stimulus by severe TBP may exhibit some parallel with the reduced NO levels found in our study. It remains to be established whether the antimycobacterial activity of these NO-producing monocytes/macrophages correlates with the amount of nitrogen-oxide generated in vitro.
Some evidence in the human system suggests that iNOS expression in monocytes/macrophages does not seem to be up-regulated by TNF-α(41), while other investigations demonstrate the generation of nitrite in response to TNF-α(42).
Thus, the production of the proinflammatory cytokine TNF-α and the highly reactive free radical nitric oxide (NO) by macrophages are considered strongly implicated in the development of the protective immune response leading to killing of phagocytosed M. tuberculosis(43). These observations confirmed that NO production is regulated by different cytokines(44,45).
The observed data showed that the pro-inflammatory cytokines and mediators induced by the activating function of HKMtb and CFP contribute to a great extent to the development of immune responses, which has been previously shown with live M. tuberculosis stimulus(46). A number of the culture filtrate proteins secreted by M. tuberculosis are known to contribute to the immunology of tuberculosis and to possess enzymatic activities associated with pathogenicity. The M. tuberculosis 80 kDa protein is an enzyme with substantial homology to hydroperoxidase I from Escherichia coli, catalaseperoxidase from Mycobacterium intracellulare, and other microbial catalase-peroxidases(47).
Mass spectrometry of peptides obtained from one member of this complex identified it as the M. tuberculosis KatG catalase/peroxidase (katG)(48). Th1 immune responses to ESAT-6 and katG have been assessed, and the cells responsible for the immune responses could be identified by several authors. The stimulatory effect of katG on the adaptative response was evidenced as increased IL-2 and IFN-γ production by CD4+ T cells(49,50).
On the other hand, the isoniazid susceptibility of M. tuberculosis is mediated by the product of the katG gene, which encodes the heme-containing enzyme catalaseperoxidase. S. Pym et al. have shown that the most commonly occurring katG mutation is associated with clinically significant levels of resistance to isoniazid (INH). The implications of this finding for the transmission and reactivation of multidrug resistant strains of M. tuberculosis are severe(51-53).
Toll-like receptor (TLR) 2 and TLR4 are particularly important in the innate immune response against M. tuberculosis(54). TLR2 recognizes microbial components of M. tuberculosis such as katG and other components. Therefore, this is interesting when postulating a role for an altered innate immune response in the pathogenesis of this disease. Activation of TLR2 results in the production of proinflammatory cytokines and up-regulation of costimulatory molecules and antigen presentation, thereby leading to priming of the ensuing adaptive immune response(54).
Moreover, CFP of M. tuberculosis have been shown to contain immunogenic components such as the 10 kDa protein, that elicits at least partial protective immunity(55,56), and could be considered a promising candidate for the development of preventive therapies and diagnostic assays.
It could be observed that the reduced oxidative response was slightly stimulated, by activating effects of HKMtb or CFP.
Depressed RB, particularly in MC, may be of relevance as to disease immunopathology. Effective phagocytosis and the subsequent production of ROIs are necessary for the intracellular killing of Mtb in phagocytes.
As host resistance to Mtb is mediated to a great extent by the bacteriostatic and bactericidal function of cytokine-activated phagocytic cells, our present results, together with the ability of the HKMtb and CFP to stimulate immune responses in vivo(57), point to their usefulness as protective immunogens.
Changes in phenotype did not depend on direct mycobacterial stimulation alone, but are a result of an autocrine-paracrine stimulation mechanism.
Going deeply into the study of the mechanisms involved in the stimulation of both MC and PMN and the cellular immune response in humans directed against several important target antigens of Mtb, and considering that some antigens are recognized by a high number of individuals, would probably lead to a better understanding of the pathogenesis of tuberculosis to get a faster and more accurate therapy.
CONCLUSIONSIn the present work, stimulation of MC and PMN improved functions associated with the early host response against mycobacterial infections, and also resulted in a substantial production of IL-12, IL-2 and TNF-α.
We also show here that HKMtb and PCF modulate the release of several protective cytokines and important inflammatory mediators that play an important role in the development of immune responses. It is therefore conceivable that the release of TNF-α by the HKMtb- and CFP stimulated MC and PMN demonstrated in the present study might contribute to induction of host protective immunity.
The strong stimulation exerted by HKMtb and CFP for IL-12 production by MC and PMN, highlights the relevance of such constituents for eliciting a Th1 response in TBP.
Analysis of nitrite levels showed that HKMtb, and particularly the CFP stimulus induced a decreased production of NO following the antigenic stimulus.
It could be observed that the reduced respiratory burst was quite stimulated, by activating effects of HKMtb or CFP. As such, defects in these functions may lead to a deficient killing of intracellular mycobacteria, favoring disease progression. The regulation of TNF-α and NO production by phagocytic cells in tuberculosis appears to be very complex, due to the ability of various mycobacterial cell wall components to stimulate the release of these inflammatory mediators(7,56).
Our findings support the hypothesis that professional phagocytes such as PMN and MC become activated at the site of mycobacterial infection and that this activation might set the stage for a subsequent antimycobacterial immune response(58).
Therefore, a better understanding of the immunomodulatory actions of M. tuberculosis-specific antigenic proteins is a prerequisite for their possible exploitation in therapeutic and diagnostic purposes.
ACKNOWLEDGMENTSWe are grateful to the Secretariat for Science and Technology of Rosario National University for financial support. We also wish to thank patients and healthy persons for agreeing to participate in the study.
CONFLICT OF INTERESTThe authors declare no financial conflict of interest.