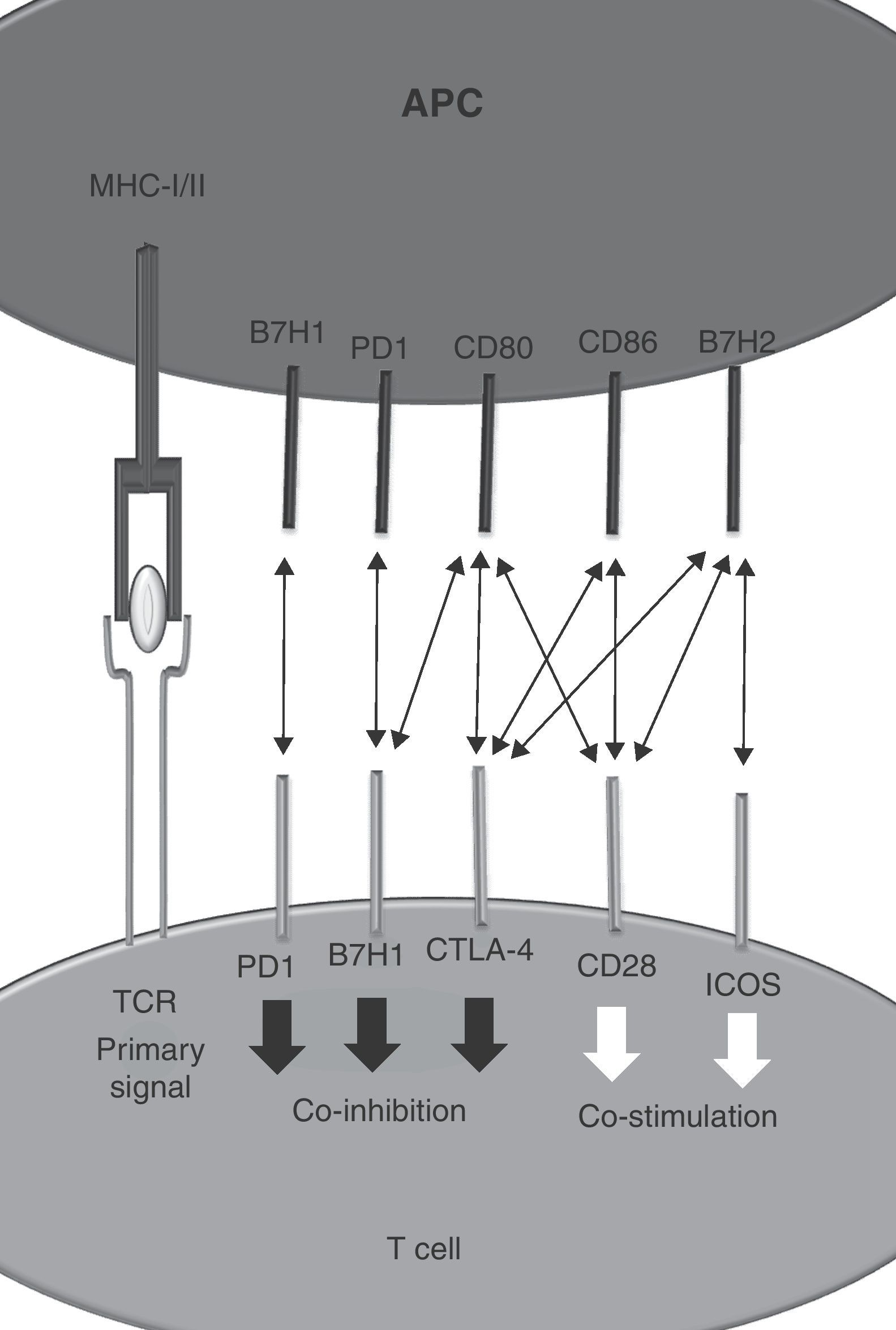
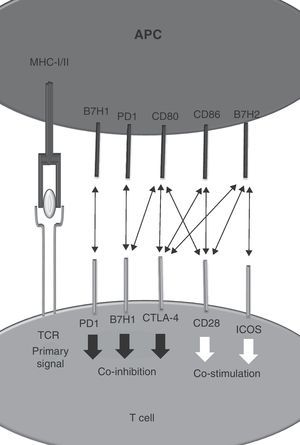
As a theoretical model it is postulated that T cells require two signals for appropriate activation.1 Signal 1 is started after T cell receptor binding to a MHC–peptide complex on the surface of an antigen presenting cell. Signal 2 is delivered by co-stimulatory molecules including CD28 that binds to CD80 and CD86.2 Negative regulators modulate the grade of T cell activation and protect the organism of deleterious effects mediated by uncontrolled T cell activation.3 CTLA-4 is one of these co-inhibitory molecules expressed on activated T cells.4,5 It recognises CD80 and CD86 with an affinity 200 times higher than CD28.6 Therapeutic strategies directed to promote or interfere with CTLA-4 function are now in clinical use for autoimmunity (i.e. Abatacept)7 and cancer (Ipilimumab).8
As new cell surface signaling molecules and interactions are being discovered, the two-signal model becomes insufficient to explain the resulting immune response. Co-stimulatory or co-inhibitory molecules belong to structurally different families of receptors including the tumor necrosis factor (TNF) receptor superfamily, immunoglobulin family or to the B7 family. Interestingly, interactions between members of different families are also possible.9 The different distribution of surface proteins between different immune populations as well as the influence of particular microenvironments provides outstanding diversity to the regulation of immunity.10 Signals from many receptors determine the intensity and the type of ensuing immune response. Integration of multiple elements determine the level of the tide (Fig. 1).
The receptor arrayThe historical approach to identify new co-stimulatory and co-inhibitory molecules started in the 60s with the usage of monoclonal antibodies used as a bait. Then in the 90s bioinformatic studies allowed identification of new surface receptors based on sequence similarity. In the 2000s the main strategy to identify new interactions between surface cosignaling molecules has been systemic monitoring of molecular events: expression cloning, immunoprecipitation and subsequent high efficiency molecular structure identification.
In a seminal paper, Chen et al. describe a new technology dubbed “receptor array” that allows a high throughoutput detection of binding partners.11 The assay starts with the cloning of more than 2500 full-length human transmembrane protein genes to be tested individually. It consists of simultaneous transfections of 293T cells on multi-well plates. Each well contains the transfectants for a single surface molecule. The screening is performed with the protein of interest tagged with a fused peptide (FLAG or HA). After incubation with a fluorescent-labeled secondary antibody against the tag, an automatized detection system screens the wells in which the plate-coating cell layer displays fluorescence. The system excludes the background signal coming from solubilized secondary antibody that has not encountered the tagged protein bound to the putative receptor expressed by the 293T transfected cells.
Identification of CD28 and CTLA-4 as new receptors for B7H2 (ICOS-L)Identification of CD28 and CTLA-4 as new receptors for B7H2 (ICOS-L) B7H2, also known as ICOS ligand, binds inducible-co-stimulator (ICOS) on activated T cells.12 Using the receptor array described before, Chen et al. were able to identify a new interactions between B7H2–CD28 and B7H2–CTLA-4. Regarding the affinity hierarchy between B7H2 and their cognate ligands, ICOS has the highest followed by CTLA-4 and CD28.
Binding of B7H2 to CD28 increases T cell proliferation and IFN-γ production on T cells. Although the interaction between B7H2 and CTLA-4 has been described, the functional consequences on T cells have not been fully explored yet due to the difficulty of isolating the effect on CTLA-4 on in vitro assays.
Targeting PD-1–PD-L1 interactions as a therapeutic approach to treat cancerMonoclonal antibodies (mAbs) provide a valuable pharmacological tool to overactivate co-stimulatory signals and inactivate co-inhibitory functions of surface receptors on T cells in the context of cancer therapeutics.13 One of the promising emerging targets in cancer immunotherapy is the pair formed by PD-L1 and PD-1.14
PD-1 is a co-inhibitory receptor that shows homology with CD28 and whose expression is induced after activation on CD4+ and CD8+ T cells as well as on other immune cells. Its cognate ligands are the members of the B7 family PDL1 (B7H1) and B7-DC (PD-L2) and other yet unreported unknown protein. PD-1 triggering on the T cell leads to a non pro-apoptotic inhibition of T cell proliferation. Because of these modulator effects it is known as an immune checkpoint along with CTLA-4.
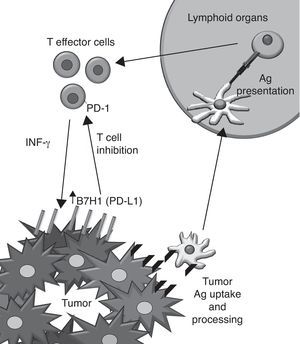
PD-L1 was discovered on the APC15 and binds PD-1, CD80 (B7-1) and other yet unreported unknown protein. Its expression can be induced in almost all nucleated cell types by the action of IFN-γ,16 TLR agonists17 and loss of PTEN/activation of PI3K.18 The PD-L1 expression has been also described on tumor cells, correlating with a poor prognosis of the disease. Interestingly, IFN-γ induces PD-L1 expression in vitro on both murine and human tumor cell lines that do not express the protein constitutively. The hypothetic implications of this induction in vivo are shown (Fig. 2).
Human anti-PD1 mAb (MDX1101) are already in early clinical trials for cancer. Preliminary data on overall response upon multiple doses of this agent indicates 30% in metastatic melanoma. The agents seem to be clinically active also on colorectal cancer, lung cancer and renal cell carcinoma.
We are grateful for support from MEC (SAF2005-03131 and SAF2008-03294), Departamento de Educación del Gobierno de Navarra, Departamento de Salud del Gobierno de Navarra (Beca Ortiz de Landázuri), Redes temáticas de investigación cooperativa RETIC (RD06/0020/0065), Fondo de investigación sanitaria (PI060932), European commission VII famework program (ENCITE), Fundacion Mutua Madrileña, and “UTE for project FIMA”. A.-P. receives an isciii predoctoral fellowship.
A summary of the closing lecture at the XXXVI SEI congress (University of Navarra) presented by Prof. Lieping Chen (Yale University).