Since the World Health Organization (WHO) announced coronavirus disease 2019 (COVID-19) had become a global pandemic on March 11, 2020, the number of infections has been increasing. The purpose of this meta-analysis was to investigate the prognosis of COVID-19 in patients with coronary heart disease.
MethodPubmed, Embase, and Cochrane Library databases were searched to collect the literature concerning coronary heart disease and COVID-19. The retrieval time was from inception to Nov 20, 2020, using Stata version 14.0 for meta-analysis.
ResultsA total of 22,148 patients from 40 studies were included. The meta-analysis revealed that coronary heart disease was associated with poor prognosis of COVID-19 (OR=3.42, 95%CI [2.83, 4.13], P<0.001). After subgroup analysis, coronary heart disease was found to be related to mortality (OR=3.75, 95%CI [2.91, 4.82], P<0.001), severe/critical COVID-19 (OR=3.23, 95%CI [2.19, 4.77], P<0.001), ICU admission (OR=2.25, 95%CI [1.34, 3.79], P=0.002), disease progression (OR=3.01, 95%CI [1.46, 6.22], P=0.003); Meta-regression showed that the association between coronary heart disease and poor prognosis of COVID-19 was affected by hypertension (P=0.004), and subgroup analysis showed that compared with the proportion of hypertension >30% (OR=2.85, 95%CI [2.33, 3.49]), the proportion of hypertension <30% (OR=4.78, 95%CI [3.50, 6.51]) had a higher risk of poor prognosis.
ConclusionCoronary heart disease is a risk factor for poor prognosis in patients with COVID-19.
Desde que la Organización Mundial de la Salud (OMS) anunció que la enfermedad por coronavirus de 2019 (COVID-19) se había convertido en una pandemia global el 11 de marzo de 2020, se ha incrementado el número de infecciones. El objetivo de este metaanálisis fue investigar el pronóstico de la COVID-19 en pacientes con cardiopatía coronaria.
MétodoSe realizó una búsqueda en las bases de datos de Pubmed, Embase y Cochrane Library para reunir la literatura relativa a cardiopatía coronaria y COVID-19. El tiempo de recuperación de datos fue desde el inicio hasta el 20 de noviembre de 2020, utilizando la versión 14.0 de Stata® para el metaanálisis.
ResultadosSe incluyó un total de 22.148 pacientes de 40 estudios. El metaanálisis reveló que la cardiopatía coronaria estaba asociada a un mal pronóstico de COVID-19 (OR: 3,42; IC 95%: 2,83-4,13; p<0,001). Tras el análisis de subgrupo, se encontró que la cardiopatía coronaria tenía relación con la mortalidad (OR: 3,75; IC 95%: 2,91-4,82; p<0,001), COVID-19 grave/crítica (OR: 3,23; IC 95%: 2,19-4,77; p<0,001), ingreso en la UCI (OR: 2,25; IC 95%: 1,34-3,79; p=0,002), progresión de la enfermedad (OR: 3,01; IC 95%: 1,46-6,22; p=0,003). La metarregresión reflejó que la asociación entre cardiopatía coronaria y mal pronóstico de la COVID-19 estaba influida por la hipertensión (p=0,004), y el análisis de subgrupo mostró que comparada con la proporción de hipertensión >30% (OR: 2,85; IC 95%: 2,33-3,49), la proporción de hipertensión <30% (OR: 4,78; IC 95%: 3,50-6,51) tenía mayor riesgo de mal pronóstico.
ConclusiónLa cardiopatía coronaria es un factor de riesgo de mal pronóstico en pacientes con COVID-19.
After the first case of coronavirus disease 2019 (COVID-19) was discovered in Wuhan, Hubei Province, China on December 8, 2019. As of November 27, 2020, a total of 61,452,584 cases have been confirmed globally, resulting in 1,440,629 deaths.1 Pathogens were severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the main manifestation were flu-like symptoms and could develop into severe respiratory failure, shock, or multiple organ failure.2,3 COVID-19 patients with underlying diseases such as diabetes, hypertension, and chronic obstructive pulmonary disease have a poor prognosis.4 However, the relationship between coronary heart disease and the prognosis of COVID-19 is still unknown. Many observational studies had been conducted to explore the factors associated with the poor prognosis of COVID-19 patients, but most of them were small sample sizes. Therefore, the purpose of this meta-analysis was to search the association between coronary heart disease and the prognosis of COVID-19 patients.
Materials and methodsThe work followed PRISMA statement for systematic reviews and meta-analysis.
Search strategyPubmed, Embase, and Cochrane Library databases were used for literature retrieval from inception to Nov 20, 2020. The search strategy in Pubmed was: 1.((((((((((((((((((“Coronary Disease”[Mesh]) OR (“Coronary Disease”)) OR (“Coronary Diseases”)) OR (“Disease, Coronary”)) OR (“Diseases, Coronary”)) OR (“Coronary Heart Disease”)) OR (“CHD”)) OR (“Coronary Heart Diseases”)) OR (“Disease, Coronary Heart”)) OR (“Diseases, Coronary Heart”)) OR (“Heart Disease, Coronary”)) OR (“Heart Diseases, Coronary”)) OR (“Coronary Artery Disease”)) OR (“CAD”)) OR (“Artery Disease, Coronary”)) OR (“Artery Diseases, Coronary”)) OR (“Coronary Artery Diseases”)) OR (“Disease, Coronary Artery”)) OR (“Diseases, Coronary Artery”); 2.((((“COVID-19”) OR (“Coronavirus disease 2019”)) OR (“2019 novel coronavirus”)) OR (“2019-nCoV”)) OR (“SARS-CoV-2”); 3.1 ADN 2.
Inclusion and exclusion criteriaThe definition of coronary heart disease in this meta-analysis were those reported in the relevant articles as coronary artery disease (CAD), coronary heart disease (CHD), or coronary disease.
Inclusion criteria: (1) The selected population was COVID-19 patients; (2) The primary outcome of the study included disease progression, severe/critical COVID-19, ICU admission, and mortality; (3) The study design should be a case–control, cross-sectional, or cohort study with detailed data of coronary heart disease for analysis; (4) The language of the study was limited to English.
Exclusion criteria: (1) Letters, comments, reviews, conference abstracts; (2)Studies describing only the children were excluded, but studies that involved both children and adults were included;
The definition of severe/critical COVID-19 was based on Diagnosis and treatment protocol for COVID-19.5
Data extraction and quality assessmentTwo authors scanned the title and abstract independently to exclude irrelevant studies, and the full-text articles were read to screen out eligible studies. Two authors independently completed the data extraction and collected the basic information of the included studies (author, study design, sample size, gender, hypertension, CHD/CAD, heart failure, primary outcome, etc.). Newcastle – Ottawa (NOS) scale was used for quality assessment.6 Any differences were resolved by the third author.
Statistical analysisStata version 14.0 was used for meta-analysis. The pooled odds ratio (OR) with 95% confidence interval (95% CI) was calculated to evaluate the relationship between coronary heart disease and the prognosis of COVID-19. P-value <0.05 was statistically significant. Heterogeneity was assessed with I2, if the heterogeneity was significant (I2>50%), the random effect model was used. A sensitivity analysis was performed by removing outlier studies, meta-regression was used to explore the source of heterogeneity. Subgroup analysis was performed based on the primary outcome and meta-regression results (P-value <0.05). We used the funnel plot and Egger test to evaluate publication bias, when the funnel plot was symmetrical and the P-value of Egger test >0.05, publication bias was accepted. If publication bias was present, the trim and fill method was used to correct for funnel plot asymmetry caused by the publication bias and to recalculate the pooled odds ratio.
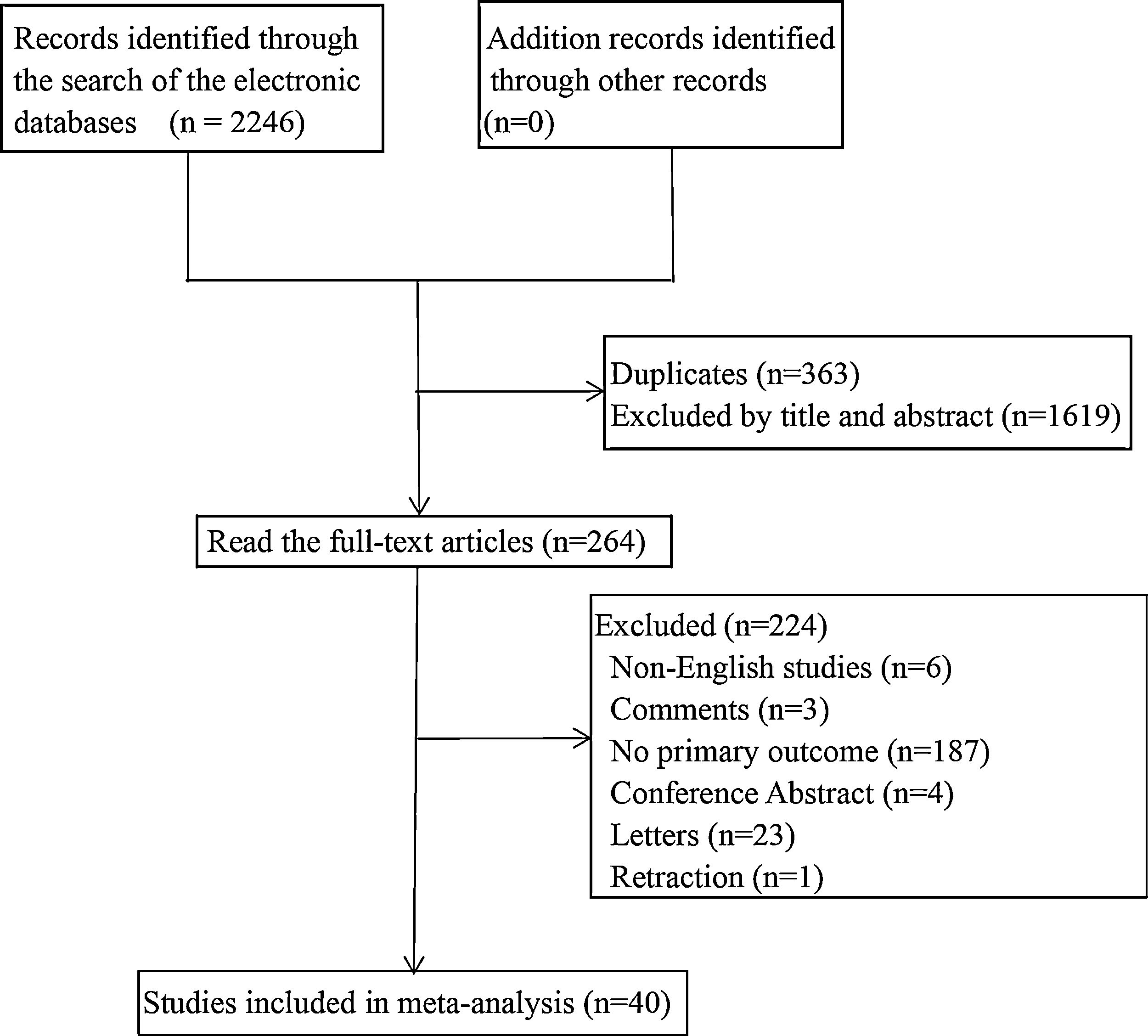
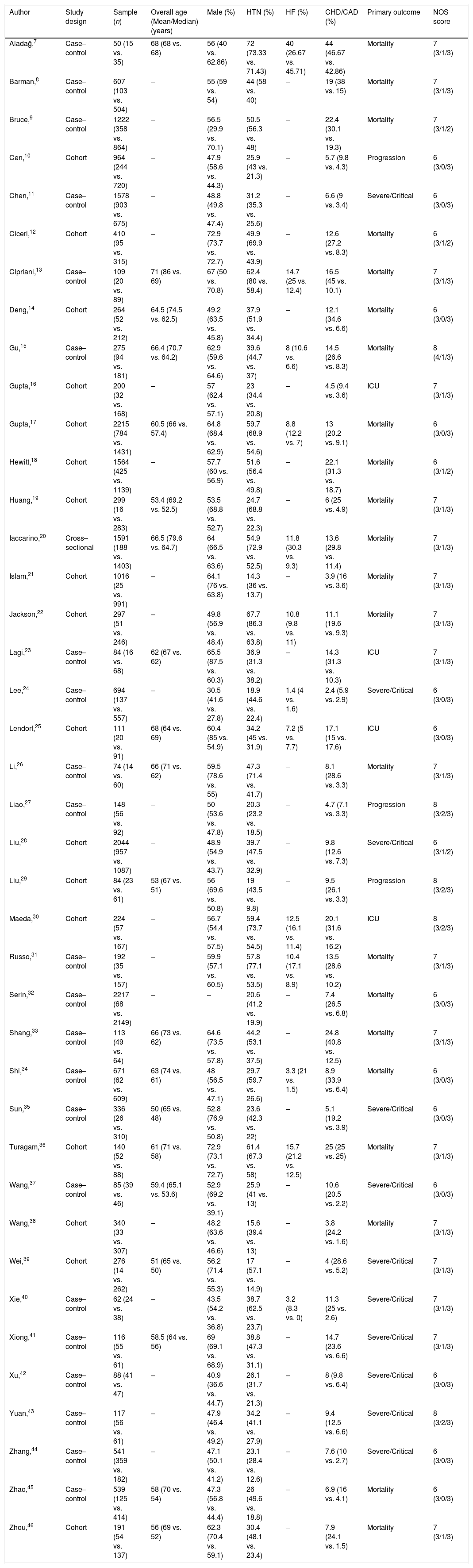
ResultsThe basic characteristics of the included studiesA total of 2246 articles were searched in the electronic database, 363 duplicate articles were excluded, 1619 articles were excluded after reading the title and abstract, 224 articles were excluded after scanning the full-text. Finally, 40 articles were involved. The literature screening process was shown in Fig. 1.
The 40 studies included 22 case–control studies, 17 cohort studies, and 1 cross-sectional study, involving 22,148 COVID-19 patients. According to the primary outcome of the study, they were divided into disease progression, severe/critical COVID-19, ICU admission, or mortality. The primary outcomes of 4 studies were ICU admission, 22 studies were mortality, 11 studies were severe/critical COVID-19, and 3 studies were disease progression. See Table 1 for the basic characteristics of the studies.
Basic characteristics of the included studies.
| Author | Study design | Sample (n) | Overall age (Mean/Median) (years) | Male (%) | HTN (%) | HF (%) | CHD/CAD (%) | Primary outcome | NOS score |
|---|---|---|---|---|---|---|---|---|---|
| Aladağ,7 | Case–control | 50 (15 vs. 35) | 68 (68 vs. 68) | 56 (40 vs. 62.86) | 72 (73.33 vs. 71.43) | 40 (26.67 vs. 45.71) | 44 (46.67 vs. 42.86) | Mortality | 7 (3/1/3) |
| Barman,8 | Case–control | 607 (103 vs. 504) | – | 55 (59 vs. 54) | 44 (58 vs. 40) | – | 19 (38 vs. 15) | Mortality | 7 (3/1/3) |
| Bruce,9 | Case–control | 1222 (358 vs. 864) | – | 56.5 (29.9 vs. 70.1) | 50.5 (56.3 vs. 48) | – | 22.4 (30.1 vs. 19.3) | Mortality | 7 (3/1/2) |
| Cen,10 | Cohort | 964 (244 vs. 720) | – | 47.9 (58.6 vs. 44.3) | 25.9 (43 vs. 21.3) | – | 5.7 (9.8 vs. 4.3) | Progression | 6 (3/0/3) |
| Chen,11 | Case–control | 1578 (903 vs. 675) | – | 48.8 (49.8 vs. 47.4) | 31.2 (35.3 vs. 25.6) | – | 6.6 (9 vs. 3.4) | Severe/Critical | 6 (3/0/3) |
| Ciceri,12 | Cohort | 410 (95 vs. 315) | – | 72.9 (73.7 vs. 72.7) | 49.9 (69.9 vs. 43.9) | – | 12.6 (27.2 vs. 8.3) | Mortality | 6 (3/1/2) |
| Cipriani,13 | Case–control | 109 (20 vs. 89) | 71 (86 vs. 69) | 67 (50 vs. 70.8) | 62.4 (80 vs. 58.4) | 14.7 (25 vs. 12.4) | 16.5 (45 vs. 10.1) | Mortality | 7 (3/1/3) |
| Deng,14 | Cohort | 264 (52 vs. 212) | 64.5 (74.5 vs. 62.5) | 49.2 (63.5 vs. 45.8) | 37.9 (51.9 vs. 34.4) | – | 12.1 (34.6 vs. 6.6) | Mortality | 6 (3/0/3) |
| Gu,15 | Case–control | 275 (94 vs. 181) | 66.4 (70.7 vs. 64.2) | 62.9 (59.6 vs. 64.6) | 39.6 (44.7 vs. 37) | 8 (10.6 vs. 6.6) | 14.5 (26.6 vs. 8.3) | Mortality | 8 (4/1/3) |
| Gupta,16 | Cohort | 200 (32 vs. 168) | – | 57 (62.4 vs. 57.1) | 23 (34.4 vs. 20.8) | – | 4.5 (9.4 vs. 3.6) | ICU | 7 (3/1/3) |
| Gupta,17 | Cohort | 2215 (784 vs. 1431) | 60.5 (66 vs. 57.4) | 64.8 (68.4 vs. 62.9) | 59.7 (68.9 vs. 54.6) | 8.8 (12.2 vs. 7) | 13 (20.2 vs. 9.1) | Mortality | 6 (3/0/3) |
| Hewitt,18 | Cohort | 1564 (425 vs. 1139) | – | 57.7 (60 vs. 56.9) | 51.6 (56.4 vs. 49.8) | – | 22.1 (31.3 vs. 18.7) | Mortality | 6 (3/1/2) |
| Huang,19 | Cohort | 299 (16 vs. 283) | 53.4 (69.2 vs. 52.5) | 53.5 (68.8 vs. 52.7) | 24.7 (68.8 vs. 22.3) | – | 6 (25 vs. 4.9) | Mortality | 7 (3/1/3) |
| Iaccarino,20 | Cross–sectional | 1591 (188 vs. 1403) | 66.5 (79.6 vs. 64.7) | 64 (66.5 vs. 63.6) | 54.9 (72.9 vs. 52.5) | 11.8 (30.3 vs. 9.3) | 13.6 (29.8 vs. 11.4) | Mortality | 7 (3/1/3) |
| Islam,21 | Cohort | 1016 (25 vs. 991) | – | 64.1 (76 vs. 63.8) | 14.3 (36 vs. 13.7) | – | 3.9 (16 vs. 3.6) | Mortality | 7 (3/1/3) |
| Jackson,22 | Cohort | 297 (51 vs. 246) | – | 49.8 (56.9 vs. 48.4) | 67.7 (86.3 vs. 63.8) | 10.8 (9.8 vs. 11) | 11.1 (19.6 vs. 9.3) | Mortality | 7 (3/1/3) |
| Lagi,23 | Case–control | 84 (16 vs. 68) | 62 (67 vs. 62) | 65.5 (87.5 vs. 60.3) | 36.9 (31.3 vs. 38.2) | – | 14.3 (31.3 vs. 10.3) | ICU | 7 (3/1/3) |
| Lee,24 | Case–control | 694 (137 vs. 557) | – | 30.5 (41.6 vs. 27.8) | 18.9 (44.6 vs. 22.4) | 1.4 (4 vs. 1.6) | 2.4 (5.9 vs. 2.9) | Severe/Critical | 6 (3/0/3) |
| Lendorf,25 | Cohort | 111 (20 vs. 91) | 68 (64 vs. 69) | 60.4 (85 vs. 54.9) | 34.2 (45 vs. 31.9) | 7.2 (5 vs. 7.7) | 17.1 (15 vs. 17.6) | ICU | 6 (3/0/3) |
| Li,26 | Case–control | 74 (14 vs. 60) | 66 (71 vs. 62) | 59.5 (78.6 vs. 55) | 47.3 (71.4 vs. 41.7) | – | 8.1 (28.6 vs. 3.3) | Mortality | 7 (3/1/3) |
| Liao,27 | Case–control | 148 (56 vs. 92) | – | 50 (53.6 vs. 47.8) | 20.3 (23.2 vs. 18.5) | – | 4.7 (7.1 vs. 3.3) | Progression | 8 (3/2/3) |
| Liu,28 | Cohort | 2044 (957 vs. 1087) | – | 48.9 (54.9 vs. 43.7) | 39.7 (47.5 vs. 32.9) | – | 9.8 (12.6 vs. 7.3) | Severe/Critical | 6 (3/1/2) |
| Liu,29 | Cohort | 84 (23 vs. 61) | 53 (67 vs. 51) | 56 (69.6 vs. 50.8) | 19 (43.5 vs. 9.8) | – | 9.5 (26.1 vs. 3.3) | Progression | 8 (3/2/3) |
| Maeda,30 | Cohort | 224 (57 vs. 167) | – | 56.7 (54.4 vs. 57.5) | 59.4 (73.7 vs. 54.5) | 12.5 (16.1 vs. 11.4) | 20.1 (31.6 vs. 16.2) | ICU | 8 (3/2/3) |
| Russo,31 | Case–control | 192 (35 vs. 157) | – | 59.9 (57.1 vs. 60.5) | 57.8 (77.1 vs. 53.5) | 10.4 (17.1 vs. 8.9) | 13.5 (28.6 vs. 10.2) | Mortality | 7 (3/1/3) |
| Serin,32 | Case–control | 2217 (68 vs. 2149) | – | – | 20.6 (41.2 vs. 19.9) | – | 7.4 (26.5 vs. 6.8) | Mortality | 6 (3/0/3) |
| Shang,33 | Case–control | 113 (49 vs. 64) | 66 (73 vs. 62) | 64.6 (73.5 vs. 57.8) | 44.2 (53.1 vs. 37.5) | – | 24.8 (40.8 vs. 12.5) | Mortality | 7 (3/1/3) |
| Shi,34 | Case–control | 671 (62 vs. 609) | 63 (74 vs. 61) | 48 (56.5 vs. 47.1) | 29.7 (59.7 vs. 26.6) | 3.3 (21 vs. 1.5) | 8.9 (33.9 vs. 6.4) | Mortality | 6 (3/0/3) |
| Sun,35 | Case–control | 336 (26 vs. 310) | 50 (65 vs. 48) | 52.8 (76.9 vs. 50.8) | 23.6 (42.3 vs. 22) | – | 5.1 (19.2 vs. 3.9) | Severe/Critical | 6 (3/0/3) |
| Turagam,36 | Cohort | 140 (52 vs. 88) | 61 (71 vs. 58) | 72.9 (73.1 vs. 72.7) | 61.4 (67.3 vs. 58) | 15.7 (21.2 vs. 12.5) | 25 (25 vs. 25) | Mortality | 7 (3/1/3) |
| Wang,37 | Case–control | 85 (39 vs. 46) | 59.4 (65.1 vs. 53.6) | 52.9 (69.2 vs. 39.1) | 25.9 (41 vs. 13) | – | 10.6 (20.5 vs. 2.2) | Severe/Critical | 6 (3/0/3) |
| Wang,38 | Cohort | 340 (33 vs. 307) | – | 48.2 (63.6 vs. 46.6) | 15.6 (39.4 vs. 13) | – | 3.8 (24.2 vs. 1.6) | Mortality | 7 (3/1/3) |
| Wei,39 | Cohort | 276 (14 vs. 262) | 51 (65 vs. 50) | 56.2 (71.4 vs. 55.3) | 17 (57.1 vs. 14.9) | – | 4 (28.6 vs. 5.2) | Severe/Critical | 7 (3/1/3) |
| Xie,40 | Case–control | 62 (24 vs. 38) | – | 43.5 (54.2 vs. 36.8) | 38.7 (62.5 vs. 23.7) | 3.2 (8.3 vs. 0) | 11.3 (25 vs. 2.6) | Severe/Critical | 7 (3/1/3) |
| Xiong,41 | Case–control | 116 (55 vs. 61) | 58.5 (64 vs. 56) | 69 (69.1 vs. 68.9) | 38.8 (47.3 vs. 31.1) | – | 14.7 (23.6 vs. 6.6) | Severe/Critical | 7 (3/1/3) |
| Xu,42 | Case–control | 88 (41 vs. 47) | – | 40.9 (36.6 vs. 44.7) | 26.1 (31.7 vs. 21.3) | – | 8 (9.8 vs. 6.4) | Severe/Critical | 6 (3/0/3) |
| Yuan,43 | Case–control | 117 (56 vs. 61) | – | 47.9 (46.4 vs. 49.2) | 34.2 (41.1 vs. 27.9) | – | 9.4 (12.5 vs. 6.6) | Severe/Critical | 8 (3/2/3) |
| Zhang,44 | Case–control | 541 (359 vs. 182) | – | 47.1 (50.1 vs. 41.2) | 23.1 (28.4 vs. 12.6) | – | 7.6 (10 vs. 2.7) | Severe/Critical | 6 (3/0/3) |
| Zhao,45 | Case–control | 539 (125 vs. 414) | 58 (70 vs. 54) | 47.3 (56.8 vs. 44.4) | 26 (49.6 vs. 18.8) | – | 6.9 (16 vs. 4.1) | Mortality | 6 (3/0/3) |
| Zhou,46 | Cohort | 191 (54 vs. 137) | 56 (69 vs. 52) | 62.3 (70.4 vs. 59.1) | 30.4 (48.1 vs. 23.4) | – | 7.9 (24.1 vs. 1.5) | Mortality | 7 (3/1/3) |
Abbreviations: HTN, hypertension; HF, heart failure; CAD, coronary artery disease; CHD, coronary heart disease.
The NOS scores of the 40 included studies were ≥6. (See Table 1).
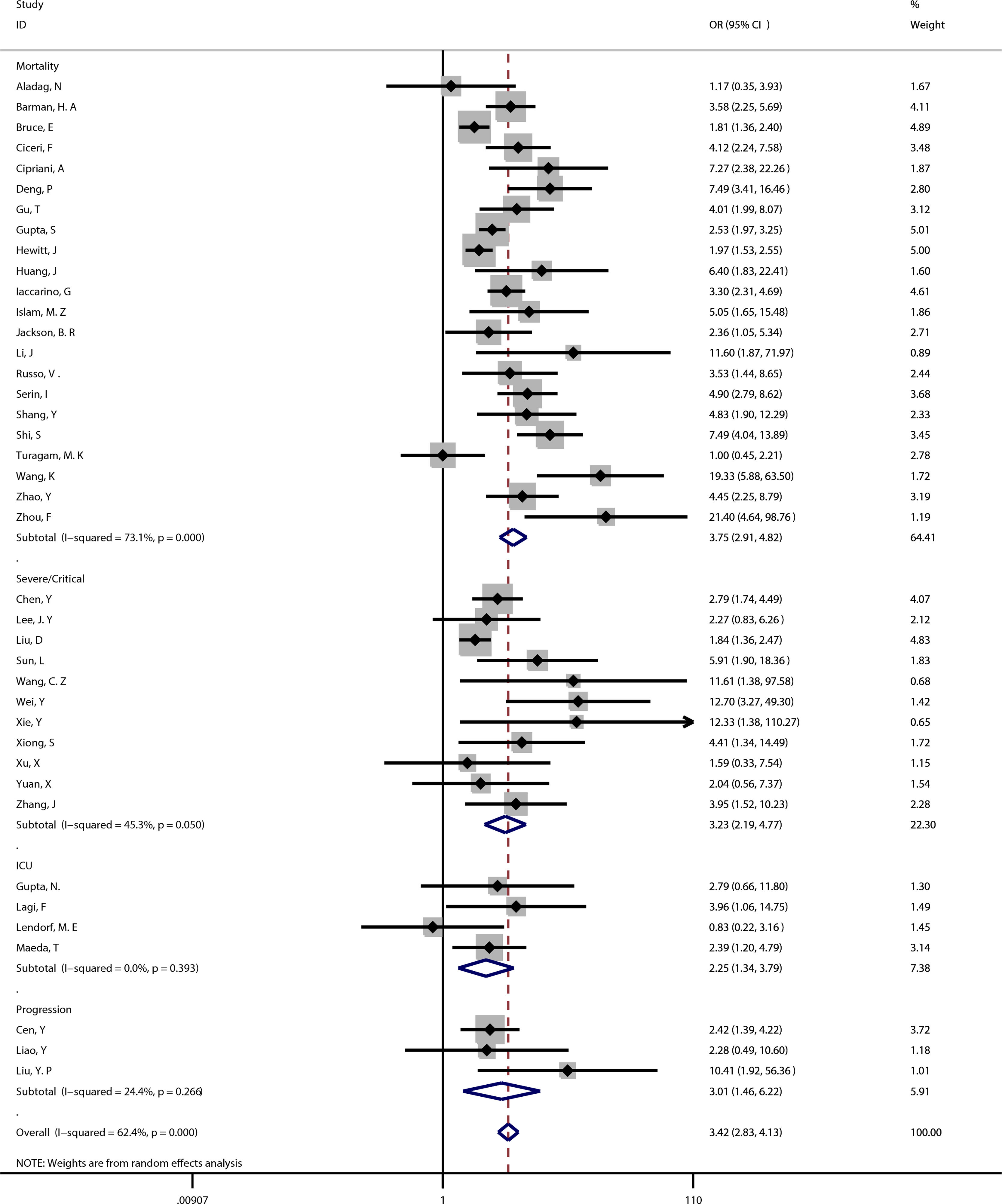
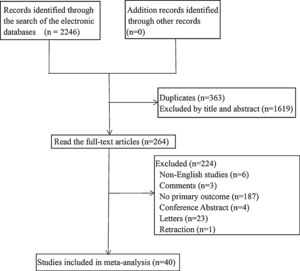
Coronary heart disease and outcomeThe meta-analysis showed coronary heart disease was associated with poor prognosis of COVID-19 (OR=3.42, 95%CI [2.83, 4.13], P<0.001; I2=62.4%, P<0.001). (See Fig. 2).
22 studies reported significant differences between coronary heart disease and mortality in COVID-19 patients (OR=3.75, 95%CI [2.91, 4.82], P<0.001), with moderately high heterogeneity (I2=73.1%, P<0.001). (See Fig. 2). A sensitivity analysis was carried out to exclude the study by Bruce,9 Gupta,17 Hewitt,18 Turagam,36 and Wang,38 resulting in a pooled odds ratio of 4.40 [3.56, 5.44; P<0.001] with low heterogeneity between studies [I2=25.5%, P=0.161].
Coronary heart disease and severe/critical COVID-1911 studies reported significant differences between coronary heart disease and severe/critical COVID-19 (OR=3.23, 95%CI [2.19, 4.77], P<0.001), with moderate heterogeneity (I2=45.3%, P=0.05). (See Fig. 2). A sensitivity analysis was carried out to exclude the studies by Liu,28 and Wei,39 resulting in a pooled OR of 3.20 [2.31, 4.43; P<0.001] with no heterogeneity between studies [I2=0.0%, P=0.595].
Coronary heart disease and ICU admission4 studies reported significant differences between coronary heart disease and ICU admission in COVID-19 patients (OR=2.25, 95%CI [1.34, 3.79], P=0.002), with no heterogeneity (I2=0.0%, P=0.393). (See Fig. 2).
Coronary heart disease and disease progression3 studies reported significant differences between coronary heart disease and disease progression in COVID-19 patients (OR=3.01, 95%CI [1.46, 6.22], P=0.003), with low heterogeneity (I2=24.4%, P=0.266). (See Fig. 2).
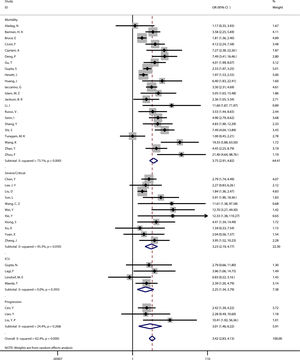
Meta-regressionDue to the significant heterogeneity between coronary heart disease and poor prognosis of COVID-19 (I2=62.4%, P<0.001), we separately conducted meta-regression of study design, sample size, overall age, gender, heart failure, hypertension, and quality score to search for the source of heterogeneity. Results showed that the association between coronary heart disease and poor prognosis of COVID-19 was affected by hypertension (P=0.004), but not study design (P=0.543), sample size (P=0.103), age (P=0.121), gender (P=0.836), heart failure (P=0.120), and quality score (P=0.924).
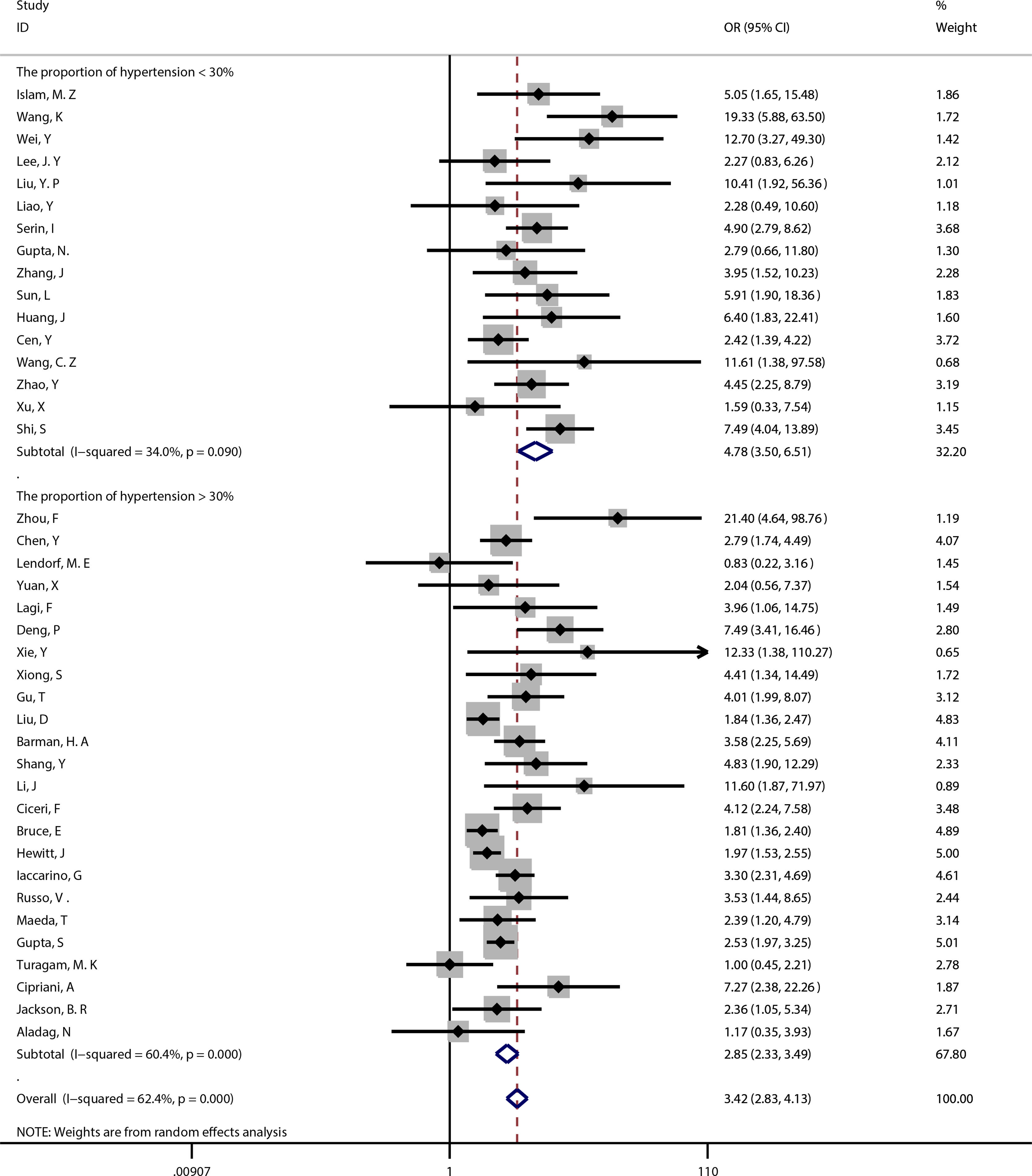
Subgroup analysisMeta-regression showed that hypertension (P=0.004) was the source of heterogeneity, subgroup analysis showed that compared with the proportion of hypertension >30% (OR=2.85, 95%CI [2.33, 3.49]), the proportion of hypertension <30% (OR=4.78, 95%CI [3.50, 6.51]) had a higher risk of poor prognosis. (See Fig. 3).
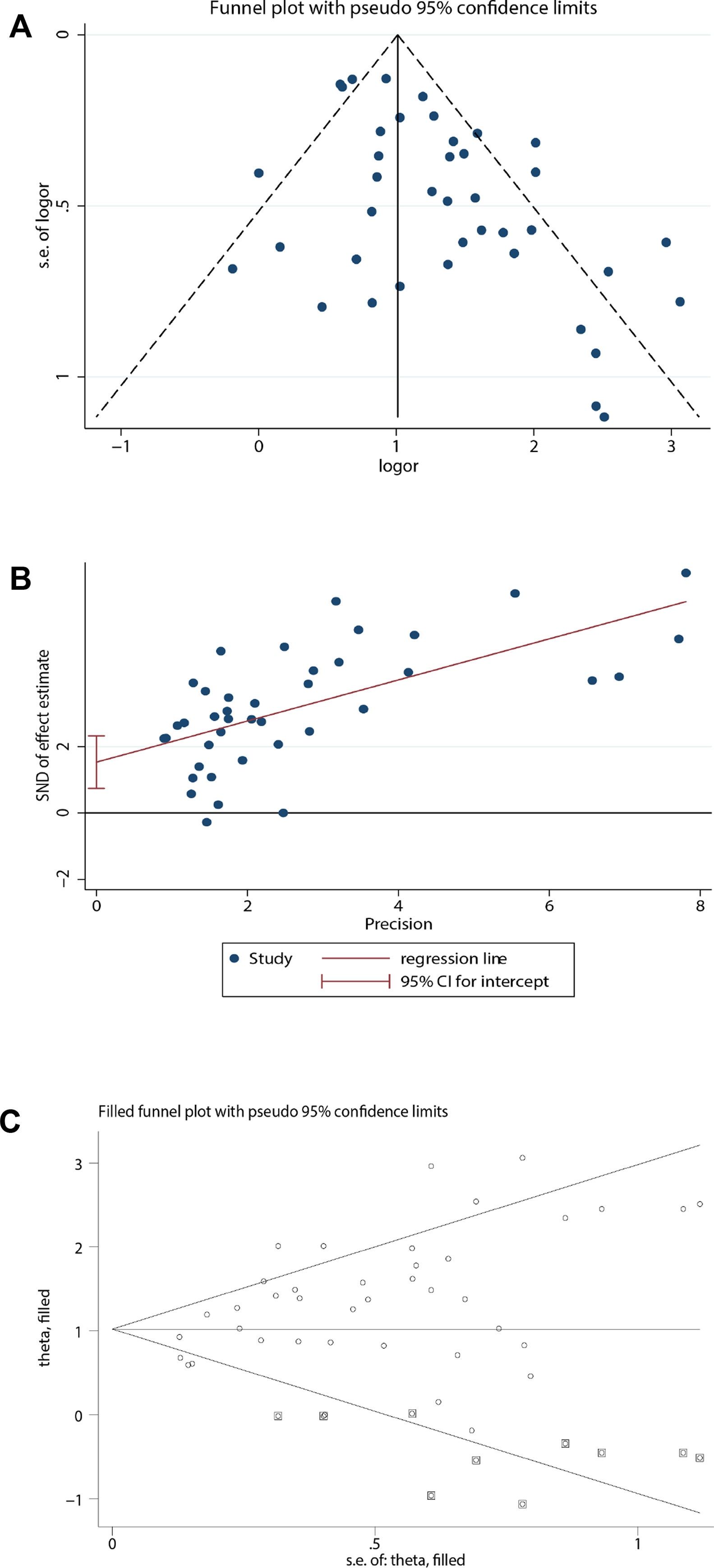
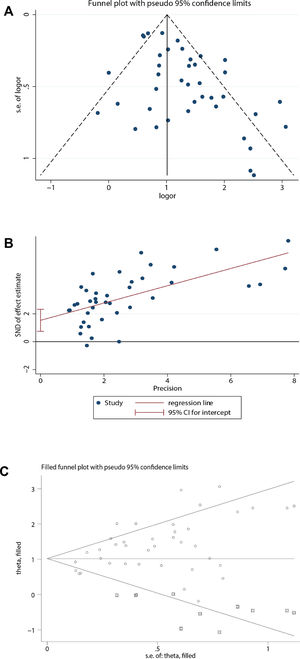
Publication biasBecause the funnel plot was asymmetric and the P-value of the Egger test <0.05, there was a publication bias in the relationship between coronary heart disease and the prognosis of COVID-19. Therefore, the trim and fill method was used to correct for funnel plot asymmetry caused by the publication bias. After adding the 10 missing hypothetical studies, the redrawn funnel plot was symmetrical and recalculated the pooled odds ratio showed that coronary heart disease was associated with poor prognosis of COVID-19 (OR=2.78, 95%CI [2.29, 3.38], P<0.001). (See Fig. 4).
Publication bias analysis. (A) For the relationship between coronary heart disease and prognosis of COVID-19, a funnel plot was used to qualitatively assess publication bias. (B) For the relationship between coronary heart disease and prognosis of COVID-19, the Egger test was used to quantitatively assess publication bias (P<0.001). (C) Trim and fill funnel plot was symmetrical after addition of 10 missing hypothetical studies.
We included a total of 40 studies in our meta-analysis, which showed that coronary heart disease was associated with poor prognosis of COVID-19, with statistically significant outcomes for mortality, disease progression, severe/critical COVID-19, and ICU admission. After correcting for publication bias, recalculation of pooled odds ratio showed that coronary heart disease was still associated with poor prognosis of COVID-19 (OR=2.78, 95%CI [2.29, 3.38], P<0.001).
At the same time, we used meta-regression to explore the source of heterogeneity. When study design, sample size, overall age, gender, heart failure, hypertension and quality score were analyzed, it found that hypertension (P=0.004) influenced the association between coronary heart disease and poor prognosis of COVID-19. Subgroup analysis showed that compared with the proportion of hypertension >30% (OR=2.85, 95%CI [2.33, 3.49]), the proportion of hypertension <30% (OR=4.78, 95%CI [3.50, 6.51]) had a higher risk of poor prognosis.
As the primary receptor for SARS-CoV-2 to enter target cells, angiotensin-converting enzyme 2 (ACE2) is found in lung cells, heart cells, renal epithelial cells, intestinal mucosal cells, immune cells, and cerebral neuronal cells, etc.3 SARS-CoV-2 can invade human cells through the interaction between spike protein and the extracellular domain of ACE2, and induce cytokine storm by down-regulating ACE2 on the infected cellular surface in various ways.47 However, the imbalance between ACE and ACE2 in lung and myocardial tissues, which is manifested as the increase of ACE activity and the decrease of ACE2 activity, can induce myocardial inflammation and acute respiratory distress syndrome.48,49 Chen50 found that the high expression of ACE2 in pericytes might be the target of SARS-CoV-2 invading cardiac cells, which could cause the dysfunction of capillary endothelial cells and induced the disorder of micro-circulation. The myocardial inflammation and microvascular dysfunction caused by SARS-CoV-2 will aggravate the imbalance between cardiac reserve and metabolic demand in patients with coronary heart disease, further promote the rupture of coronary plaques.49 This may be the reason why COVID-19 patients with coronary heart disease are more likely to have a poor prognosis.
The use of angiotensin receptor blocker (ARB) and angiotensin-converting enzyme inhibitor (ACEI) in animal experiments can reduce lung injury caused by SARS-COV infection. But on the other hand, the use of large doses of ACEI/ARB can up-regulate ACE2 expression in animal studies, thereby arising concern about whether ACEI/ARB can increase the risk of COVID-19.51 Feng et al.52 included a multicenter study and found that COVID-19 patients taking ACEI/ARB were more in the moderate group than severe and the critical group. Meng et al.53 included 42 COVID-19 patients with hypertension to evaluate the effect of ACEI/ARB and found ACEI/ARB could reduce inflammatory response by inhibiting the level of IL-6 in peripheral blood. Besides, Meng et al.53 also found ACEI/ARB could reduce the peak viral load and increased the count of CD3 and CD8 T cells in peripheral blood to avoid the consumption of peripheral T cells. This evidence supports that ACEI/ARB can improve the prognosis of COVID-19 patients with hypertension. Our meta-analysis showed a higher proportion of hypertension among COVID-19 patients with coronary heart disease seemed to have a better prognosis. The possible reason was the use of ACEI/ARB drugs in patients with hypertension. Unfortunately, due to the limitation of data, the association between COVID-19 patients with coronary heart disease and ACEI/ARB drugs was not analyzed. The influence of ACEI/ARB on the prognosis of COVID-19 patients still needs further study.
In conclusion, people with pre-existing coronary heart disease are more likely to have more severe COVID-19 disease, as compared to people without coronary heart disease. The association is affected by hypertension. Therefore, people with coronary heart disease should pay more attention to self-prevention to avoid infection with the virus, and doctors should also give more notice to COVID-19 patients with coronary heart disease to avoid the occurrence of adverse events.
LimitationsThe restrictions of this meta-analysis are as follows: 1. The factors of coronary heart disease included in this analysis were simply combined with the data without adjusting the confounding factors; 2. Due to the limitation of data, the association between COVID-19 patients with coronary heart disease and ACEI/ARB drugs was not analyzed.
ConclusionCoronary heart disease is a risk factor for poor prognosis in COVID-19 patients. The relationship was more powerful in studies with a lower proportion of hypertensive patients.
FundingThis work was supported by special projects for the guidance of the transformation of scientific and technological achievements in Shanxi Province (Project number: 201804D131045) and the transformation and cultivation projects of scientific and technological achievements in colleges and universities of Shanxi Province (Project number: 2020CG028).
Author contributionsGQ proposed and designed the study. CDL and WJZ contributed to literature research and data extraction. CDL performed statistical analysis. CDL and WJZ drafted the manuscript. SZL played a guiding role in this meta-analysis. GQ contributed to the revision of the manuscript and determined the final version.
Conflicts of interestNone declared.