Tumor Budding (TB), defined as the presence of individual neoplastic cells or isolated groups of up to 4 cells at the front of tumor invasion, has become an adverse prognostic marker in colorectal cancer (CRC) in recent decades. The prognostic impact of TB in CRC remains not clearly defined and histological methods for its evaluation vary depending on the center. The objective of this study is to investigate the association between TB and CRC, in terms of oncological evolution and pathological stage.
MethodsA retrospective observational study was conducted, including patients undergoing curative oncological surgery for CRC between January 2017 and December 2022. The effects of TB on disease-free survival (DFS) and overall survival (OS) were evaluated according to the Kaplan-Meier curves.
ResultsIn 78 cases TB was described in the pathology report. TB was present in 56 patients (71.8%), divided into the following categories: low grade in 22 (39.3%), intermediate grade in 17 (30.4%) and high grade in 17 (30.4%). The proportion of patients who presented lymph node metastases, lympho-vascular and perineural invasion was significantly higher in patients with TB (26.8% vs 0%, P = .008; 41.1% vs 4.5%, P = .002; 16.1% vs 0% P = .054; respectively). DFS was 86.3% in low-grade TB, 75.3% in intermediate-grade TB, and 70.3% in high-grade TB. Cases with intermediate and high grade were associated with a shorter OS compared to the low grade group (93.7% and 75.4% vs 100% P = .012, respectively).
ConclusionThese results suggest that TB expression may be a useful risk factor as a prognostic factor for the detection of lymph node metastasis, local recurrence, and distant metastasis in CRC.
El tumor budding (TB), definido como la presencia de células neoplásicas individuales o en grupos aislados de hasta 4 células en el frente de invasión del tumor, se ha convertido en las últimas décadas en un marcador pronóstico adverso en el cáncer colorrectal (CCR). El impacto pronóstico del TB en el CCR sigue sin estar claramente definido y los métodos histológicos para su evaluación varían según el centro. El objetivo de este estudio es investigar la asociación entre el TB y el CCR, en términos de evolución oncológica y estadio patológico.
MétodosSe realizó un estudio observacional retrospectivo, incluyendo los pacientes sometidos a cirugía oncológica curativa de CCR entre enero del 2017 y diciembre del 2022. Se evaluaron los efectos del TB sobre la supervivencia libre de enfermedad (SLE) y la supervivencia global (SG) según las curvas de Kaplan-Meier.
ResultadosEn 78 casos se describió el TB en el informe de anatomía patológica. El TB estuvo presente en 56 pacientes (71,8%), repartido en las siguientes categorías: bajo grado en 22 (39,3%), grado intermedio en 17 (30,4%) y alto grado en 17 (30,4%). La proporción de pacientes que presentaron metástasis ganglionares, invasión linfo-vascular y perineural fue significativamente mayor en pacientes con TB (26,8% vs 0%, P = ,008; 41,1% vs 4,5%, P = ,002; 16,1% vs 0% P = ,054; respectivamente). La SLE fue del 86,3% en el TB de bajo grado, del 75,3% en el TB de grado intermedio y del 70,3% en el TB de alto grado. Los casos que reunían el grado intermedio y alto se asociaban a una SG más corta en comparación con el grupo de bajo grado (93,7% y 75,4% vs 100% P = ,012, respectivamente).
ConclusiónEstos resultados sugieren que la expresión del TB puede ser un factor de riesgo útil como factor pronóstico para la detección de metástasis en los ganglios linfáticos, de la recurrencia local y de metástasis a distancia en el CCR.
Tumour budding (TB) is defined as neoplastic cells, individually or in clusters of up to 4, present at the tumour invasive front. This histological finding, which represents the dissociation of malignant cells from the main tumour population, has captured the interest of pathologists since it was first described in the 1950s.1
Although TB is currently the most common term used in published articles, the terms “focal dedifferentiation” and “epithelial-mesenchymal transition” indicate biological processes that lead to the detection of this histological feature.2 TB does not only mean that a simple detachment of tumour cells has occurred, but also provides information about the progression of a malignant tumour from an initially localised disease to a systemic spread.3 This process requires the acquisition of specific genetic characteristics and the subsequent modification of the expression of cell adhesion molecules.4,5
The utility of TB in the context of colorectal cancer (CRC) has been recognised in some recent clinical guidelines; indeed, it has been associated with other aggressive pathological features, including lymph node metastases, venous invasion, poor tumour differentiation and recently, expression of DNA mismatch repair system proteins.6 In 2016, the International Tumour Budding Consensus Conference (ITBCC) developed an international scoring system, based on evidence in CRC.7 However, until recently, the absence of a standardised scoring system hindered the implementation of TB in clinicopathological practice. Subsequent to ITBCC, the literature has also validated the association between intermediate/high grade TB with adverse clinicopathological features8 and disease-free survival (DFS) and overall survival (OS).9
Several studies and systematic reviews have found that TB was independently associated with disease recurrence, cancer-related death and reduced OS. A comprehensive review, conducted in 2020, demonstrated a worse prognosis in the setting of advanced stage TB, applying multivariate analysis (5-year DFS of 89%–98% vs. 52%–80% in low vs. high grade, respectively).10 Some studies have provided different scoring systems to assess the prognostic role of TB; however, these studies had limitations, including confounding bias between groups, limited sample size and restrictions to certain disease stages.11,12
Due to the novel nature and the relatively recent introduction of this parameter, endorsed by an international consensus, TB is not widely used in clinical practice. In this sense, the aim of this study is to provide new information on the association between TB and CRC, in terms of tumour progression, and its characteristics according to pathological stage.
MethodsThe present study was approved by the Fundación Jiménez Díaz ethics committee and conducted according to the STROBE recommendations for observational studies.13 We retrospectively included data from patients diagnosed with CRC, between 2017 and 2022, who underwent surgery with curative intent in the General Surgery Department of the Hospital Universitario Ruber Juan Bravo.
Inclusion criteria were as follows: over 18 years of age, pre-surgical and/or post-surgical diagnosis of colorectal cancer, elective or emergency surgery (in cases of stricture, contained perforation). Exclusion criteria consisted of: gastrointestinal tumours other than classic adenocarcinoma, stage M1, presence of other tumours in the surgical specimen, lack of TB description in the anatomical pathology report.
Patient demographics, type of resection, tumour location and size, degree of differentiation, TNM classification, IUCC staging, lymph nodes and expression of microsatellite instability were collected from the electronic hospital database, retrospectively and pseudonymised. TB data were analysed from anatomical pathology reports, accessible through the electronic hospital record system.
Survival data were collected through outpatient services and in-hospital clinics, with the collaboration of the oncology department of our hospital. The last update of the follow-up data was performed on 31/01/2023.
Sample size calculationPrevious publications describe that 36.8% of CRC patients have TB at the surgical specimen and that the 5-year mortality probability of these cases is 4.5 times higher (95% CI: 2.5–8.0) compared to non-TB patients.14 If the 5-year mortality of CRC without TB is expected to be 36%,15,16 a minimum of 52 patients (40 patients without TB and 12 patients with TB) should be included in the study in order to detect a relative risk of 5-year mortality greater than or equal to 2.5, with a statistical power of 80% and a significance level of 95%.
Pathological studyA standardised protocol was used to evaluate the resected specimens.17 The resection margin was reported according to the R-stage definition, where R0 corresponds to a tumour-free margin of 1 mm. Tumours were classified according to the current TNM staging system. T stage was defined according to the maximum diameter of the invasive component. Classification of the adenocarcinoma component followed WHO recommendations.18 Prognostic staging was performed according to the 8th edition of the AJCC/UICC cancer staging manual.17
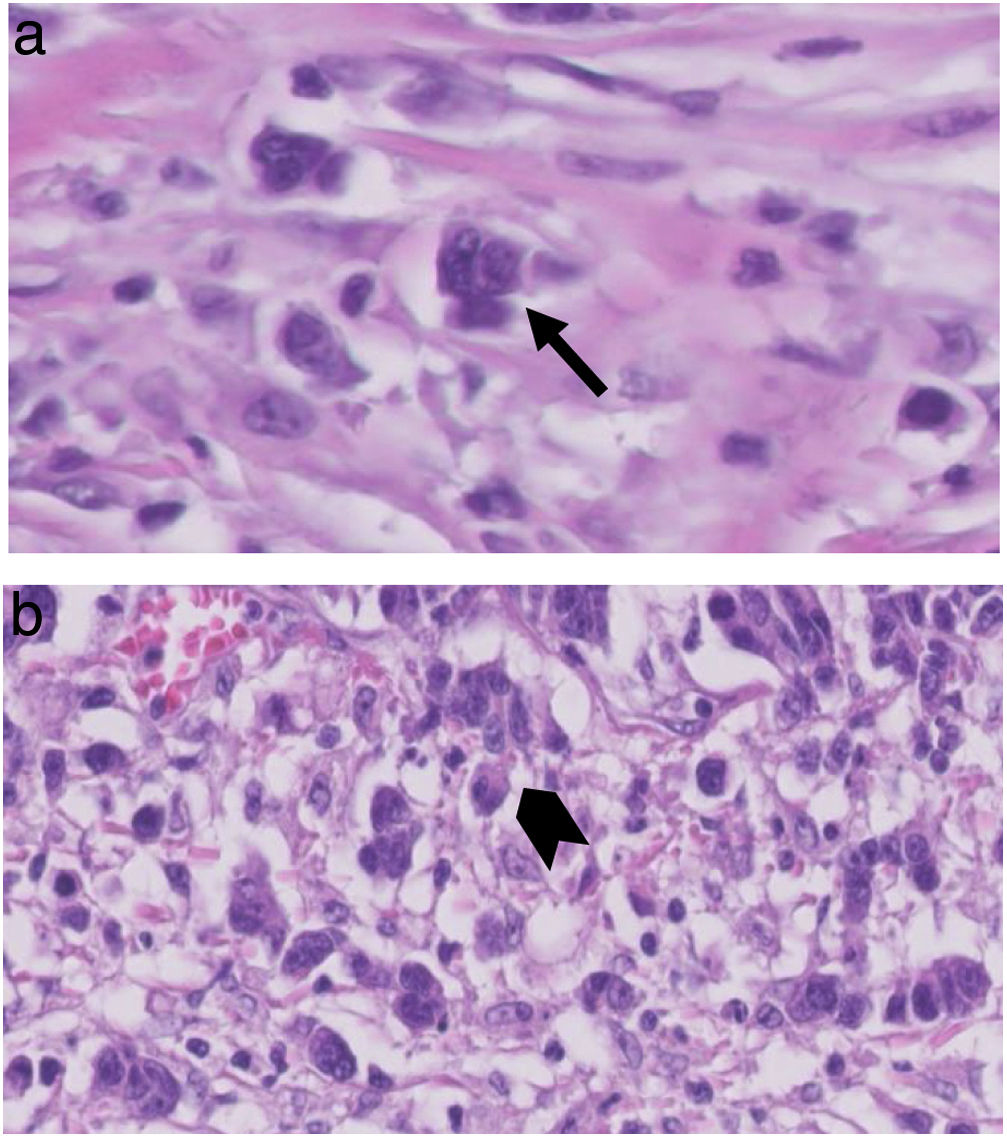
TB was graded according to the 2016 ITBCC7; TB counts, in H&E sections, were selected at a “hotspot”, chosen after reviewing available slides from the area with the most tumour infiltration. The total number of TB was reported in an area of 0.785 mm2; thus, both the total number and a score in three grades (low, 0–4; intermediate, 5–9; high, equal or more than 10; Fig. 1) were reported.
Statistical analysisQuantitative parameters had non-normal distribution and were expressed as median and interquartile range (IQR), unless otherwise indicated. Categorical parameters were represented as absolute numbers and relative frequencies. Comparisons between the 2 groups, with or without TB, according to categorical histopathological parameters were performed using the Chi-square test or Fisher's exact test. Survival curves were created according to the Kaplan-Meier method. Differences in survival were assessed using the log-rank test. OS at 5 years was defined as the time from resection to death from any cause or last follow-up; DFS at 5 years was defined as the absence of metastasis or local recurrence during follow-up. Patients alive at last follow-up were censored.
Statistical significance was set at P < .05. For statistical analysis, the IBM SPSS statistical software version 23.0 (IBM Corp., 2021, Armond, NY, USA) was used.
ResultsIn total, 129 patients undergoing colectomy or rectal resection were identified between January 2017 and December 2022. Fifty-one patients were excluded because TB data were missing from the pathology report. The final study cohort consisted of 78 patients, all with full description of TB, its presence or absence, and with the different grades. Forty-eight males (61.5%) and 30 females (38.4%) were described, with a median age of 70.9 years.
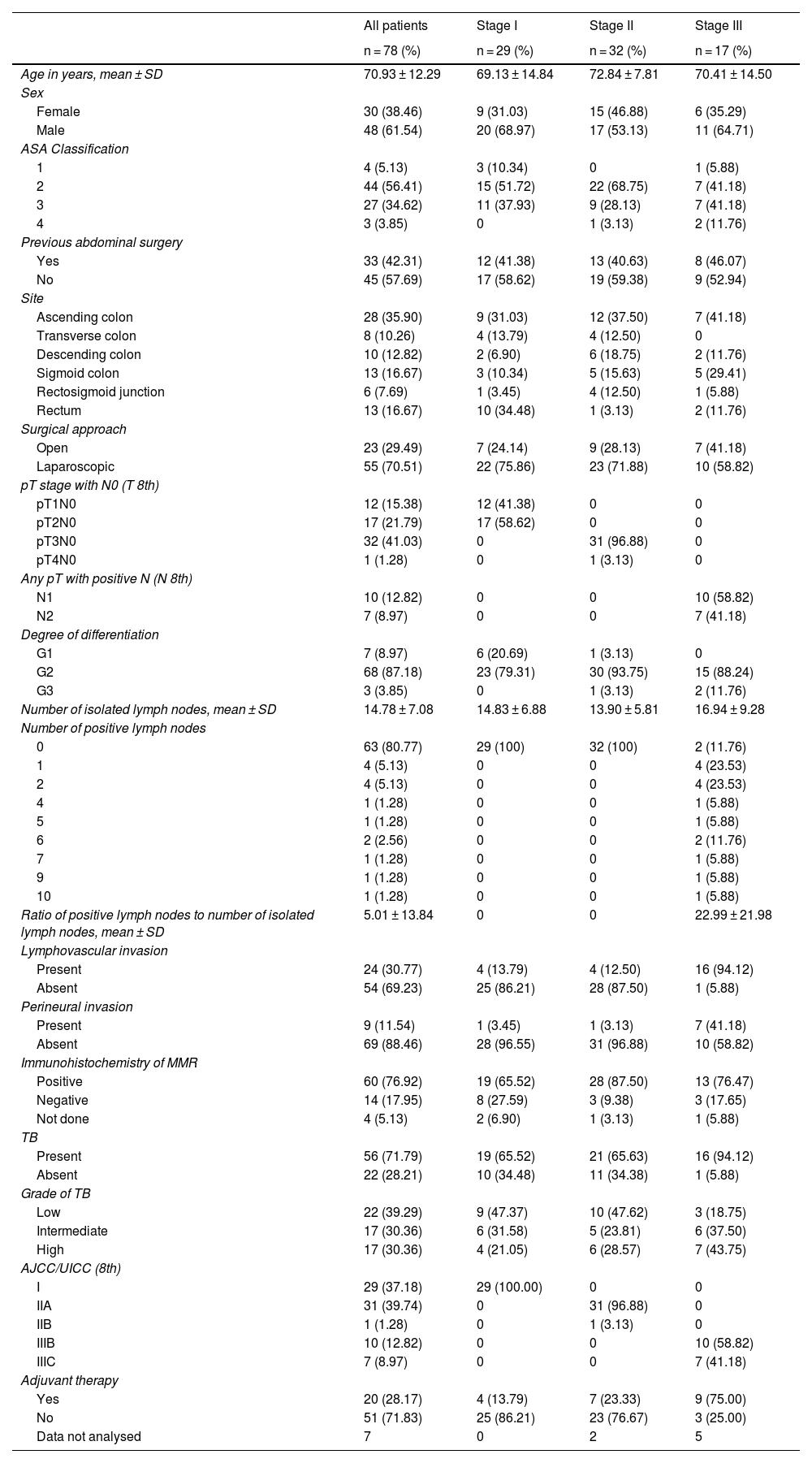
Table 1 summarises the main demographic and clinicopathological characteristics.
Clinical/pathological characteristics of the 78 patients according to clinical stage AJCC/UICC.
| All patients | Stage I | Stage II | Stage III | |
|---|---|---|---|---|
| n = 78 (%) | n = 29 (%) | n = 32 (%) | n = 17 (%) | |
| Age in years, mean ± SD | 70.93 ± 12.29 | 69.13 ± 14.84 | 72.84 ± 7.81 | 70.41 ± 14.50 |
| Sex | ||||
| Female | 30 (38.46) | 9 (31.03) | 15 (46.88) | 6 (35.29) |
| Male | 48 (61.54) | 20 (68.97) | 17 (53.13) | 11 (64.71) |
| ASA Classification | ||||
| 1 | 4 (5.13) | 3 (10.34) | 0 | 1 (5.88) |
| 2 | 44 (56.41) | 15 (51.72) | 22 (68.75) | 7 (41.18) |
| 3 | 27 (34.62) | 11 (37.93) | 9 (28.13) | 7 (41.18) |
| 4 | 3 (3.85) | 0 | 1 (3.13) | 2 (11.76) |
| Previous abdominal surgery | ||||
| Yes | 33 (42.31) | 12 (41.38) | 13 (40.63) | 8 (46.07) |
| No | 45 (57.69) | 17 (58.62) | 19 (59.38) | 9 (52.94) |
| Site | ||||
| Ascending colon | 28 (35.90) | 9 (31.03) | 12 (37.50) | 7 (41.18) |
| Transverse colon | 8 (10.26) | 4 (13.79) | 4 (12.50) | 0 |
| Descending colon | 10 (12.82) | 2 (6.90) | 6 (18.75) | 2 (11.76) |
| Sigmoid colon | 13 (16.67) | 3 (10.34) | 5 (15.63) | 5 (29.41) |
| Rectosigmoid junction | 6 (7.69) | 1 (3.45) | 4 (12.50) | 1 (5.88) |
| Rectum | 13 (16.67) | 10 (34.48) | 1 (3.13) | 2 (11.76) |
| Surgical approach | ||||
| Open | 23 (29.49) | 7 (24.14) | 9 (28.13) | 7 (41.18) |
| Laparoscopic | 55 (70.51) | 22 (75.86) | 23 (71.88) | 10 (58.82) |
| pT stage with N0 (T 8th) | ||||
| pT1N0 | 12 (15.38) | 12 (41.38) | 0 | 0 |
| pT2N0 | 17 (21.79) | 17 (58.62) | 0 | 0 |
| pT3N0 | 32 (41.03) | 0 | 31 (96.88) | 0 |
| pT4N0 | 1 (1.28) | 0 | 1 (3.13) | 0 |
| Any pT with positive N (N 8th) | ||||
| N1 | 10 (12.82) | 0 | 0 | 10 (58.82) |
| N2 | 7 (8.97) | 0 | 0 | 7 (41.18) |
| Degree of differentiation | ||||
| G1 | 7 (8.97) | 6 (20.69) | 1 (3.13) | 0 |
| G2 | 68 (87.18) | 23 (79.31) | 30 (93.75) | 15 (88.24) |
| G3 | 3 (3.85) | 0 | 1 (3.13) | 2 (11.76) |
| Number of isolated lymph nodes, mean ± SD | 14.78 ± 7.08 | 14.83 ± 6.88 | 13.90 ± 5.81 | 16.94 ± 9.28 |
| Number of positive lymph nodes | ||||
| 0 | 63 (80.77) | 29 (100) | 32 (100) | 2 (11.76) |
| 1 | 4 (5.13) | 0 | 0 | 4 (23.53) |
| 2 | 4 (5.13) | 0 | 0 | 4 (23.53) |
| 4 | 1 (1.28) | 0 | 0 | 1 (5.88) |
| 5 | 1 (1.28) | 0 | 0 | 1 (5.88) |
| 6 | 2 (2.56) | 0 | 0 | 2 (11.76) |
| 7 | 1 (1.28) | 0 | 0 | 1 (5.88) |
| 9 | 1 (1.28) | 0 | 0 | 1 (5.88) |
| 10 | 1 (1.28) | 0 | 0 | 1 (5.88) |
| Ratio of positive lymph nodes to number of isolated lymph nodes, mean ± SD | 5.01 ± 13.84 | 0 | 0 | 22.99 ± 21.98 |
| Lymphovascular invasion | ||||
| Present | 24 (30.77) | 4 (13.79) | 4 (12.50) | 16 (94.12) |
| Absent | 54 (69.23) | 25 (86.21) | 28 (87.50) | 1 (5.88) |
| Perineural invasion | ||||
| Present | 9 (11.54) | 1 (3.45) | 1 (3.13) | 7 (41.18) |
| Absent | 69 (88.46) | 28 (96.55) | 31 (96.88) | 10 (58.82) |
| Immunohistochemistry of MMR | ||||
| Positive | 60 (76.92) | 19 (65.52) | 28 (87.50) | 13 (76.47) |
| Negative | 14 (17.95) | 8 (27.59) | 3 (9.38) | 3 (17.65) |
| Not done | 4 (5.13) | 2 (6.90) | 1 (3.13) | 1 (5.88) |
| TB | ||||
| Present | 56 (71.79) | 19 (65.52) | 21 (65.63) | 16 (94.12) |
| Absent | 22 (28.21) | 10 (34.48) | 11 (34.38) | 1 (5.88) |
| Grade of TB | ||||
| Low | 22 (39.29) | 9 (47.37) | 10 (47.62) | 3 (18.75) |
| Intermediate | 17 (30.36) | 6 (31.58) | 5 (23.81) | 6 (37.50) |
| High | 17 (30.36) | 4 (21.05) | 6 (28.57) | 7 (43.75) |
| AJCC/UICC (8th) | ||||
| I | 29 (37.18) | 29 (100.00) | 0 | 0 |
| IIA | 31 (39.74) | 0 | 31 (96.88) | 0 |
| IIB | 1 (1.28) | 0 | 1 (3.13) | 0 |
| IIIB | 10 (12.82) | 0 | 0 | 10 (58.82) |
| IIIC | 7 (8.97) | 0 | 0 | 7 (41.18) |
| Adjuvant therapy | ||||
| Yes | 20 (28.17) | 4 (13.79) | 7 (23.33) | 9 (75.00) |
| No | 51 (71.83) | 25 (86.21) | 23 (76.67) | 3 (25.00) |
| Data not analysed | 7 | 0 | 2 | 5 |
AJCC/UICC: American Joint Committee on Cancer/Union for International Cancer Control; ASA: American Society of Anaesthesiologists classification; MMR: Mismatch Repair (DNA mismatch repair system); SD: standard deviation; TB: Tumor Budding.
Of the 78 cases, TB was present in 56 patients (71.8%), divided into the following categories: low grade in 22 (39.3%), intermediate grade in 17 (30.4%) and high grade in 17 (30.4%). The tumours were located in the ascending colon (35.9%), transverse colon (10.2%), descending colon (12.8%), sigma (16.6%), rectosigmoid junction (7.6%) and rectum (16.6%). There were 29 cases (37.1%) in stage I, 32 cases (41%) in stage II and 17 cases (21.7%) in stage III. The majority (68%) were moderately differentiated adenocarcinomas, while 3 cases (3.8%) were poorly differentiated adenocarcinomas. Microsatellite instability status was available in 74 cases, with deficiency of repair protein expression in 17.9% of patients. Neoadjuvant treatment was given in 20 cases (28.1%).
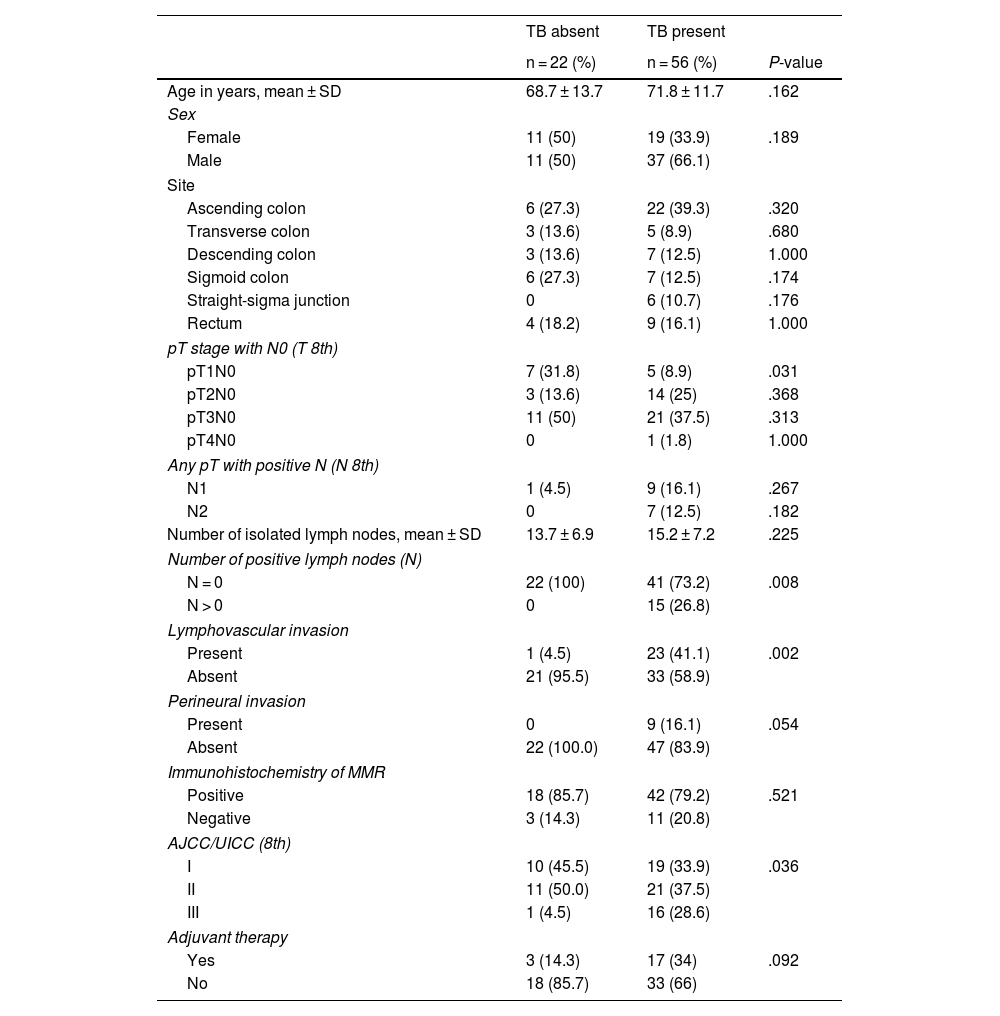
Clinical characteristics of patients according to tumour buddingRegardless of grade, there were no significant associations between TB and variables such as sex, age, tumour location, histological differentiation, microsatellite instability; while strong associations were observed between TB and pT1 stage, pN stage and lymphovascular invasion (Table 2). The presence of lymphovascular invasion and the number of positive lymph nodes had a statistically significant association with TB (P = .008, P = .001, respectively).
Clinical characteristics of the patients according to TB.
| TB absent | TB present | ||
|---|---|---|---|
| n = 22 (%) | n = 56 (%) | P-value | |
| Age in years, mean ± SD | 68.7 ± 13.7 | 71.8 ± 11.7 | .162 |
| Sex | |||
| Female | 11 (50) | 19 (33.9) | .189 |
| Male | 11 (50) | 37 (66.1) | |
| Site | |||
| Ascending colon | 6 (27.3) | 22 (39.3) | .320 |
| Transverse colon | 3 (13.6) | 5 (8.9) | .680 |
| Descending colon | 3 (13.6) | 7 (12.5) | 1.000 |
| Sigmoid colon | 6 (27.3) | 7 (12.5) | .174 |
| Straight-sigma junction | 0 | 6 (10.7) | .176 |
| Rectum | 4 (18.2) | 9 (16.1) | 1.000 |
| pT stage with N0 (T 8th) | |||
| pT1N0 | 7 (31.8) | 5 (8.9) | .031 |
| pT2N0 | 3 (13.6) | 14 (25) | .368 |
| pT3N0 | 11 (50) | 21 (37.5) | .313 |
| pT4N0 | 0 | 1 (1.8) | 1.000 |
| Any pT with positive N (N 8th) | |||
| N1 | 1 (4.5) | 9 (16.1) | .267 |
| N2 | 0 | 7 (12.5) | .182 |
| Number of isolated lymph nodes, mean ± SD | 13.7 ± 6.9 | 15.2 ± 7.2 | .225 |
| Number of positive lymph nodes (N) | |||
| N = 0 | 22 (100) | 41 (73.2) | .008 |
| N > 0 | 0 | 15 (26.8) | |
| Lymphovascular invasion | |||
| Present | 1 (4.5) | 23 (41.1) | .002 |
| Absent | 21 (95.5) | 33 (58.9) | |
| Perineural invasion | |||
| Present | 0 | 9 (16.1) | .054 |
| Absent | 22 (100.0) | 47 (83.9) | |
| Immunohistochemistry of MMR | |||
| Positive | 18 (85.7) | 42 (79.2) | .521 |
| Negative | 3 (14.3) | 11 (20.8) | |
| AJCC/UICC (8th) | |||
| I | 10 (45.5) | 19 (33.9) | .036 |
| II | 11 (50.0) | 21 (37.5) | |
| III | 1 (4.5) | 16 (28.6) | |
| Adjuvant therapy | |||
| Yes | 3 (14.3) | 17 (34) | .092 |
| No | 18 (85.7) | 33 (66) | |
AJCC/UICC: American Joint Committee on Cancer/Union for International Cancer Control; MMR: Mismatch Repair (DNA mismatch repair system); SD: standard deviation.
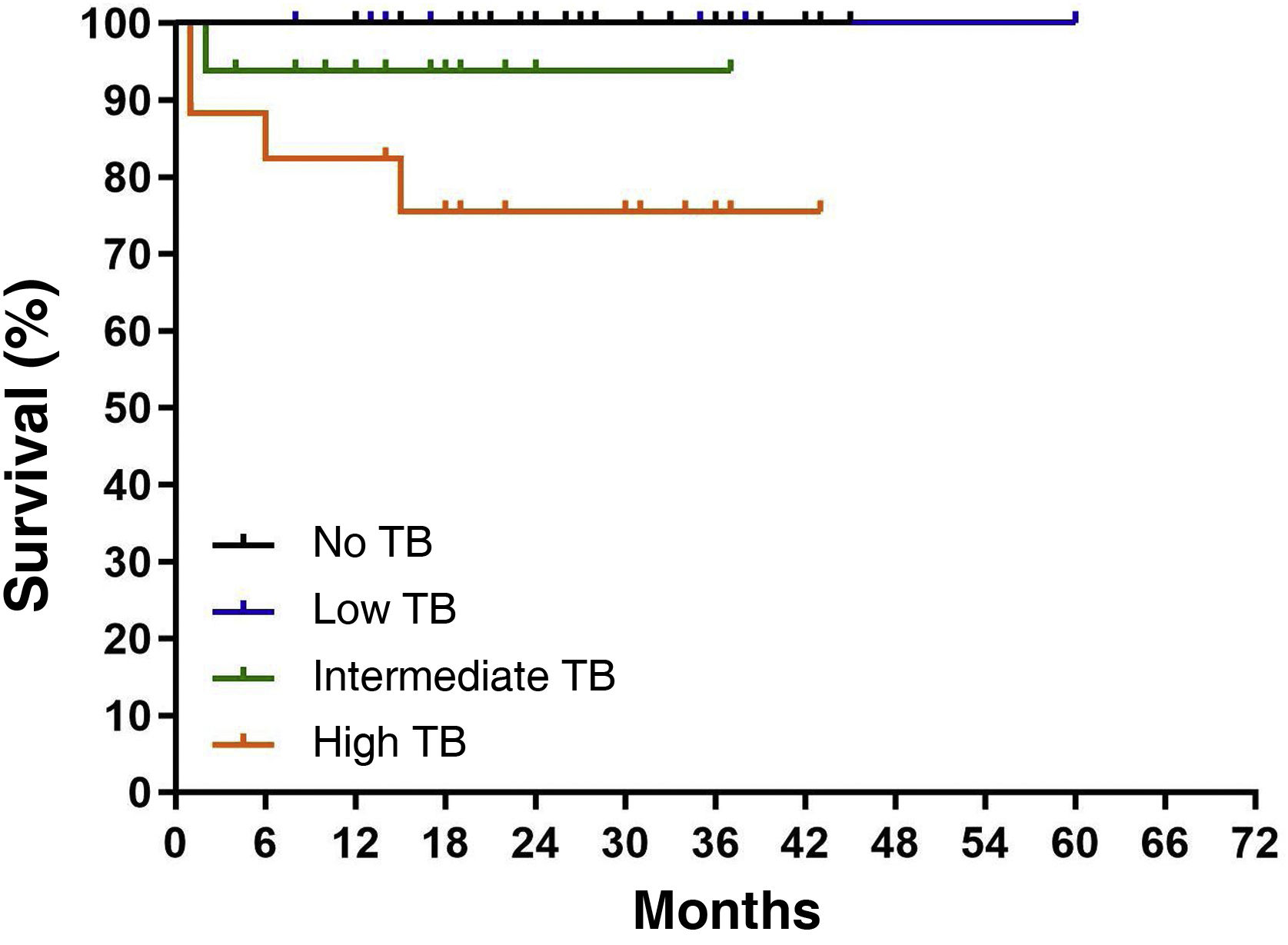
There was a total of 7 deaths (91% of the entire series): one case died in the group without TB expression and 6 patients in the group with TB; comparing OS between the 2 groups (95.5% vs. 89.3%, respectively), no statistically significant differences were observed (P = .143). However, TB showed worse disease survival at 5 years with each increase in grade; in fact, intermediate- and high-grade TB were associated with shorter OS compared with that of low-grade TB (93.7 and 75.4% vs. 100%, respectively; P = .012; Fig. 2).
Twelve patients were diagnosed with any type of recurrence, metastasis or local recurrence, with an DFS of 84.6% for the entire series. In 2 cases, the coexistence of distant metastasis and local relapse at intra-abdominal and anastomotic level was observed. In the TB group there were 11 patients with recurrence compared to only one case in the non-TB group (80.4% vs. 95.5% respectively; P = .096).
DFS was observed with worse outcomes with increasing grade: 70% in high grade, 75.3% in intermediate grade and 86.3% in low grade. A trend towards higher local recurrence according to TB grade was described, without reaching a statistically significant difference (P = .071; Fig. 3a). However, combining metastasis and local recurrence rate with TB grade, Kaplan-Meier survival curves for DFS showed significant differences between grades (P = .034; Fig. 3b).
DiscussionTB describes the presence of isolated or small clusters of tumour cells at the invasive front of carcinomas. It is now believed that this morphological aspect, resulting from the transition from an epithelial to a mesenchymal phenotype, may represent the first step for tumour invasion. For this reason, TB has been considered as a possible prognostic factor related to colorectal cancer. The prognostic relevance of TB is beginning to be reflected in the latest UICC publication of 2017, as well as its inclusion in guidelines for CRC screening, diagnosis and treatment in Europe and Japan.19,20 ESMO (European Society for Medical Oncology) guidelines have included it as a high-risk feature, along with lymphatic or venous invasion.21
Although previous studies have described an association between high-grade TB and lymphovascular invasion, recognising its importance as a poor prognostic factor, there are now discrepancies in the scientific community; in particular, TB is not yet currently included as a high-risk adverse feature in other guidelines, including NICE (National Institute for Health and Clinical Excellence) guidelines.
The oncological outcomes of TB in CRC have been studied, suggesting a worse outcome and a higher rate of lymph node metastasis.10 A 2016 review based on retrospective studies showed that TB was associated with a worse outcome after curative resection for stage II CRC2; although retrospective cohort studies analysed how TB was associated with worse survival in treated patients with stage II CRC, a study of 477 patients with stage III CRC did not demonstrate that TB was an independent prognostic factor.22
In the presence of metastases, a retrospective study analysed a 5-year survival rate of 18.4% for high grade compared to 40.5% for low and intermediate grade (HR: 1.51; P < .009) in patients with metastatic CRC.9 In contrast, some studies demonstrated that the prognostic significance of TB was not significant in multivariate analysis; in fact, a 2010 retrospective analysis questioned the prognostic utility of TB in CRC patients with lymph node metastases, suggesting that once the tumour had spread to the lymph nodes, its grade was not significant for future biological behaviour.22
Given the heterogeneity of results and the lack of robust uniformity in clinical practice, this study investigates the association between TB at the invasive front and oncological outcome in patients with surgical resection of CRC.
Our study enrolled a consecutive number of patients with CRC without metastases at diagnosis, thus excluding patients with clinical stage IV. Although the literature suggests TB is most useful in early CRC, its role may apply to all stages of CRC. In stage III, a retrospective analysis showed a significant association between high-grade TB and chemoresistance.23 It is true that the prognostic utility of TB is greatest in patients with stage I and II CRC, where it may allow the identification of patients at increased risk of lymph node metastasis.24
Our study analysed the cases according to AJCC/UICC stages; taking into account the clinical stage and TB expression, 33.9% were stage I, 37.5% stage II, 28.6% stage III compared to 45.5% stage I, 50% stage II, 4.5% stage III in the group without TB, showing a statistically significant difference (P = .036) (Table 2). Looking exclusively at the anatomical pathology “T” stage with N0, obtained after analysing the surgical specimens, the distribution in the TB group was as follows: 8.9% were classified as pT1, 25% as pT2, 37.5% as pT3, 1.8% as pT4. It is interesting to note a statistically significant difference when comparing the pT1N0 stage in the two study groups, with or without TB (8.9 vs. 31.8%; P = .031, respectively) (Table 2).
The increasing proportion of TB in more advanced T-stage tumours would be justified by the fact that tumours with more T infiltration tend to be more aggressive and advanced neoplasms and, therefore, with higher TB expression. Following this hypothesis, a strong association was found between TB expression and the negative/positive status of the isolated lymph nodes of the surgical specimen (P = .008) and a higher rate of lymphovascular (P = .002) and perineural (P = .054) invasion (Table 2).
With a median follow-up of 5 years, only one case died in the group without TB expression and 6 patients died in the group with TB expression, with an OS rate of 95.5% and 89.3%, respectively. When differentiating by grade, we observed an OS of 100% in low grade, 93.7% in intermediate grade and 75.4% in high grade, respectively, with a statistically significant difference (P = .012) (Fig. 2).
The most relevant aspect is the analysis of the results according to DFS. In a prospective multicentre study, the 5-year relapse-free survival rate was 90.9, 85.1 and 74.4% in low, intermediate and high grades, respectively (P < .001), and TB grade was significantly correlated with recurrence in the group with liver, lung, lymph node and peritoneal metastases.25 These results corroborate the data we found in our series; DFS was 86.3% in low, 70.6% in intermediate and 76.5% in high grade respectively, with a statistically significant difference (P = .034) (Fig. 3b).
Similar results have been shown in patients with colorectal cancer treated with neoadjuvant therapy.26 Although most studies address the prognostic impact in previously untreated CRC, controversy exists in the post-neoadjuvant setting of rectal tumours treated with chemoradiotherapy.14 In our study, we analysed patients with colorectal cancer (16.67% of the sample) together with the rest of the cases, taking into account that the effect of neoadjuvant treatment may hinder the assessment of TB due to radiotherapy-induced fibrosis and cellular sparsity in the case of a complete response to treatment.
Another important aspect is based on the type of TB observation method chosen and the size of the microscopic field, recommending a standardised method for future studies.20 For this reason, the 2016 consensus meeting established that TB observation should be performed on H&E sections, which is more cost-effective and at the same time sufficient compared to the immunohistochemistry technique using pancytokeratins. The TB count should be obtained by first selecting an area defined as a “hotspot” after scanning 10 microscopic fields at medium power and then analysing this area with a ×20 objective lens, which corresponds to a microscopic field of 0.785 mm2. In our series, thanks to the collaboration of 2 pathologists with expertise in gastrointestinal pathology, we were able to establish a protocol for the evaluation of TB in all the slides of the patients included, according to the guidelines described above.
It is noteworthy that, despite the prognostic implications of TB in the literature, its implementation in the anatomical pathology report is not homogeneous and universally established. In our experience, its use has become more protocolised in recent years, with study samples being reviewed by the same pathologists, experts in gastrointestinal oncology. This meant that 51 of the 129 patients who underwent curative surgery for CRC were excluded because they did not have such a description. We are aware that this would introduce a selection bias in that the excluded patients could have altered the results found. However, once the description of TB has been incorporated in a much more consolidated way, a high rate for this histological feature has been reached in the specimens analysed and significant conclusions have been drawn regarding oncological prognosis. For this reason, we insist on the need to establish protocols for TB in surgical oncology departments in order to be able to conduct future research.
There are some limitations to our study. Firstly, it was conducted retrospectively at a single institution; therefore, the retrospective nature by definition entails a selection bias. In selecting the method of TB observation, we did not systematically compare H&E with other histological methods, such as immunohistochemistry. After completion of the study, there was a difference between the expected proportion of TB patients described in the literature and the proportion of TB patients in our study (56 patients, 71% of the sample).
In spite of these limitations, our study highlights a number of relevant aspects. We distinguished the characteristics of TB according to the three stages of CRC and analysed OS, taking into account the different grades. Although the sample consisted of a small number of patients, the results were homogeneous thanks to the surgical and clinical management by the same team and the systematic analysis of all slides.
ConclusionIn recent decades, TB has been considered a prognostic marker in CRC; higher TB expression would be associated with more aggressive tumour behaviour and less favourable outcomes in terms of disease progression and survival.
This study provides information on TB morphology according to tumour stage and the clinical significance of TB as a poor prognostic factor; in fact, this work confirms the published evidence associating TB with shorter survival. To improve the power of the study in terms of subgroup analysis, due to the limited number of patients, future multicentre studies are needed that enrol a larger number of patients in a given time frame.
A standardised and routine application of TB is suggested and the need for future studies to clarify its usefulness in early-stage prognosis and decision making in the oncological treatment of CRC patients is recommended and encouraged.
Ethical approvalThis study protocol was reviewed and approved by the Research Ethics Committee of the Fundación Jiménez Díaz, approval file n° 22/21 (14/12/21) and n° 06/22 (04/12/22), with Protocol Code PGG-CAD-2022-01. This study was conducted in accordance with the Declaration of Helsinki of the World Medical Association. It was conducted in accordance with the World Medical Association Declaration of Helsinki, the Belmont Report, the Oviedo Convention on Human Rights and Biomedicine and Law 14/2007 of 3 July on Biomedical Research. The study was conducted in accordance with EU legislation on personal data, specifically Organic Law 3/2018 of 5 December on the Protection of Personal Data and the Guarantee of Digital Rights, Royal Decree 1720/2007, Law 41/2002 of 14 November, the basic law regulating patient autonomy and rights and obligations regarding clinical information and documentation.
FundingThis research has not received specific support from public, private or non-profit organisations.
AuthorsPietro Giovanni Giordano: conceptualisation, data curation, formal analysis, methodology, visualisation, writing the original draft, revising and editing the final draft. Ana Gabriela Díaz Zelaya: conceptualisation, data curation, formal analysis, methodology, writing the original draft. Yari Yuritzi Aguilera Molina: data curation, formal analysis, supervision, review and editing of the final draft. Nestor Orlando Taboada Mostajo: data curation, formal analysis, methodology, review and editing of the final draft. Yelene Ajete Ramos: data curation, formal analysis, methodology, review and editing of the final draft. Ricardo Ortega García: data curation, formal analysis, methodology, review and editing of the final draft. Esteban Peralta De Michelis: data curation, formal analysis, methodology, review and editing of the final draft. Juan Carlos Meneu Díaz: conceptualisation, data curation, formal analysis, methodology, visualisation, writing of the original draft, revision and editing of the final draft.
Conflict of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank Dr Cristina Andreu Vázquez, Dr Ana Isabel Castillo Varón and Dr Israel John Thuissard Vasallo (Methodological and Statistical Advisory Group, European University of Madrid) for their advice on the statistical analysis.






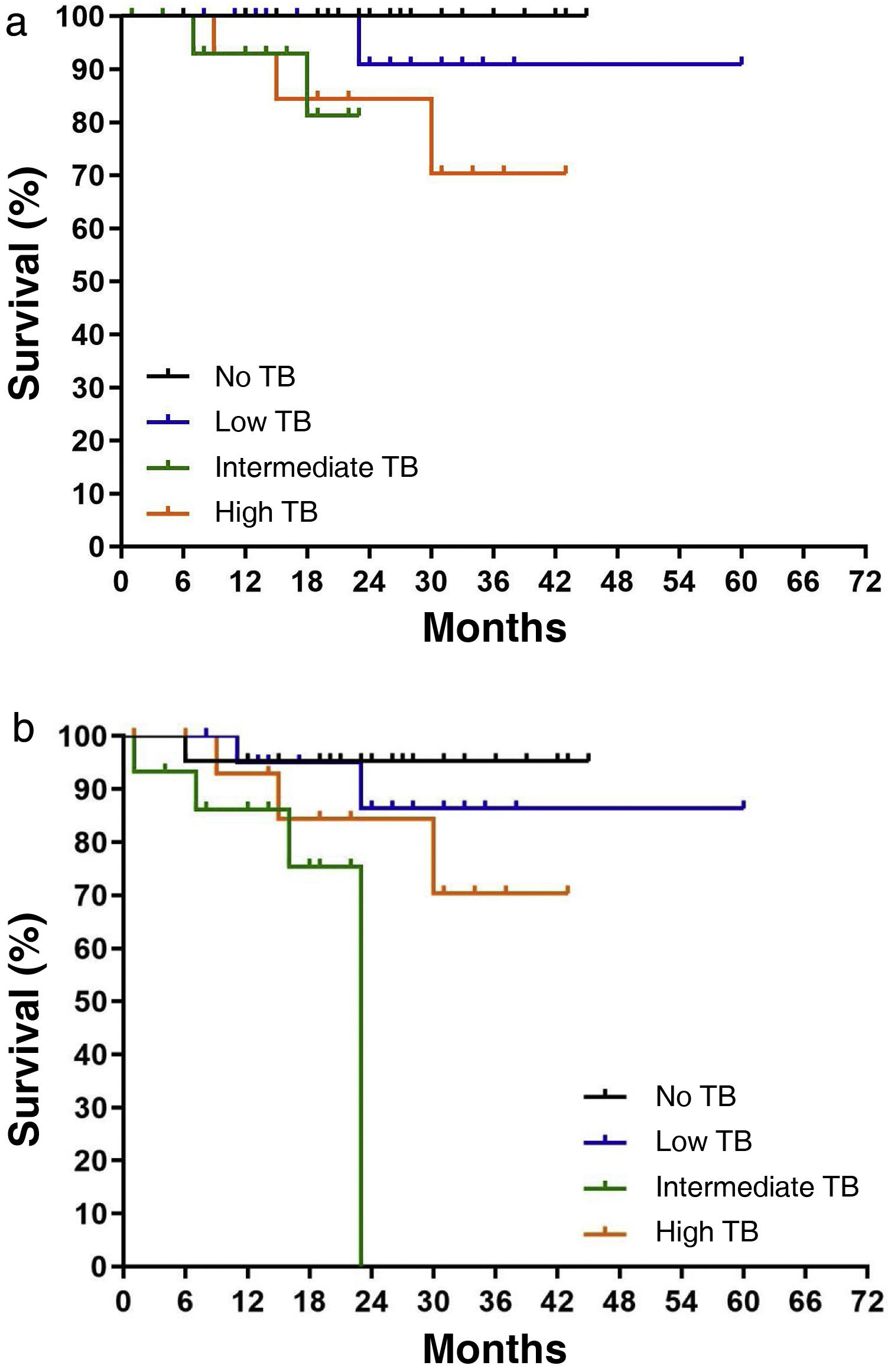


![Survival based on local recurrence rate and combined rate of metastasis and local recurrence (disease-free survival [DFS]): a) Survival based on local recurrence rate and TB grade (P = .071). b) DFS and TB grade (P = .034). Survival based on local recurrence rate and combined rate of metastasis and local recurrence (disease-free survival [DFS]): a) Survival based on local recurrence rate and TB grade (P = .071). b) DFS and TB grade (P = .034).](https://static.elsevier.es/multimedia/23870206/0000016300000004/v2_202409040637/S2387020624003383/v2_202409040637/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
