Modulation of the immune system to prevent lung injury is being widely used against the new coronavirus disease (COVID-19). The primary endpoint was mortality at 7 days after tocilizumab administration. Secondary endpoints were admission to the intensive care unit, development of ARDS and respiratory insufficiency among others.
MethodsWe report the preliminary results from the Vall d’Hebron cohort study at Vall d’Hebron University Hospital, in Barcelona (Spain), including all consecutive patients who had a confirmed SARS-CoV-2 infection and who were treated with tocilizumab until March 25th.
Results82 patients with COVID-19 received at least one dose of tocilizumab. The mean (± SD) age was 59.1 (19.8) years, 63% were male, 22% were of non-Spanish ancestry, and the median (IQR) age-adjusted Charlson index at baseline was 3 (1–4) points. Respiratory failure and ARDS developed in 62 (75.6%) and 45 (54.9%) patients, respectively. Median time from symptom onset to ARDS development was 8 (5–11) days. Mortality at 7 days was 26.8%. Hazard ratio for mortality was 3.3; 95% CI, 1.3–8.5 (age-adjusted hazard ratio for mortality 2.1; 95% CI, 0.8–5.8) if tocilizumab was administered after the onset of ARDS.
ConclusionEarly administration of tocilizumab in patients needing oxygen supplementation may be critical to patient recovery. Our preliminary data could inform bedside decisions until more data regarding the precise timing in of initiation of the treatment with tocilizumab.
Los tratamientos inmunomoduladores para la prevención del daño pulmonar están siendo ampliamente estudiados contra la COVID-19. El objetivo primario es evaluar la mortalidad a los 7 días después de la administración de tocilizumab. El objetivo secundario es el ingreso en UCI, el desarrollo de distrés respiratorio agudo e insuficiencia respiratoria aguda entre otros.
MétodosInformamos sobre los resultados preliminares de la cohorte del Hospital Universitario Vall d’Hebron en Barcelona (España), que incluye todos los pacientes consecutivos con infección confirmada por SARS-CoV-2 y que recibieron tratamiento con tocilizumab hasta el 25 de marzo 2020.
ResultadosOchenta y dos pacientes con COVID-19 recibieron al menos una dosis de tocilizumab. La edad media (±DE) fue de 59,1 (±19,8) años, el 63% eran hombres, 22% correspondía a paciente nacidos fuera de España, y la mediana (RIC) del índice de Charlson ajustado por edad en el momento basal fue de 3 (1-4) puntos. Sesenta y dos pacientes (75,6%) y 45 pacientes (54,9%) desarrollaron insuficiencia respiratoria y distrés respiratorio agudo respectivamente. La mediana de tiempo desde el inicio de los síntomas hasta el desarrollo de ditrés fue de 8 días (5-11). La mortalidad a los 7 días fue del 26,8% La hazard ratio de mortalidad fue del 3,3; IC 95% 1,3-8,5 (la hazard ratio de mortalidad ajustada por edad fue de 2,1; IC 95% 0,8-5,8) si el tocilizumab se administraba después del inicio del distrés respiratorio.
ConclusiónLa administración precoz de tocilizumab en pacientes con suplementos de oxígeno podría ser crítica para la recuperación de los pacientes. Nuestros datos podrían ayudar a tomar decisiones clínicas hasta que se disponga de más información sobre el momento adecuado para iniciar el tratamiento con tocilizumab.
Coronavirus disease 2019 (COVID-19) is a novel illness caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). It was first reported in December 2019 in Hubei province, China.1 Since then, SARS-CoV-2 has rapidly spread worldwide.
According to the World Health Organization (WHO), as of April 13th, 2021, there have been 135.057.587 laboratory-confirmed cases and 2.919.932 deaths.2 The crude case fatality rate, estimated to be between 2.3% and 3.3%, is highly dependent on age and underlying conditions.3,4 Death is mainly due to respiratory failure caused by an acute respiratory distress syndrome (ARDS). As the pathophisiology behind lung injury is progressively elucidated, several therapies have been proposed on the basis of pre-clinical studies and the previous experience with the severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East Respiratory Syndrome Coronavirus (MERS-CoV).5,6 Many of them are being used off-label in a desperate attempt to improve patient outcomes, including antiviral therapies, coagulation-modifying drugs and immune-modulating therapies.7–11
In COVID-19, an excessive immune response inducing disproportionate release of cytokines and hyperinflammation has been proposed as a cause for the lung damage, mimicking a secondary haemophagocytic lymphohistiocytosis.12 Host-directed therapies have immune-modulating properties with higher precision than steroids and other immune-modulating therapies.13 Tocilizumab is a humanized monoclonal antibody that inhibits interleukin-6 (IL-6) receptor with a well-known safety profile and is approved for the treatment of rheumatoid arthritis and, since 2017, the treatment of chimeric antigen receptor (CAR) T cell-induced severe or life-threatening cytokine release syndrome (CRS).14,15 It has been used with promising results in clinical trials.16,17
However, a proper characterization of the subset of patients who will benefit most from host-directed therapy and defining the precise timing for host-directed therapies administration has not yet been performed and is critical to allocate limited drug stocks and reduce COVID-19 associated mortality. We aim to describe a cohort of SARS-CoV-2 infected patients treated with tocilizumab and define risk factors associated with 7-days mortality.
MethodsStudy setting and populationThe Vall d’Hebron COVID-19 Cohort Study includes all consecutive adult patients (≥18 years old) treated for COVID-19 at Vall d’Hebron University Hospital, a 1100-bed public tertiary care hospital in Barcelona, Spain. For this study we selected the subgroup of patients with laboratory-confirmed COVID-19 and radiologically confirmed pneumonia who received at least one dose of tocilizumab. Identification and inclusion of patients receiving tocilizumab was performed from the Pharmacy Department registry.
Standard of care and tocilizumab administration criteriaAt admission, all patients were initially evaluated with chest radiography and blood tests including complete cell count, coagulation studies, biochemistry and inflammatory parameters. Treatment with lopinavir/ritonavir, azithromycin and hydroxychloroquine was initiated according to Vall d’Hebron University Hospital protocol. Tocilizumab was considered as additional treatment in patients with the following criteria: (1) respiratory failure defined as a ratio of arterial oxygen tension to fraction of inspired oxygen (PaO2/FiO2 ratio) of <300, a ratio of arterial oxygen saturation measured by pulse oximetry to fraction of inspired oxygen (SpO2/FiO2 ratio) of <315 or pO2<60mmHg or oxygen saturation measured by pulse oximetry less than 90% when breathing room air or rapidly progressive clinical worsening according to treating physician and (2) interleukin-6 (IL-6) levels>40pg/mL (reference 0–4.3pg/mL) or a D-dimer levels>1500ng/mL (reference 0–243pg/mL). Two dosing regimens based on weight were considered for tocilizumab. Patients over 75kg received 600mg, otherwise 400mg was the preferred dose. A second dose was considered in patients with a poor early response. Patients with liver enzymes (aspartate aminotransferase and alanine aminotransferase) 5 times over the upper limit of normality or concomitant severe bacterial infection were not eligible for tocilizumab treatment.
Data sourcesData were collected retrospectively from the medical charts of tpatients from the 13th of March 2020 to the 18th of March 2020, when the protocol was submitted to the institutional review board, and prospectively thereafter. Inclusion and follow-up are still ongoing. The cut-off data for inclusion in this sub-study was the 25th of March 2020. All patients included were followed for at least 7 days. The institutional review board provided ethical clearance (local review board code number: PR(AG)183/2020). Patients were asked for an oral consent. The institutional review board granted an informed consent waiver if patients were unable to give oral consent. Written consent was waived because of the crisis context and concerns about safety when introducing a physical support for the consent in the isolation areas. The institutional review board granted a consent waiver for the patients included between 13th and 18th of March.
A Laboratory-confirmed case was defined as a patient with a real-time reverse-transcriptase-polymerase-chain-reaction (RT-PCR) SARS-CoV-2 positive result in any respiratory sample (nasopharyngeal swab, sputum, bronchoalveolar lavage or aspirate, tracheal aspirate).
We collected sociodemographic characteristics, past medical history, Charlson comorbidity score, concomitant medication, current therapy, adverse drug events, blood test results, imaging studies, microbiological tests other than SARS-CoV-2 RT-PCR on respiratory samples when available, and supportive measures needed. Vital signs, symptoms and physical examination were evaluated on admission, at 48h and weekly during hospital admission. Laboratory, microbiology and imaging studies were performed on admission and thereafter according to the clinical care needs of each patient. Laboratory assessments consisted of a complete blood count, coagulation testing including D-dimer measurement, liver and renal tests, electrolyte profile, and inflammatory profile including C-reactive protein, fibrinogen, ferritin and IL-6. All radiographs were reviewed by the investigators and computed tomography (CT) scans were recorded according to the radiology department reports. The COVID-19 severity was measured with the CURB-65 scale for community acquired pneumonia and other scales.18,19 Data was recorded in the Research Electronic Data Capture software (REDCap, Vanderbilt University).
Laboratory confirmationFrom the onset of the outbreak until 15th of March the microbiological diagnosis was based on a homebrew RT-PCR assay targeting two viral targets (N1 and N2) in the viral nucleocapsid (N) gene and one in the envelope (E) gene of SARS-CoV-2, as well as the human RNase P (RP) gene as an internal control of the whole process, according to the CDC and ECDC Real-Time RT-PCR Diagnostic Panels with minor modifications.20 Since March 15th, commercial Allplex™ 2019-nCoV multiplex RT-PCR (Seegene, South Korea) were used for the detection of three viral targets (E; N; and, RNA-dependent RNA polymerase, RdRp) and an internal control. First SARS-CoV-2 laboratory-confirmations were confirmed by RdRp sequencing.21,22 Total nucleic acids (DNA/RNA) were extracted from respiratory specimens using NucliSENS easyMAG (BioMerieux, France) and STARMag Universal Cartridge Kit (Seegene, South Korea) according to the manufacturer's instructions. All microbiological procedures were carried out in the laboratory under Biosafety Level 2 conditions.
Study outcomesThe primary simple endpoint was defined as death at 7 days after first dose of tocilizumab. Secondary outcomes were admission to Intensive Care Unit (ICU), acute Respiratory Distress Syndrome (ARDS) and respiratory insufficiency. We also assessed acute myocardial infarction, septic shock, acute kidney injury and secondary infections. Berlin criteria for the ARDS were adapted, as many of the patients did not have an available arterial O2 pressure due to the overwhelming volume of admitted patients that precluded us from performing arterial blood samples on all patients. Instead, we used oxygen saturation by pulse oximetry and its correlation to the inspired fraction of oxygen (SpO2/FiO2 ratio<315).23,24 One patient died a few hours after receiving tocilizumab and was excluded from the primary endpoint analysis.
Statistical analysisContinuous variables were expressed as mean and standard deviation or medians and interquartile range, as appropriate. Categorical variables were summarized as absolute number and percentages. Comparisons among groups was performed with Chi squared test and Fisher's test for categorical variables; and ANOVA, Student's T test and Mann–Whitney U test for continuous variables. Box plots and bar plots are also provided for some associations. Mortality in the cohort was described with the use of Kaplan–Meier analysis. Tests were considered significant when the two-tailed p-value was<0.05. We did not correct for multiple comparisons; hence, the widths of the confidence intervals should not be interpreted as definitive for the associations with the outcomes. Association between time to tocilizumab administration and mortality were assessed by means of Cox proportional hazards regression. Missing urea and bilirubin levels on admission were assumed normal for CURB-65 and SOFA calculation; no other imputation was made for missing data. Analysis was performed with Stata 15.1 software (StataCorp).
Study oversightThe study was designed and conducted by the investigators from the Vall d’Hebron COVID-19 Cohort Study. No specific funding was provided to conduct the study. Data were collected, debugged, analyzed and interpreted by the authors. All the authors reviewed the manuscript and vouch for the accuracy and completeness of the data and for the adherence of the study to the protocol.
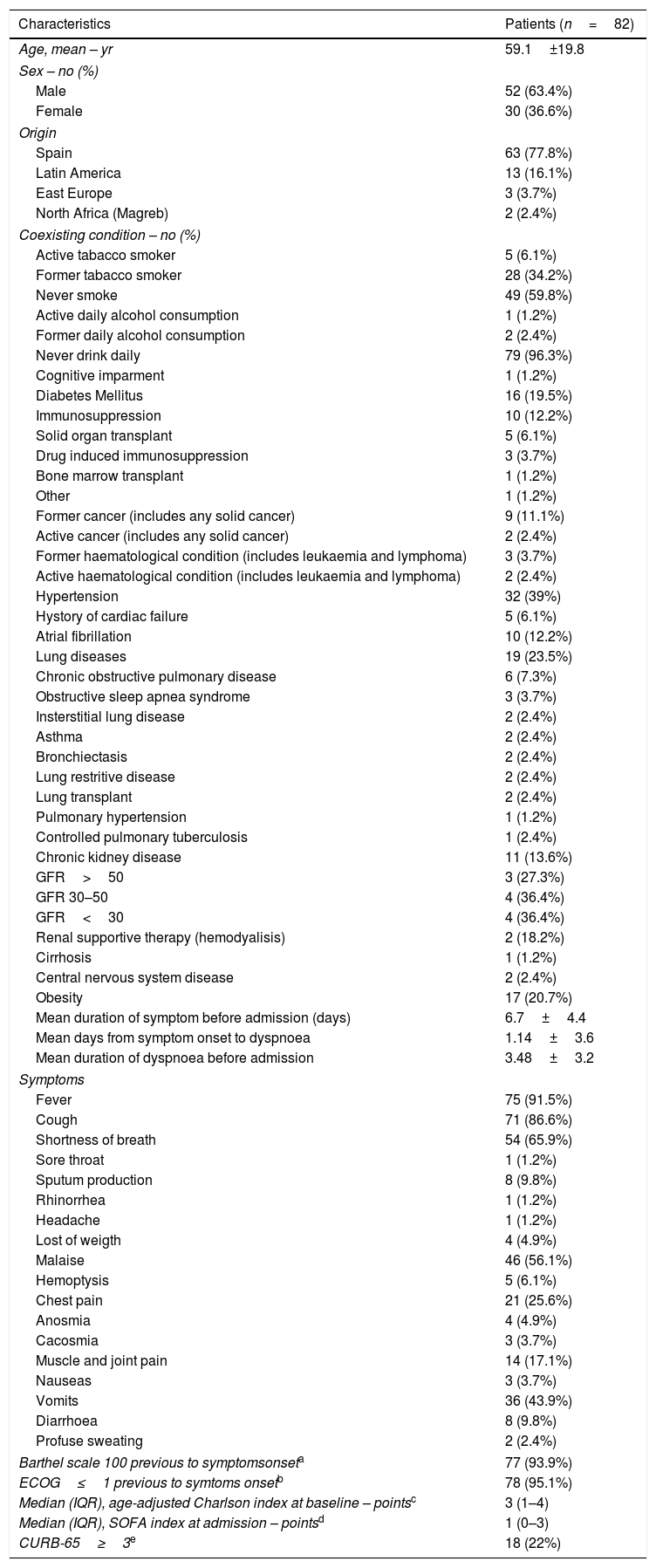
ResultsDemographic and clinical characteristicsSince the onset of the COVID-19 outbreak until March 25th, 3242 respiratory-derived samples have been requested from our institution for COVID-19 diagnosis. Samples were requested from the emergency room and hospital wards, as well as from the health care worker surveillance strategy plan. From them, 941 were positive (29%). During this period, 82 SARS-CoV-2 infected patients received at least one dose of tocilizumab. The mean (±SD) age was 59.1 (±19.8) years. Fifty-two patients were male (63.5%). Eighteen (21.9%) patients were born abroad, 13 (16.1%) in Latin America, 3 (3.7%) in Eastern Europe and 2 (2.4%) in North Africa. The mean (±SD) duration of symptoms before hospital admission was 6.7 (±4.4) days. Fever and cough were the main symptoms on admission, occurring in 75 (91.5%) and 71 (86.6%) cases respectively.
Thirty-three (40.3%) patients were former or active tobacco smokers. Coexisting conditions were as follows: 32 (39.0%) had hypertension, 19 (23.5%) had lung diseases (2 (2.4%) asthma, 6 (7.3%) chronic obstructive pulmonary disease among others), 17 (20.7%) had obesity, 16 (19.5%) had diabetes mellitus, 11 (13.6%) had chronic kidney disease, 5 (6.1%) a history of cardiac failure, 1 (1.2%) had cirrhosis. Ten (12.5%) patients were immunosuppressed because of different conditions. Seventy-seven (95.1%) patients had a Barthel scale index of 100 points previous to hospital admission. Table 1 shows demographic and clinical characteristics at baseline.
Demographic and clinical characteristics of the patients at baseline.f
| Characteristics | Patients (n=82) |
|---|---|
| Age, mean – yr | 59.1±19.8 |
| Sex – no (%) | |
| Male | 52 (63.4%) |
| Female | 30 (36.6%) |
| Origin | |
| Spain | 63 (77.8%) |
| Latin America | 13 (16.1%) |
| East Europe | 3 (3.7%) |
| North Africa (Magreb) | 2 (2.4%) |
| Coexisting condition – no (%) | |
| Active tabacco smoker | 5 (6.1%) |
| Former tabacco smoker | 28 (34.2%) |
| Never smoke | 49 (59.8%) |
| Active daily alcohol consumption | 1 (1.2%) |
| Former daily alcohol consumption | 2 (2.4%) |
| Never drink daily | 79 (96.3%) |
| Cognitive imparment | 1 (1.2%) |
| Diabetes Mellitus | 16 (19.5%) |
| Immunosuppression | 10 (12.2%) |
| Solid organ transplant | 5 (6.1%) |
| Drug induced immunosuppression | 3 (3.7%) |
| Bone marrow transplant | 1 (1.2%) |
| Other | 1 (1.2%) |
| Former cancer (includes any solid cancer) | 9 (11.1%) |
| Active cancer (includes any solid cancer) | 2 (2.4%) |
| Former haematological condition (includes leukaemia and lymphoma) | 3 (3.7%) |
| Active haematological condition (includes leukaemia and lymphoma) | 2 (2.4%) |
| Hypertension | 32 (39%) |
| Hystory of cardiac failure | 5 (6.1%) |
| Atrial fibrillation | 10 (12.2%) |
| Lung diseases | 19 (23.5%) |
| Chronic obstructive pulmonary disease | 6 (7.3%) |
| Obstructive sleep apnea syndrome | 3 (3.7%) |
| Insterstitial lung disease | 2 (2.4%) |
| Asthma | 2 (2.4%) |
| Bronchiectasis | 2 (2.4%) |
| Lung restritive disease | 2 (2.4%) |
| Lung transplant | 2 (2.4%) |
| Pulmonary hypertension | 1 (1.2%) |
| Controlled pulmonary tuberculosis | 1 (2.4%) |
| Chronic kidney disease | 11 (13.6%) |
| GFR>50 | 3 (27.3%) |
| GFR 30–50 | 4 (36.4%) |
| GFR<30 | 4 (36.4%) |
| Renal supportive therapy (hemodyalisis) | 2 (18.2%) |
| Cirrhosis | 1 (1.2%) |
| Central nervous system disease | 2 (2.4%) |
| Obesity | 17 (20.7%) |
| Mean duration of symptom before admission (days) | 6.7±4.4 |
| Mean days from symptom onset to dyspnoea | 1.14±3.6 |
| Mean duration of dyspnoea before admission | 3.48±3.2 |
| Symptoms | |
| Fever | 75 (91.5%) |
| Cough | 71 (86.6%) |
| Shortness of breath | 54 (65.9%) |
| Sore throat | 1 (1.2%) |
| Sputum production | 8 (9.8%) |
| Rhinorrhea | 1 (1.2%) |
| Headache | 1 (1.2%) |
| Lost of weigth | 4 (4.9%) |
| Malaise | 46 (56.1%) |
| Hemoptysis | 5 (6.1%) |
| Chest pain | 21 (25.6%) |
| Anosmia | 4 (4.9%) |
| Cacosmia | 3 (3.7%) |
| Muscle and joint pain | 14 (17.1%) |
| Nauseas | 3 (3.7%) |
| Vomits | 36 (43.9%) |
| Diarrhoea | 8 (9.8%) |
| Profuse sweating | 2 (2.4%) |
| Barthel scale 100 previous to symptomsonseta | 77 (93.9%) |
| ECOG≤1 previous to symtoms onsetb | 78 (95.1%) |
| Median (IQR), age-adjusted Charlson index at baseline – pointsc | 3 (1–4) |
| Median (IQR), SOFA index at admission – pointsd | 1 (0–3) |
| CURB-65≥3e | 18 (22%) |
Barthel index total scores range from 0 to 100, with higher scores indicating a better performance of 10 basic daily self-care activities.
The Eastern Cooperative Oncolgy Group (ECOG) performance scale range from 0 (fully active) to 4 (completely disabled).
The Charlson risk index score ranges from 0 to 37 with higher scores indicating a higher risk of death.
Sequential Organ Failure Assessment (SOFA) score ranges from 0 to 24 with higher ranges indicating a higher risk or morbidity; individuals with a score of 15 or more have a mortality rate of 90%. Its calculation is missing in two patients.
Community acquired pneumonia severity index assessing Confusion, Urea, Respiratory rate, Blood pressure and age over 65 years (CURB-65) ranges from 0 to 5 depending on the number of risk factors present in the same patient.
Plus-minus values are means (±SD). Rounding has been applied to percentages. Total may no be 100 because of rounding.
GFR: glomerular filtration rate.
One patient had insterstitial lung disease and pulmonary hypertension and another patient had obtructive sleep apnea syndrome and lung restrictive disease.
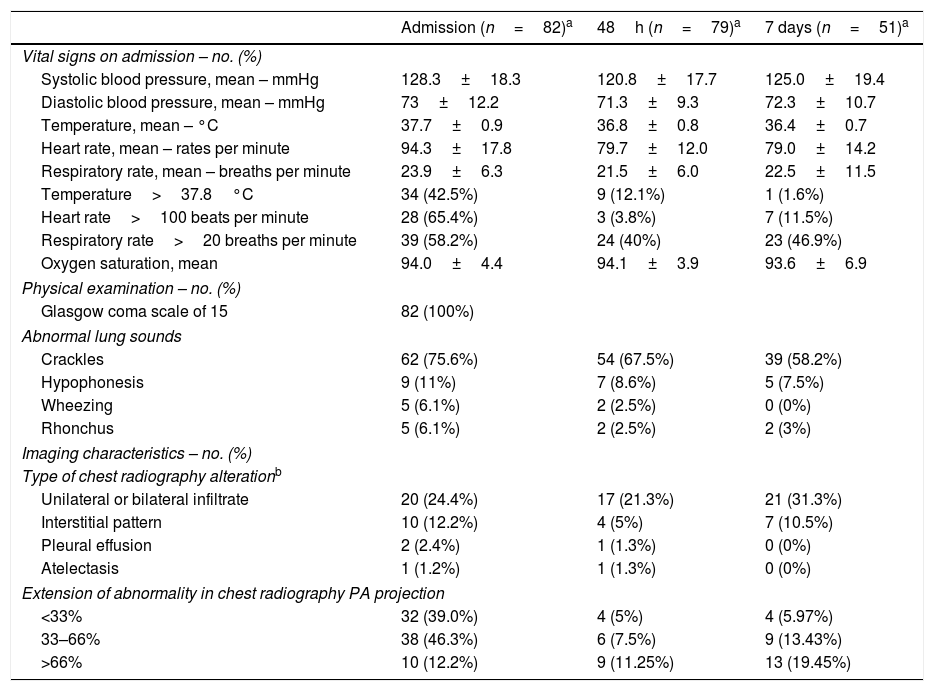
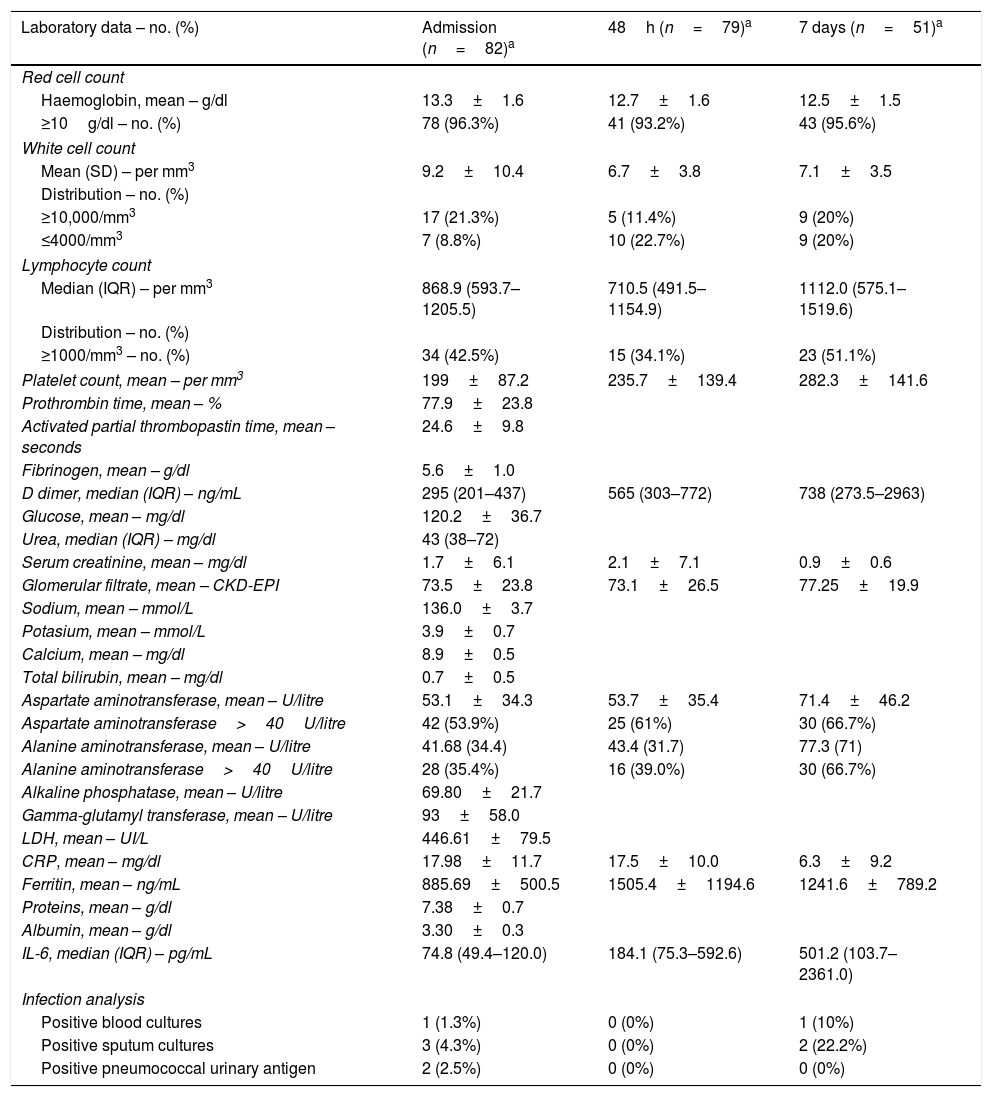
On admission, mean (±SD) white cell count was 9.2 (10.4) with 17 (21.3%) patients having more than 10,000 per cubic millilitre white cells. Lymphocytopenia (<1000cells per cubic millilitre) was present in 46 (57.5%) patients. Interleukin-6 median (IQR) plasma level on admission was 74.8 (49.4–120.0) ng/mL. Liver enzymes were below five times the upper normal value in all patients. Pneumonia was radiologically proven in all patients on admission or during follow up. Tables 2 and 3 describe laboratory and radiologic findings on admission and during follow up.
Status on admission and follow up.c
| Admission (n=82)a | 48h (n=79)a | 7 days (n=51)a | |
|---|---|---|---|
| Vital signs on admission – no. (%) | |||
| Systolic blood pressure, mean – mmHg | 128.3±18.3 | 120.8±17.7 | 125.0±19.4 |
| Diastolic blood pressure, mean – mmHg | 73±12.2 | 71.3±9.3 | 72.3±10.7 |
| Temperature, mean – °C | 37.7±0.9 | 36.8±0.8 | 36.4±0.7 |
| Heart rate, mean – rates per minute | 94.3±17.8 | 79.7±12.0 | 79.0±14.2 |
| Respiratory rate, mean – breaths per minute | 23.9±6.3 | 21.5±6.0 | 22.5±11.5 |
| Temperature>37.8°C | 34 (42.5%) | 9 (12.1%) | 1 (1.6%) |
| Heart rate>100 beats per minute | 28 (65.4%) | 3 (3.8%) | 7 (11.5%) |
| Respiratory rate>20 breaths per minute | 39 (58.2%) | 24 (40%) | 23 (46.9%) |
| Oxygen saturation, mean | 94.0±4.4 | 94.1±3.9 | 93.6±6.9 |
| Physical examination – no. (%) | |||
| Glasgow coma scale of 15 | 82 (100%) | ||
| Abnormal lung sounds | |||
| Crackles | 62 (75.6%) | 54 (67.5%) | 39 (58.2%) |
| Hypophonesis | 9 (11%) | 7 (8.6%) | 5 (7.5%) |
| Wheezing | 5 (6.1%) | 2 (2.5%) | 0 (0%) |
| Rhonchus | 5 (6.1%) | 2 (2.5%) | 2 (3%) |
| Imaging characteristics – no. (%) | |||
| Type of chest radiography alterationb | |||
| Unilateral or bilateral infiltrate | 20 (24.4%) | 17 (21.3%) | 21 (31.3%) |
| Interstitial pattern | 10 (12.2%) | 4 (5%) | 7 (10.5%) |
| Pleural effusion | 2 (2.4%) | 1 (1.3%) | 0 (0%) |
| Atelectasis | 1 (1.2%) | 1 (1.3%) | 0 (0%) |
| Extension of abnormality in chest radiography PA projection | |||
| <33% | 32 (39.0%) | 4 (5%) | 4 (5.97%) |
| 33–66% | 38 (46.3%) | 6 (7.5%) | 9 (13.43%) |
| >66% | 10 (12.2%) | 9 (11.25%) | 13 (19.45%) |
Laboratory data at admission and follow up.b
| Laboratory data – no. (%) | Admission (n=82)a | 48h (n=79)a | 7 days (n=51)a |
|---|---|---|---|
| Red cell count | |||
| Haemoglobin, mean – g/dl | 13.3±1.6 | 12.7±1.6 | 12.5±1.5 |
| ≥10g/dl – no. (%) | 78 (96.3%) | 41 (93.2%) | 43 (95.6%) |
| White cell count | |||
| Mean (SD) – per mm3 | 9.2±10.4 | 6.7±3.8 | 7.1±3.5 |
| Distribution – no. (%) | |||
| ≥10,000/mm3 | 17 (21.3%) | 5 (11.4%) | 9 (20%) |
| ≤4000/mm3 | 7 (8.8%) | 10 (22.7%) | 9 (20%) |
| Lymphocyte count | |||
| Median (IQR) – per mm3 | 868.9 (593.7–1205.5) | 710.5 (491.5–1154.9) | 1112.0 (575.1–1519.6) |
| Distribution – no. (%) | |||
| ≥1000/mm3 – no. (%) | 34 (42.5%) | 15 (34.1%) | 23 (51.1%) |
| Platelet count, mean – per mm3 | 199±87.2 | 235.7±139.4 | 282.3±141.6 |
| Prothrombin time, mean – % | 77.9±23.8 | ||
| Activated partial thrombopastin time, mean – seconds | 24.6±9.8 | ||
| Fibrinogen, mean – g/dl | 5.6±1.0 | ||
| D dimer, median (IQR) – ng/mL | 295 (201–437) | 565 (303–772) | 738 (273.5–2963) |
| Glucose, mean – mg/dl | 120.2±36.7 | ||
| Urea, median (IQR) – mg/dl | 43 (38–72) | ||
| Serum creatinine, mean – mg/dl | 1.7±6.1 | 2.1±7.1 | 0.9±0.6 |
| Glomerular filtrate, mean – CKD-EPI | 73.5±23.8 | 73.1±26.5 | 77.25±19.9 |
| Sodium, mean – mmol/L | 136.0±3.7 | ||
| Potasium, mean – mmol/L | 3.9±0.7 | ||
| Calcium, mean – mg/dl | 8.9±0.5 | ||
| Total bilirubin, mean – mg/dl | 0.7±0.5 | ||
| Aspartate aminotransferase, mean – U/litre | 53.1±34.3 | 53.7±35.4 | 71.4±46.2 |
| Aspartate aminotransferase>40U/litre | 42 (53.9%) | 25 (61%) | 30 (66.7%) |
| Alanine aminotransferase, mean – U/litre | 41.68 (34.4) | 43.4 (31.7) | 77.3 (71) |
| Alanine aminotransferase>40U/litre | 28 (35.4%) | 16 (39.0%) | 30 (66.7%) |
| Alkaline phosphatase, mean – U/litre | 69.80±21.7 | ||
| Gamma-glutamyl transferase, mean – U/litre | 93±58.0 | ||
| LDH, mean – UI/L | 446.61±79.5 | ||
| CRP, mean – mg/dl | 17.98±11.7 | 17.5±10.0 | 6.3±9.2 |
| Ferritin, mean – ng/mL | 885.69±500.5 | 1505.4±1194.6 | 1241.6±789.2 |
| Proteins, mean – g/dl | 7.38±0.7 | ||
| Albumin, mean – g/dl | 3.30±0.3 | ||
| IL-6, median (IQR) – pg/mL | 74.8 (49.4–120.0) | 184.1 (75.3–592.6) | 501.2 (103.7–2361.0) |
| Infection analysis | |||
| Positive blood cultures | 1 (1.3%) | 0 (0%) | 1 (10%) |
| Positive sputum cultures | 3 (4.3%) | 0 (0%) | 2 (22.2%) |
| Positive pneumococcal urinary antigen | 2 (2.5%) | 0 (0%) | 0 (0%) |
All included patients had a positive RT-PCR for SARS-CoV-2 in a respiratory-derived sample. On admission, 2 patients out of 56 had a positive pneumococcal urinary antigen result. Sputum samples from 13 patients were sent on admission, bacterial growth was demonstrated in 3 samples, two with Haemophilus influenzae that were considered clinically significant and one was deemed contamination with oral bacteria. During the first 7 days follow up, 2 more positive results were retrieved: one Extended-Spectrum Beta-Lactamase (ESBL)-producing Escherichia coli (considered clinically significant) and one Staphylococcus epidermidis (considered non-clinically significant). Blood cultures from 65 patients were sent, and one positive bacterial growth (coagulase-negative Staphylococcus) was observed, although considered a contamination. Detailed microbiologic data are shown in Table 3.
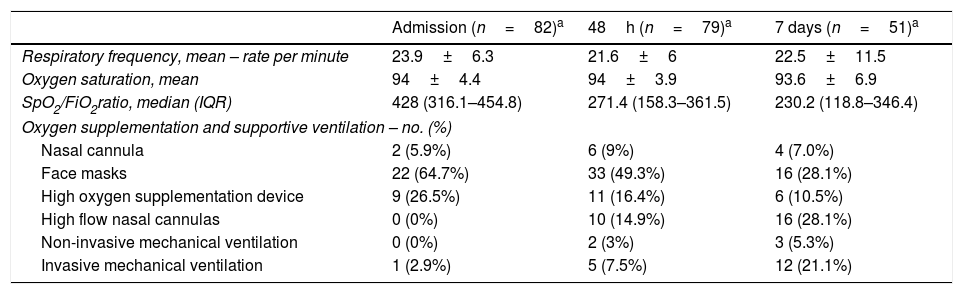
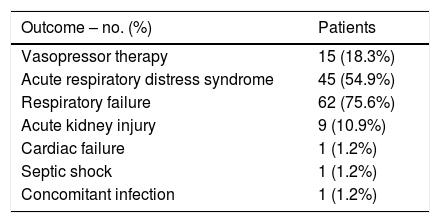
Oxygen supplementation and secondary outcomesTable 4 shows oxygen saturation, oxygen supplementation and ventilation support. On admission, mean FiO2 oxygen supplementation was 0.36 (±0.26) and mean oxygen saturation was 94% (±4.39). Regarding oxygen supplementation devices on admission, 34 (41.5%) patients were on oxygen supplementation: two (5.8%) patients were on nasal cannulas, 22 (64.7%) were using face masks, 9 (26.5%) patients were using high oxygen supplementation devices and 1 (2.9%) patient required endotracheal intubation with mechanical ventilation. Over time, SpO2/FiO2 ratio deteriorated from a median (IQR) of 428 (316.1–454.8) on admission, 271.4 (158.3–361.5) at 48h and 230.2 (118.8–346.4) at 7 days follow up. Fifty-five (69.6%) patients required intensive oxygen therapy, including high flow oxygen delivery systems, high flow nasal cannula, non-invasive mechanical ventilation or invasive ventilation during the study period. Median (IQR) days on high flow oxygen delivery systems, high flow nasal cannula, non-invasive mechanical ventilation or invasive ventilation before progression to other intensive oxygen therapy, outcome attainment or data censoring were 2.0 (1.0–3.0), 4.0 (2.0–6.0), 3.0 (2.0–4.0) and 9.0 (9.0–9.0) respectively. The median (IQR) days from admission to first intensive oxygen therapy was 2.0 (1.0–4.5). Only one (1.2%) patient required vasopressor therapy due to hypotension. No patient required renal replacement therapy. Respiratory failure and ARDS developed in 62 (75.6%) and 45 (54.9%) patients, respectively. Median (IQR) days from symptoms to respiratory failure and ARDS were 8 (6.0–11.0) and 8 (5.0–11.0) respectively. Median (IQR) days from admission to respiratory failure and ARDS were 1 (0.0–3.0) and 2 (1.0–4.0) respectively. Secondary outcomes can be found in Table 5.
Oxygen supplementation and supportive ventilation on admission and follow up.b
| Admission (n=82)a | 48h (n=79)a | 7 days (n=51)a | |
|---|---|---|---|
| Respiratory frequency, mean – rate per minute | 23.9±6.3 | 21.6±6 | 22.5±11.5 |
| Oxygen saturation, mean | 94±4.4 | 94±3.9 | 93.6±6.9 |
| SpO2/FiO2ratio, median (IQR) | 428 (316.1–454.8) | 271.4 (158.3–361.5) | 230.2 (118.8–346.4) |
| Oxygen supplementation and supportive ventilation – no. (%) | |||
| Nasal cannula | 2 (5.9%) | 6 (9%) | 4 (7.0%) |
| Face masks | 22 (64.7%) | 33 (49.3%) | 16 (28.1%) |
| High oxygen supplementation device | 9 (26.5%) | 11 (16.4%) | 6 (10.5%) |
| High flow nasal cannulas | 0 (0%) | 10 (14.9%) | 16 (28.1%) |
| Non-invasive mechanical ventilation | 0 (0%) | 2 (3%) | 3 (5.3%) |
| Invasive mechanical ventilation | 1 (2.9%) | 5 (7.5%) | 12 (21.1%) |
SpO2/FiO2 ratio: arterial oxygen saturation measured by pulse oximetry to fraction of inspired oxygen.
Secondary outcomes at 7-day follow up from tocilizumab administration.
| Outcome – no. (%) | Patients |
|---|---|
| Vasopressor therapy | 15 (18.3%) |
| Acute respiratory distress syndrome | 45 (54.9%) |
| Respiratory failure | 62 (75.6%) |
| Acute kidney injury | 9 (10.9%) |
| Cardiac failure | 1 (1.2%) |
| Septic shock | 1 (1.2%) |
| Concomitant infection | 1 (1.2%) |
Eighty-one (98.9%) patients received hydroxychloroquine, 63 (76.8%) lopinavir/ritonavir, 21 (25.61%) darunavir/cobicistat, and 79 (96.34%) azithromycin. All patients were initially treated with antibiotic therapy, mainly ceftriaxone (77 (93.9%) patients). As expressed before, all patients received at least one dose of tocilizumab. Median (IQR) time in days from symptom onset to tocilizumab administration was 9.0 (6.0–11.0) and from admission to tocilizumab administration was 2.0 (1.0–3.0)). Other treatments include cytokine hemoadsorption therapy in 2 (2.4%) patients in ICU.
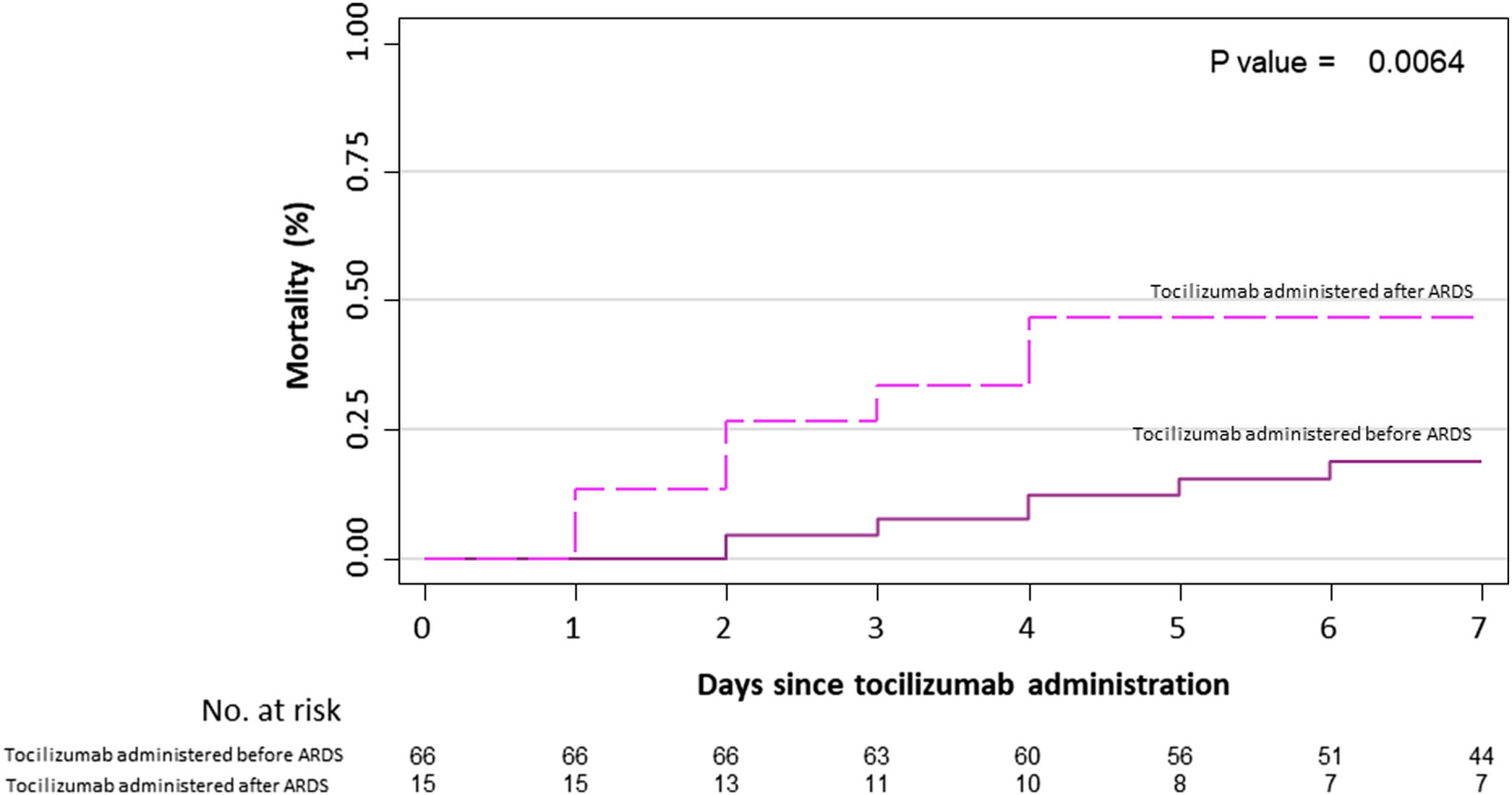
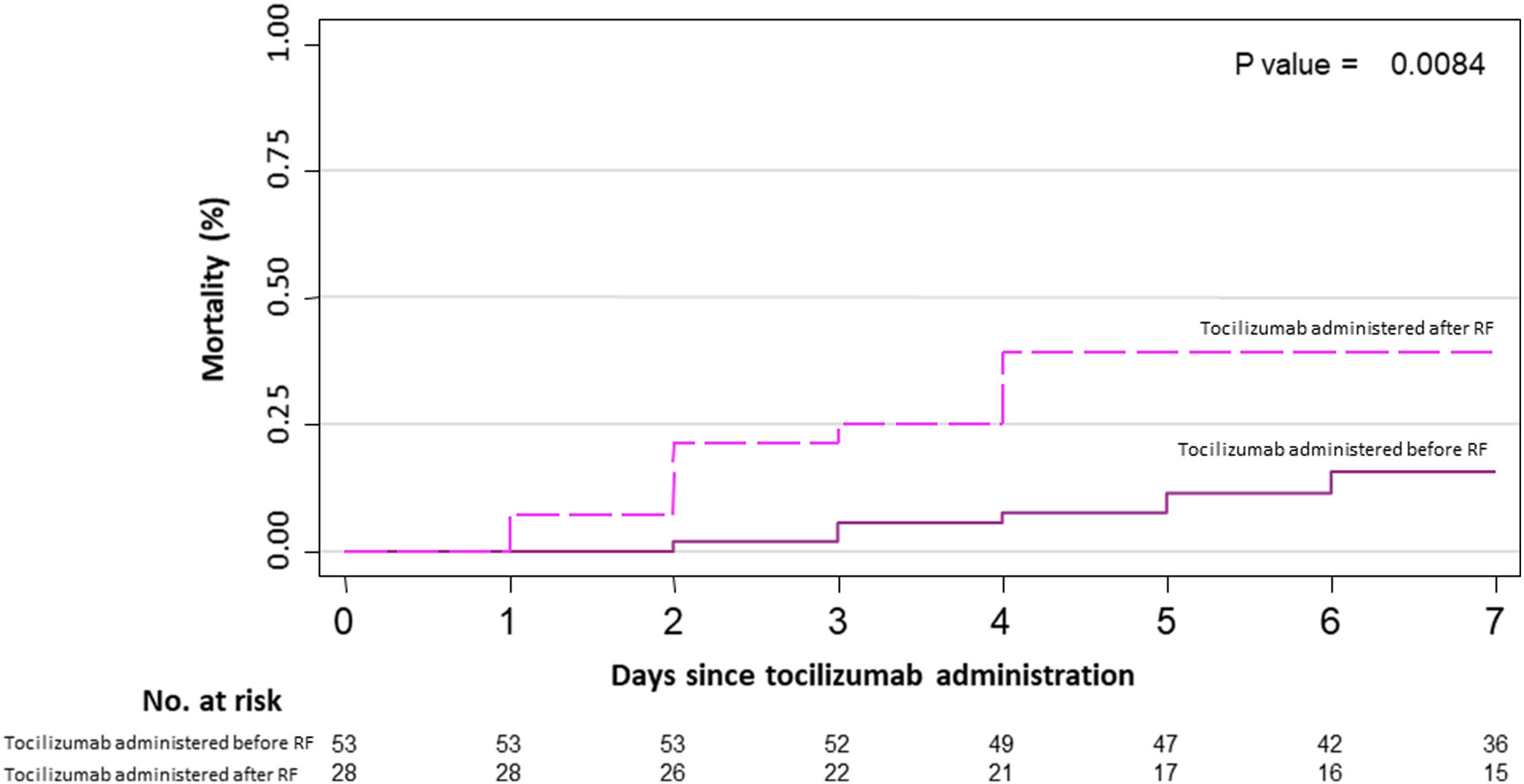
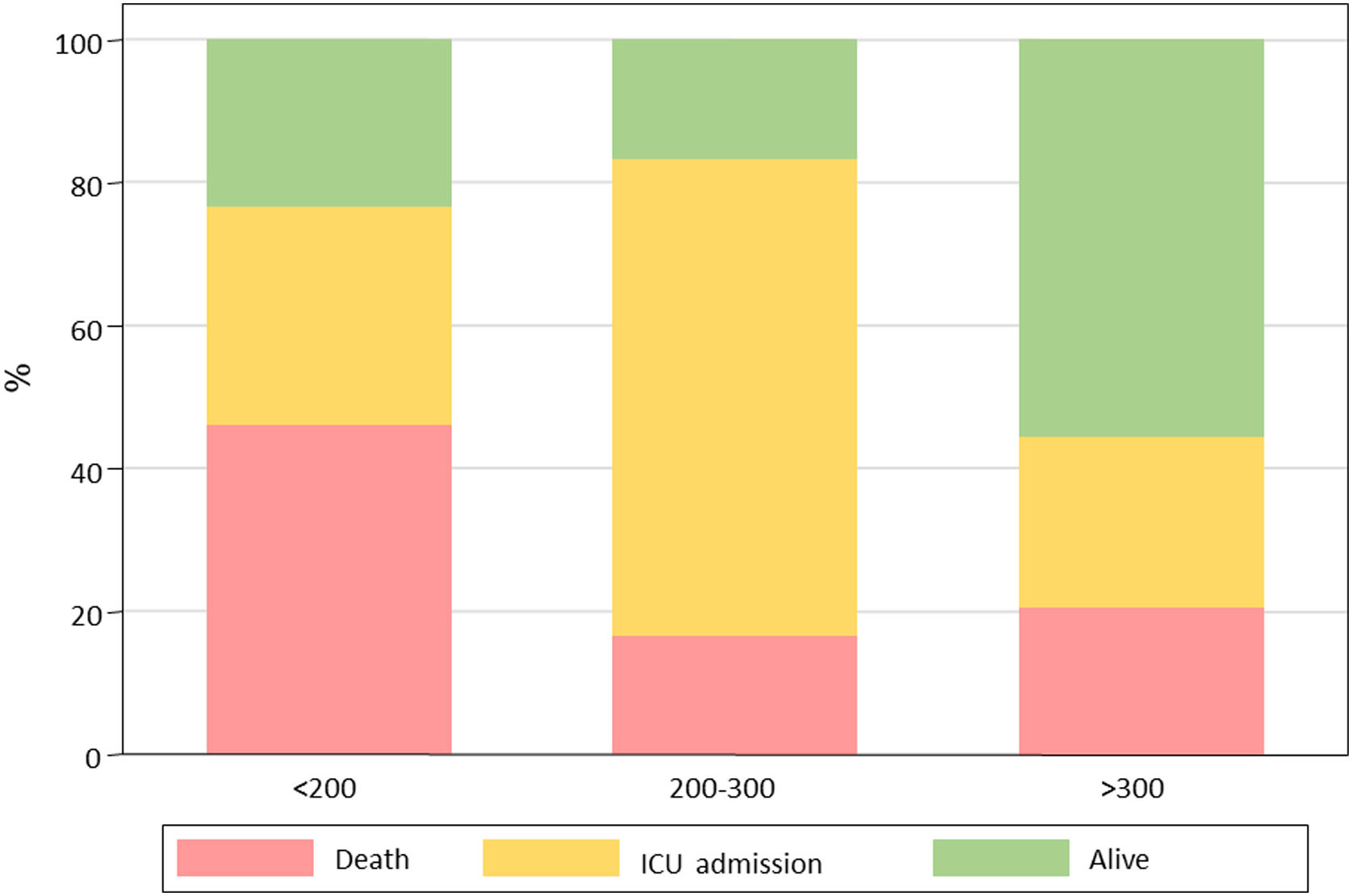
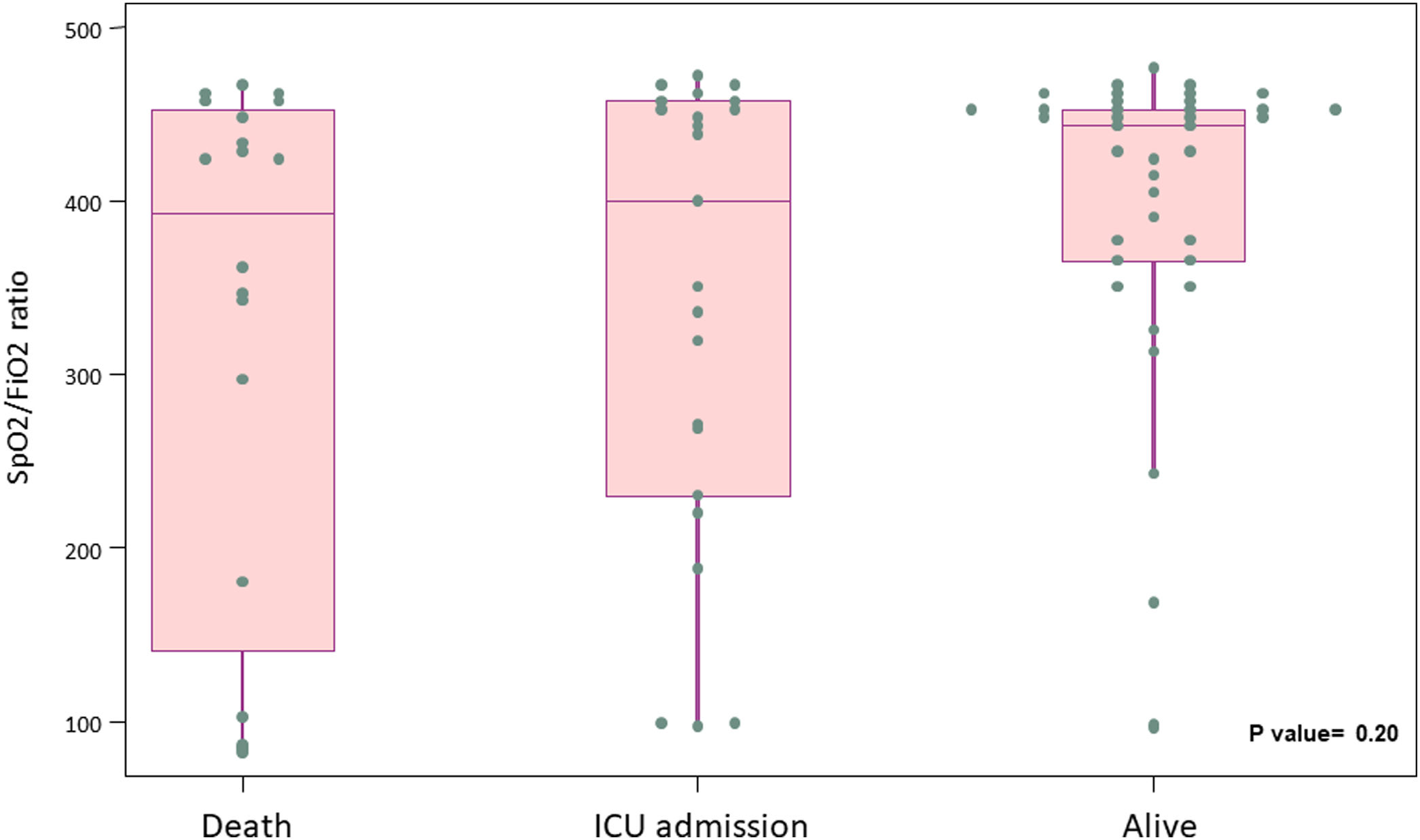
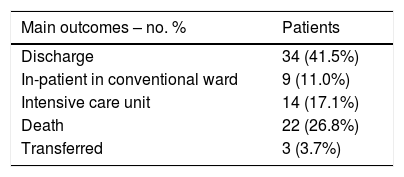
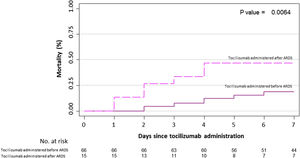
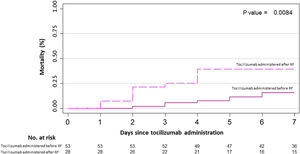
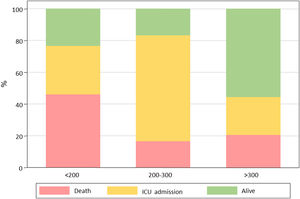
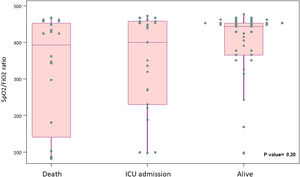
Primary outcome and mortality risk factorsTable 6 summarizes primary outcome in the study population. At the end of the follow up period, of the 82 patients 34 (41.5%) had been discharged, 22 (26.8%) had died, 14 (17.1%) were hospitalized in ICU, 9 (11.0%) were hospitalized in medical wards, and 3 (3.7%) had been transferred to another institution. In the univariate analysis age, age-adjusted Charlson comorbidity index, medical history of active or former solid cancer, hypertension, history of heart failure, chronic kidney disease and worse age-adjusted Charlson index at baseline were associated with increased risk of mortality (Table 7). By 7-day follow-up, the mortality rate was 4.0% per person-day (95% confidence interval [CI], 2.4% to 6.2%) by Kaplan–Meier analysis. Mortality was more frequent in patients receiving tocilizumab once ARDS was present (hazard ratio for mortality 3.3; 95% CI, 1.3–8.5; age-adjusted hazard ratio for mortality 2.1; 95% CI, 0.8–5.8) (Fig. 1) or respiratory failure was present (hazard ratio for mortality 3.13; 95% CI, 1.3–7.8; age-adjusted hazard ratio for mortality 2.4; 95% CI, 0.9–6.4) (Fig. 2). When dividing patients according to the nearest SpO2/FiO2 ratio to tocilizumab administration, mortality was higher among patients with lower SpO2/FiO2 ratio (SpO2/FiO2 ratio<200, 46.2%; SpO2/FiO2 ratio 200–300, 16.7%; SpO2/FiO2 ratio>300, 20.6%; p=0.03) (Fig. 3). Distribution of the nearest SpO2/FiO2 ratio to tocilizumab administration depending on outcome groups was not statistically significant (mean (SD) SpO2/FiO2 ratio: 321.3 (154.7) dead, 343.1 (132.7) ICU, 396.9 (96.2) alive; p=0.2) (Fig. 4). No correlation was observed between nearest IL-6 levels to tocilizumab administration and main outcome (median (IQR) IL-6 levels: 79.7 (48.2–128.1) dead, 77.5 (55–120) ICU admission, 71.4 (49.4–116) alive; p=0.92). Basal characteristics of patients stratified by ARDS and respiratory failure can be found in the Supplementary Appendix.
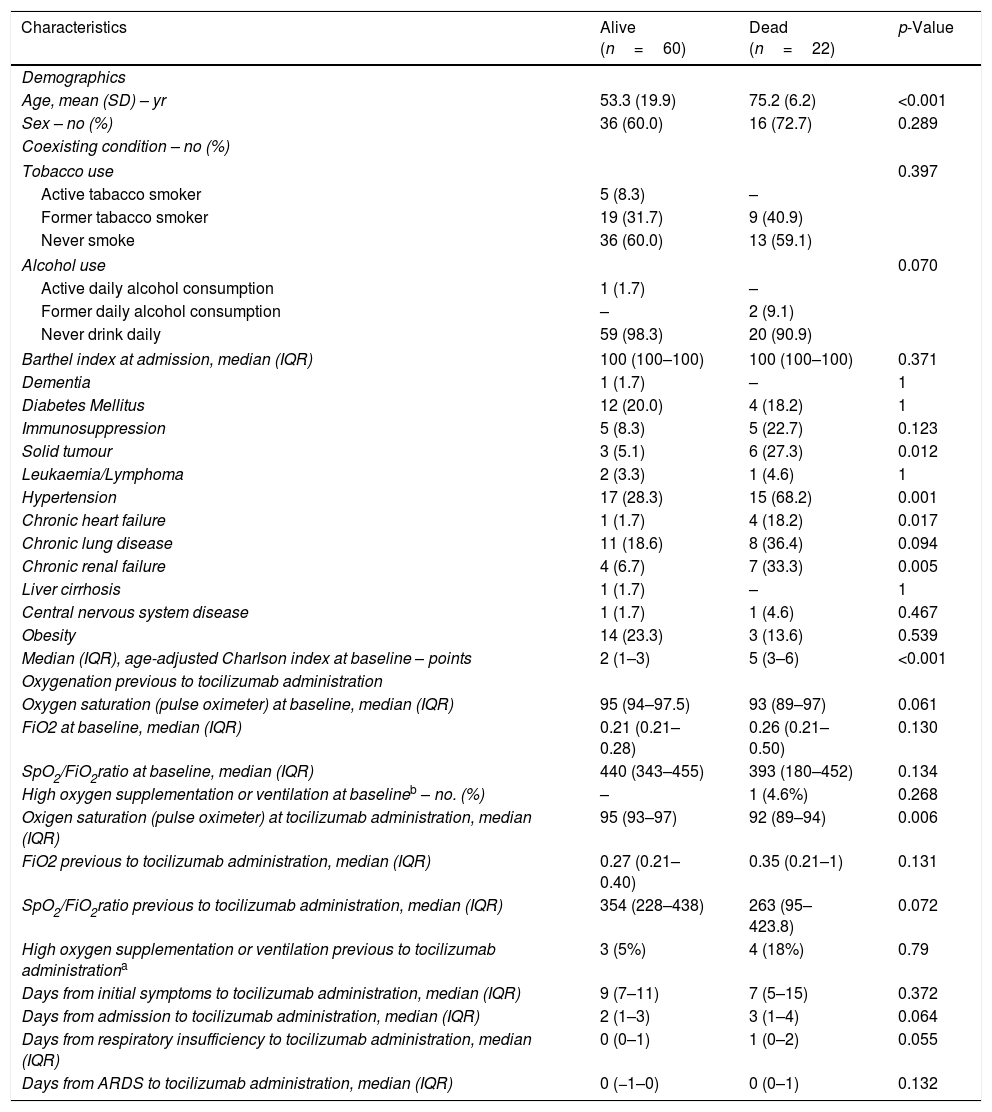
Comparison of risk factors by in-hospital mortality.
| Characteristics | Alive (n=60) | Dead (n=22) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age, mean (SD) – yr | 53.3 (19.9) | 75.2 (6.2) | <0.001 |
| Sex – no (%) | 36 (60.0) | 16 (72.7) | 0.289 |
| Coexisting condition – no (%) | |||
| Tobacco use | 0.397 | ||
| Active tabacco smoker | 5 (8.3) | – | |
| Former tabacco smoker | 19 (31.7) | 9 (40.9) | |
| Never smoke | 36 (60.0) | 13 (59.1) | |
| Alcohol use | 0.070 | ||
| Active daily alcohol consumption | 1 (1.7) | – | |
| Former daily alcohol consumption | – | 2 (9.1) | |
| Never drink daily | 59 (98.3) | 20 (90.9) | |
| Barthel index at admission, median (IQR) | 100 (100–100) | 100 (100–100) | 0.371 |
| Dementia | 1 (1.7) | – | 1 |
| Diabetes Mellitus | 12 (20.0) | 4 (18.2) | 1 |
| Immunosuppression | 5 (8.3) | 5 (22.7) | 0.123 |
| Solid tumour | 3 (5.1) | 6 (27.3) | 0.012 |
| Leukaemia/Lymphoma | 2 (3.3) | 1 (4.6) | 1 |
| Hypertension | 17 (28.3) | 15 (68.2) | 0.001 |
| Chronic heart failure | 1 (1.7) | 4 (18.2) | 0.017 |
| Chronic lung disease | 11 (18.6) | 8 (36.4) | 0.094 |
| Chronic renal failure | 4 (6.7) | 7 (33.3) | 0.005 |
| Liver cirrhosis | 1 (1.7) | – | 1 |
| Central nervous system disease | 1 (1.7) | 1 (4.6) | 0.467 |
| Obesity | 14 (23.3) | 3 (13.6) | 0.539 |
| Median (IQR), age-adjusted Charlson index at baseline – points | 2 (1–3) | 5 (3–6) | <0.001 |
| Oxygenation previous to tocilizumab administration | |||
| Oxygen saturation (pulse oximeter) at baseline, median (IQR) | 95 (94–97.5) | 93 (89–97) | 0.061 |
| FiO2 at baseline, median (IQR) | 0.21 (0.21–0.28) | 0.26 (0.21–0.50) | 0.130 |
| SpO2/FiO2ratio at baseline, median (IQR) | 440 (343–455) | 393 (180–452) | 0.134 |
| High oxygen supplementation or ventilation at baselineb – no. (%) | – | 1 (4.6%) | 0.268 |
| Oxigen saturation (pulse oximeter) at tocilizumab administration, median (IQR) | 95 (93–97) | 92 (89–94) | 0.006 |
| FiO2 previous to tocilizumab administration, median (IQR) | 0.27 (0.21–0.40) | 0.35 (0.21–1) | 0.131 |
| SpO2/FiO2ratio previous to tocilizumab administration, median (IQR) | 354 (228–438) | 263 (95–423.8) | 0.072 |
| High oxygen supplementation or ventilation previous to tocilizumab administrationa | 3 (5%) | 4 (18%) | 0.79 |
| Days from initial symptoms to tocilizumab administration, median (IQR) | 9 (7–11) | 7 (5–15) | 0.372 |
| Days from admission to tocilizumab administration, median (IQR) | 2 (1–3) | 3 (1–4) | 0.064 |
| Days from respiratory insufficiency to tocilizumab administration, median (IQR) | 0 (0–1) | 1 (0–2) | 0.055 |
| Days from ARDS to tocilizumab administration, median (IQR) | 0 (−1–0) | 0 (0–1) | 0.132 |
ARDS: acute respiratory distress syndrome.
Twelve (14.63%) out of 82 patients reported a total of 14 adverse events during the follow up. Thirteen (92.9%) adverse events were considered related to lopinavir/ritonavir, 9 (75.0%) patients discontinued lopinavir/ritonavir treatment due to gastrointestinal symptoms. Diarrhoea was the most common reported adverse event. Other adverse events included nausea and dysuria. There were no adverse events attributed to tocilizumab. No serious adverse events were reported during follow up, and only 2 (14.3%) were considered moderate. Eleven (91.7%) patients recovered without medical sequelae and one patient had an unknown outcome. No tocilizumab discontinuation was reported due to adverse events.
DiscussionThis preliminary report from the Vall d’Hebron COVID-19 Cohort Study describes the characteristics and clinical outcomes of patients who were hospitalized in non-ICU wards and received treatment with tocilizumab. Our results show that a timely administration of immune-modulating therapies, before the onset of respiratory insufficiency or ARDS, may improve severe COVID-19 patients’ outcomes.
Therapies to improve outcomes of patients with COVID-19 focus on viral-directed therapies and host-directed therapies. There is still lack of evidence about the efficacy of any of these therapies, although this does not prevent physicians to use all sorts of off-label therapies despite the risk of serious adverse events.25 Therapies to curb uncontrolled cytokine release have been proposed and are being widely used. Randomized controlled trials with tocilizumab have shown promising results, although the proper timing of administration and the subpopulation with the best risk-benefit ratio is still unknown.16,17 Besides, data from prospective studies can help to improve COVID-19 patient management.26 In our study, the 7-day mortality was 26.8%. Other studies reported mortalities ranging between 13% and 22.1%, although follow up times are not homogeneous.27,28 It is important to mention that the patients in our cohort had more coexisting conditions, including potential mortality risk factors such as hypertension, other cardiovascular and metabolic diseases, chronic kidney disease and cancer.
The understanding of mortality risk factors in patients with COVID-19 is an evolving matter. Age, specific coexisting conditions and laboratory parameters may help identify patients with poor outcome.29 As expressed before, in our cohort of patients treated with tocilizumab, hypertension, history of cardiac failure and chronic kidney disease were associated with higher mortality in the univariate analysis. Antihypertensive agents, such as angiotensin-converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB), have been suggested to be associated with the increased mortality observed in this subset of patients. Angiotensin converting enzyme 2 plays an important role in SARS-CoV-2 viral entry as co-receptor.30 Hypothesis outline that the interactions between these drugs and co-receptors may increase viral spreading in the lung and increase risk of death. However, the evidence is limited and no specific recommendations could be drawn from current evidence, especially when ACEI and ARB have shown to reduce mortality in this at risk population.31 The Vall d’Hebron COVID-19 Cohort Study has among its main objectives to analyze the role of these and other drugs used to treat chronic conditions in the prognosis of patients with COVID-19.
Host-directed therapies aiming at blocking an unrestrained immune response and an excessive inflammation have been proposed as potential therapies to prevent acute lung injury and subsequent ARDS. SARS-CoV-2 infection severity has been associated with an increase in IL-6 and D dimer levels, and the cytokine profile resembles that of other conditions in which host-directed therapies have been successfully used.13,29,32 Timely use of host-directed therapies may curb uncontrolled cytokine release and prevent damage inflicted by hyperinflammation. Tocilizumab has shown to be safe in multicentre clinical trials. In the RECOVERY study participants were eligible if they have hypoxia and levels of C-reactive protein higher than 75mg/dl, 28-day mortality in the tocilizumab group was 29% vs 33% in the control arm, with an incidence ratio of 0.86 (95%CI 0.77–0.96). Another clinical trial (REMAP CAP) also showed mortality reduction in critically ill hospitalized patients with COVID19 pneumonia treated with IL-6 receptors antagonists.17 Although, other clinical trials in hospitalized patients with COVID19 pneumonia did not show mortality reduction or clinical improvement when receiving tocilizumab.33,34 it is important to highlight the heterogeneity in time of initiation of the intervention, since tocilizumab may be more active at the initial stage of the inflammatory cascade and the lack of IL-6 guided therapy.
As in other infections and inflammation-driven diseases, timely initiation of precise therapy is the mainstay of patient management and directly affects mortality and morbidity. Our study showed that patients receiving prompt treatment with tocilizumab before lung injury is established have less 7-day mortality, a benefit that may be sustained in the long-term.35
The safety profile of tocilizumab has been extensively studied in clinical trials with patients suffering autoimmune diseases and recently in COVID-19 patients. The most common adverse events of intravenous tocilizumab in a pooled analysis of 3 clinical trials were upper respiratory infections, nasopharyngitis, headache, hypertension and increase in liver enzymes. Serious adverse events occurred in 12% of the patients, infectious diseases being the most common.36 In our study we did not report any serious adverse events, although the symptoms of systemic viral infections may mimic any adverse event and make its identification difficult. Tozilizumab-related bacterial infections were not reported in our study. Two factors may have contributed to this: first, many patients were under antibiotic treatment, and second, the short follow up period precludes us from any further analysis. Nevertheless, the low cumulative dose administered in this subset of patients may diminish the likelihood of infectious adverse events.
In a time of scarce medical resources, including limited stock of host-directed therapies, hard medical decisions have to be done by front-line physicians. Allocation of therapies to patients with the highest chances of a favourable outcome should be encouraged, maximizing the benefit of the intervention.37 Evidence-based decision-making and benefit-maximizing allocation of the available resources should be promoted. In this regard our study can help physicians to better allocate host-directed therapy in patients with COVID-19 prioritizing moderate-to-severe ill patients over critically ill patients.
This preliminary exploratory study has several limitations. First, there is no control group and the minimum follow up period was 7 days. Therefore, at this point it is not possible to evaluate the differences between patients receiving tocilizumab or not and, consequently, it is not possible to evaluate solidly what is the overall benefit of administering this drug. However, the urgency of obtaining data on new therapies justifies the early communication of these results. Second, ICU admission is not a very robust endpoint since it depends on the attitudes of the treating physicians as well as the availability of beds at times of resource scarcity and overwhelming demand. For this reason, mortality was selected as the main outcome in our study. Besides, the subsequent analysis of all patients included in the Vall d’Hebron COVID-19 Cohort Study may solve this limitation and inform results with a longer follow up period. Finally, our data is limited to a single centre. While our results may not be extrapolated to other populations or other standards of care, the management of patients was homogeneous avoiding the centre effect of multicentric studies. Multivariate analysis is limited by sample size.
ConclusionIn summary, we found a mortality of 26.8% in this subset of patients with COVID-19 receiving tocilizumab for the treatment of inflammatory-related lung injury. Early administration of tocilizumab in patients needing oxygen supplementation may be critical to patient recovery. Our results may help front-line physician to make evidence-based decisions in times of scarce resources and operationalized fair and transparent allocation procedures, maximizing the benefit of the intervention. Future and current host-directed clinical trials for patients with COVID-19 should consider our preliminary data in their design. Host-directed therapies need further investigation.
Contribution statementConceived the idea: ASM, JEP, JSN, NFH, BA.
Reviewed medical chart and collected data: ASM, JSM, JEP, NFH, FS, XD, MM, AA, SEE, ASG, AMP, PBM, SA, JS, AGdC.
Supervised the findings: ASM, JEP, JSN, NFH, BA.
Performed statistical analysis: ASM, JEP, NFH, SPH.
Wrote the manuscript with inputs from all authors: ASM, JEP, NFH.
Supervised the project: ASM, JS, BA.
Interpretation of the results: ASM, JSM, JEP, NFH, FS, XD, MM, AA, SEE, ASG, AMP, PBM, SA, JS, AGdC.
Critically reviewed the manuscript: ASM, JSM, JEP, NFH, FS, XD, MM, AA, SEE, ASG, AMP, PBM, SA, JS, AGdC.
Sources of fundingNone.
Summary of the article's main pointIn patient with COVID-19 and lung injury, time from lung injury onset to tocilizumab administration may be critical to patient recovery. Early administration of host-directed therapies may improve patient outcome.
Conflicts of interestAdrián Sánchez-Montalvá is supported a grant from Spanish ministry of Health through the Program for consolidated researcher from Instituto de Salud Carlos II (Juan Rodés; JR18/00022).