To study Chlamydophila pneumoniae DNA (CP-DNA) in leukocytes measured by real-time polymerase chain reaction (PCR) in patients with type 2 diabetes mellitus (DM2) with different degrees of atherosclerosis, a cross-sectional protocol was performed.
Patients and methodsWe included 135 patients with DM2. Clinical, metabolic and inflammatory variables were measured. Previous clinical macrovascular disease was recorded and carotid ultrasound and real-time PCR for CP-DNA were performed.
ResultsMean age was 62 (7) years and mean diabetes duration 16 (9) years; 40.7% of patients presented clinical atherosclerosis, 32.5% subclinical atherosclerosis and 26.6% no evidence of atherosclerosis. Anthropometric data were homogeneous in the three groups. Patients with clinical atherosclerosis had greater carotid intima-media thickness compared to the other two groups. No CP-DNA was detected in any patient.
ConclusionsThe lack of detection of CP-DNA in blood leukocytes suggests that C. pneumoniae plays no active, systemic role in the pathogenesis of atherosclerosis in DM2 patients and is not a reliable marker of atherosclerosis in high-risk patients.
En un estudio transversal, se evaluó la presencia de ADN de Chlamydophila pneumoniae (ADN-CP) en leucocitos de sangre periférica mediante reacción en cadena de la polimerasa (PCR) en tiempo real en pacientes con diabetes mellitus tipo 2 (DM2) y diferentes grados de aterosclerosis carotídea.
Pacientes y métodoSe incluyeron 135 pacientes con DM2. Se determinaron variables clínicas, metabólicas e inflamatorias. Se registraron los antecedentes de enfermedad macrovascular clínica, se realizó ecografía carotídea y PCR en tiempo real para el ADN-CP.
ResultadosLa edad fue de 62 (7) años. La duración de la diabetes fue de 16 (9) años. El 40,7% de los pacientes presentaban aterosclerosis clínica, el 32,5% aterosclerosis subclínica y el 26,6% no evidencia de aterosclerosis. Todos los grupos fueron homogéneos en los datos antropométricos. Los pacientes con aterosclerosis clínica tenían mayor grosor de la íntima-media carotídea en comparación con los otros dos grupos. No se detectó ADN-CP en ninguno de los casos estudiados.
ConclusionesLa falta de detección de ADN-CP en leucocitos de sangre periférica sugiere que esta bacteria no parece tener un papel activo sistémico en la patogénesis de la aterosclerosis en pacientes con DM2 y no sería un marcador fiable de aterosclerosis en pacientes de alto riesgo.
Diabetes mellitus is associated with an increased risk of clinical macrovascular disease, including cardiovascular disease, peripheral macroangiopathy and stroke. It is unclear whether infectious agents such as Chlamydophila pneumoniae are involved in the atherosclerotic process. C. pneumoniae, a strict intracellular bacterium, has been proposed as a vector of atherosclerosis growth and progression using peripheral blood mononuclear cells as carriers.1,2 Studies in animals and humans have reported the presence of C. pneumoniae, but the prevalence varies widely.2 Serological detection of immunoglobulins against C. pneumoniae is unsuitable for the evaluation of chronic infection.3 Using polymerase chain reaction (PCR), both the absence and presence of C. pneumoniae-DNA in atherosclerotic plaques or blood leukocytes has been reported.4,5 However, most studies used nested PCR, yielding a high rate of false positive results.6 Recently, no C. pneumoniae-DNA was found using a probe-based real-time PCR (RT-PCR) in leukocytes from patients with atherosclerotic plaques7 or coronary artery disease.4 However, no diabetic patients were specifically included in these studies.
The objective of this study was to evaluate C. pneumoniae-DNA by high-sensitive RT-PCR in a cohort of patients with type 2 diabetes mellitus (DM2) with and without ultrasound-confirmed atherosclerosis.
MethodsConsecutive DM2 patients from two outpatient clinics were included. Exclusion criteria were: acute cardiovascular or peripheral vascular disease in the six months before inclusion, acute or chronic systemic infectious or inflammatory disease, past or current malignancy, and pregnancy. Clinical macrovascular disease was defined as any documented episode of myocardial infarction, angina, cerebrovascular disease or peripheral artery disease. The study was approved by local ethics committees according to the Declaration of Helsinki, and written informed consent was obtained from all participants. Blood samples were drawn in the morning after an overnight fast and biochemical variables were measured by routine clinical chemistry immediately after extraction.
C. pneumoniae-DNA determinationSamples were drawn in trisodium EDTA tubes (Becton Dickinson) for genotype studies. Genomic DNA was extracted from 200μL of whole blood by a silica gel column method (QIAamp DNA blood mini kit, Qiagen® GmbH, Hilden, Germany) according to the manufacturer's protocol. For C. pneumoniae-DNA detection, a RT-PCR protocol was used (LightMix® for the detection of C. pneumoniae, Tib Molbiol, Berlin, Germany) in a LightCycler® 2.0 thermocycler (Roche Applied Science, Basel, Switzerland). The protocol, primes and probes were previously validated with positive controls. A 140bp fragment of the C. pneumoniae genome was amplified with specific primers and detected with probes labelled with LC Red 640. The PCR product was identified by running a melting curve with a specific melting point (Tm) of 66.5°C in channel 640. An internal positive control was amplified with each sample and generated a PCR product of 278bp, and was detected with probes labelled with LC 750 dye and identified by running a melting curve with a specific Tm of 67-69°C in channel 750. A negative control (replacing the DNA template with water) was included in each run. The standards provided from 101 to 106 target equivalents per reaction of DNA and permitted absolute quantification of the unknown samples. Test sensitivity permitted the detection of 101 copies of C. pneumoniae DNA. PCR was performed in a reaction volume of 20 uL. After an initial denaturation at 95°C for 10min, amplification was performed using 55 cycles of denaturation (95°C for 0.5 sec.), annealing (62°C for 0.5 sec.) and extension (72°C for 0.5 sec.). The temperature transition rates were programmed at 20°C/sec. from denaturation to annealing, 10°C/sec. from annealing to extension and 20°C/sec. from extension to denaturation. After amplification was complete, a final melting curve was recorded by cooling to 40°C for 20 sec., and then heating slowly at 0.2°C/sec. up to 85°C. Fluorescence was measured continuously during the slow temperature rise to monitor the dissociation of the detection probes. PCR for C. pneumoniae was performed for replicate aliquots of each extracted sample in separate PCR runs.
Carotid ultrasoundUltrasonographic images were acquired using high-resolution B-mode ultrasound (Acuson® Sequoia C-256) as previously described.8 Carotid plaques were evaluated and intima-media thickness (IMT) was measured from the media-adventitia interface to the intima-lumen interface.
Patients were classified in three groups: clinical atherosclerosis (CA) included patients with carotid plaques and documented clinical macrovascular disease; subclinical atherosclerosis (SA) included patients with carotid plaques but no previous episode of clinical macrovascular disease, and patients with no carotid plaques and without a history of macrovascular disease were considered as diabetic controls (C).
Statistical analysisContinuous variables were expressed as mean (SD) or median (interquartile range) and categorical variables as frequencies and percentages. Differences were evaluated by the Student's t-test, Mann-Whitney U test or Chi-square-test. The one-tailed 95% confidence interval (95% CI) for binomial data was calculated by an exact method using the binomial distribution.
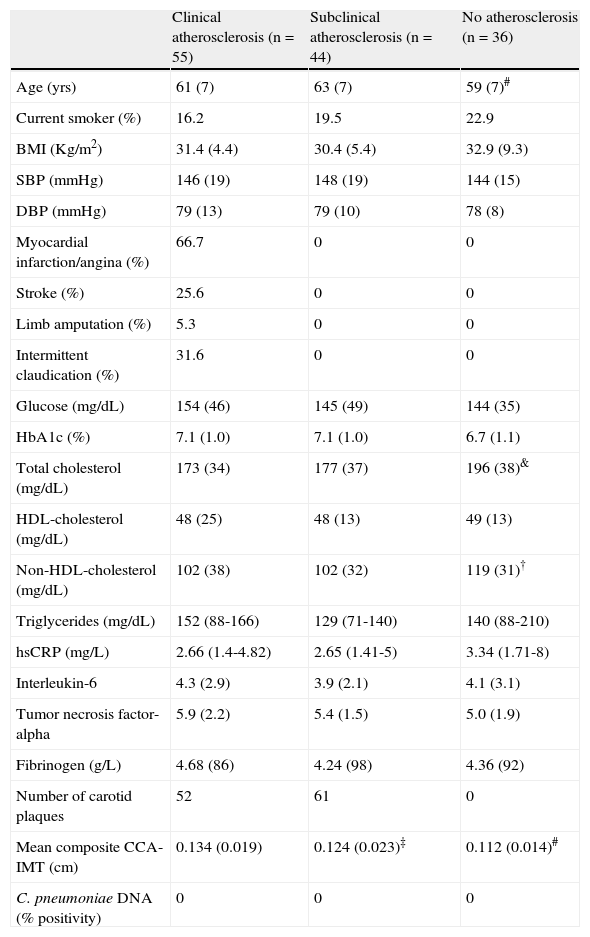
ResultsWe included 135 patients with DM2 (58% male, mean age 62 [7] years, mean diabetes duration 16 [9] years). Fifty-five patients were classified as CA, 44 as SA and 36 as C. As shown in table 1, the groups were homogeneous in clinical and biochemical data except for age (diabetic controls were younger than SA patients), and total cholesterol and non-HDL-cholesterol, which were significantly higher in controls than in the other two groups. Differences were found in hypercholesterolemia treatment (79% in CA patients, 61% in SA patients and 46% in controls), but were only significant between controls and CA patients (P=.003). The leukocyte count and inflammatory markers, such as TNF-alpha, IL-6 and hsCRP, did not differ between the three groups. Mean IMT was higher in CA but the number of carotid plaques was similar in CA and SA patients.
Clinical, analytical and carotid ultrasound characteristics of diabetic patients with symptomatic, asymptomatic or no atherosclerosis.
| Clinical atherosclerosis (n=55) | Subclinical atherosclerosis (n=44) | No atherosclerosis (n=36) | |
| Age (yrs) | 61 (7) | 63 (7) | 59 (7)# |
| Current smoker (%) | 16.2 | 19.5 | 22.9 |
| BMI (Kg/m2) | 31.4 (4.4) | 30.4 (5.4) | 32.9 (9.3) |
| SBP (mmHg) | 146 (19) | 148 (19) | 144 (15) |
| DBP (mmHg) | 79 (13) | 79 (10) | 78 (8) |
| Myocardial infarction/angina (%) | 66.7 | 0 | 0 |
| Stroke (%) | 25.6 | 0 | 0 |
| Limb amputation (%) | 5.3 | 0 | 0 |
| Intermittent claudication (%) | 31.6 | 0 | 0 |
| Glucose (mg/dL) | 154 (46) | 145 (49) | 144 (35) |
| HbA1c (%) | 7.1 (1.0) | 7.1 (1.0) | 6.7 (1.1) |
| Total cholesterol (mg/dL) | 173 (34) | 177 (37) | 196 (38)& |
| HDL-cholesterol (mg/dL) | 48 (25) | 48 (13) | 49 (13) |
| Non-HDL-cholesterol (mg/dL) | 102 (38) | 102 (32) | 119 (31)† |
| Triglycerides (mg/dL) | 152 (88-166) | 129 (71-140) | 140 (88-210) |
| hsCRP (mg/L) | 2.66 (1.4-4.82) | 2.65 (1.41-5) | 3.34 (1.71-8) |
| Interleukin-6 | 4.3 (2.9) | 3.9 (2.1) | 4.1 (3.1) |
| Tumor necrosis factor-alpha | 5.9 (2.2) | 5.4 (1.5) | 5.0 (1.9) |
| Fibrinogen (g/L) | 4.68 (86) | 4.24 (98) | 4.36 (92) |
| Number of carotid plaques | 52 | 61 | 0 |
| Mean composite CCA-IMT (cm) | 0.134 (0.019) | 0.124 (0.023)‡ | 0.112 (0.014)# |
| C. pneumoniae DNA (% positivity) | 0 | 0 | 0 |
Data are expressed as mean (SD) or median (interquartile range). CA: clinical atherosclerosis; SA: subclinical atherosclerosis.
#P=.01 with respect to SA. &P=.03 with respect to CA and SA. †P=.02 with respect to CA and SA. ‡P=.02 with respect to CA.
BMI: body mass index; CCA-IMT: common carotid artery-intima-media thickness; DBP: diastolic blood pressure; hsCRP: high sensitivity C-reactive protein; SBP: systolic blood pressure.
No C. pneumoniae DNA was detected in any patient (95% CI: 0-2.19%).
DiscussionThis study found no detectable C. pneumoniae DNA copies in peripheral blood leukocytes in type 2 diabetic patients. To our knowledge, no previous reports have combined the inclusion of a high-risk population such as diabetics, the use of a highly-sensitive PCR technology and the evaluation of carotid plaques by B-mode ultrasound.
The utility of serologic determination of C. pneumoniae as a predictor of future cardiovascular events has not been demonstrated.9 When traditional cardiovascular risk factors are included in the multivariate analysis, the possible association is only weak or even negative.1 The variability in the interpretation of results, the inability to discriminate between chronic, active or past infection and the high prevalence of C. pneumoniae in healthy people (75% in the elderly) makes C. pneumoniae serology unreliable at present.3 DNA detection by the most-sensitive PCR techniques has been used in both atherosclerotic arteries and peripheral leukocytes. The prevalence of DNA detection varied between 0 to 100% in arteries2 and 0 to 86% in blood leukocytes.4,7 In most of these studies, PCR was performed by methods that could amplify non-C. pneumoniae DNA: RT-PCR could minimize this risk.10 Our study confirms previous results in non-diabetic subjects7. In our opinion, the strict protocol used, with appropriate internal positive and negative controls, minimizes the possibility that the results obtained may be due to hidden methodological problems. Our results indicate that chronic active C. pneumoniae infection seems not to be common in DM2 patients, regardless of the atherosclerotic status, suggesting that C. pneumoniae may not play an active role in the atherosclerotic process and is not responsible for its systemic dissemination. Our results, together with those of other reports4,7, suggest that there may be a publication bias towards the positive implication of C. pneumoniae in atherosclerosis.
However, the lack of detection of C. pneumoniae in the circulation does not exclude past infection or its presence in atherosclerotic plaques. In histopathological studies, C. pneumoniae was detected in less than 46% of atheromatous arteries. Furthermore, detection of C. pneumoniae within arteriosclerotic lesions only demonstrates an association, but not a specific role, because the bacterium could infiltrate atherosclerotic plaques after they have begun to develop. It has been proposed that C. pneumoniae-DNA in mononuclear cells might identify currently-infected patients who are at higher risk for atherosclerosis.1 However, in our study, C. pneumoniae-DNA was not found in blood cells in DM2 patients, a group with generalized, severe arteriosclerosis.
There are limitations to our study, including those inherent to all cross-sectional and case-control studies. The statistical power of the study is limited; however, because the results were uniformly negative, this limitation is not significant.
In conclusion, no detectable C. pneumoniae DNA was found in peripheral blood leukocytes in type 2 diabetic patients with clinical, subclinical or no ultrasound-confirmed atherosclerosis, measured by highly-sensitive real-time PCR. This suggests that C. pneumoniae does not play an active, systemic role in the atherosclerotic process in diabetes and that the technology used is not a reliable marker for the development of atherosclerosis in high-risk patients such as diabetics.
Conflict of interestThe authors declare no conflicts of interest.
Chlamydophila pneumoniae detection kits were kindly supplied by Roche Diagnostics.