On 14th March 2020, a state of alarm was declared in Spain due to the SARS-CoV-2 virus pandemic. After 12 weeks since the beginning of the pandemic, confirmed cases in Aragon reached 5,781, 46% requiring hospitalisation, 5% admission to ICU and 15% died.
The rapid progression of the pandemic and the absence of effective treatment and clinical trials prompted the initiation of multiple therapeutic combinations. Most of the prescription proposals were based on Chinese publications, after their experience in patients with COVID-19.
Although there is currently no effective treatment for COVID-19 infection, different therapeutic approaches have been proposed since the beginning of the pandemic: antivirals that inhibit enzyme systems with the aim of reducing viral replication, those that inhibit SARS-CoV-2 entry in the cell, and immunomodulators that try to reduce the cytokine storm and associated lung damage.1
With the “evidence generated” in clinical practice and with data from ongoing studies, the Ministry of Health issues, and updates recommendations for the treatment of patients with the infection.2 Likewise, in Aragon, a regional protocol for pharmacological management has been established incorporating these recommendations for use.
In order to have a clear picture of the treatments used during the pandemic, the most common combinations and whether they were in line with the recommendations based on the available evidence, a retrospective descriptive study in the consumption and dispensing of in-hospital drugs was carried out in a sample of patients admitted for COVID- 19 (PCR positive), covering all of Aragon’s Health Service hospitals, between 1st March to 8th May 2020 (phases 0–1). Data from 1,482 patients admitted with confirmed infection (60% of hospitalized COVID-19 patients) were analysed: 813 (54.9%) men and 669 (45.1%) women, with a median age of 75 years IQR (62–85). No statistically significant differences were observed in sex according to province, except in Huesca (39% women and 61% men, p = 0.04). 12% required ICU admission.
The behaviour of the analysed sample is similar to the reports from other national hospitals during the study period.3 46% of patients required hospitalization (45% Spain), predominantly males (54% Aragon, 57% Spain), and a median age above the national level (75 years IQR 62–85, compared to 70 IQR 55–81 in Spain).
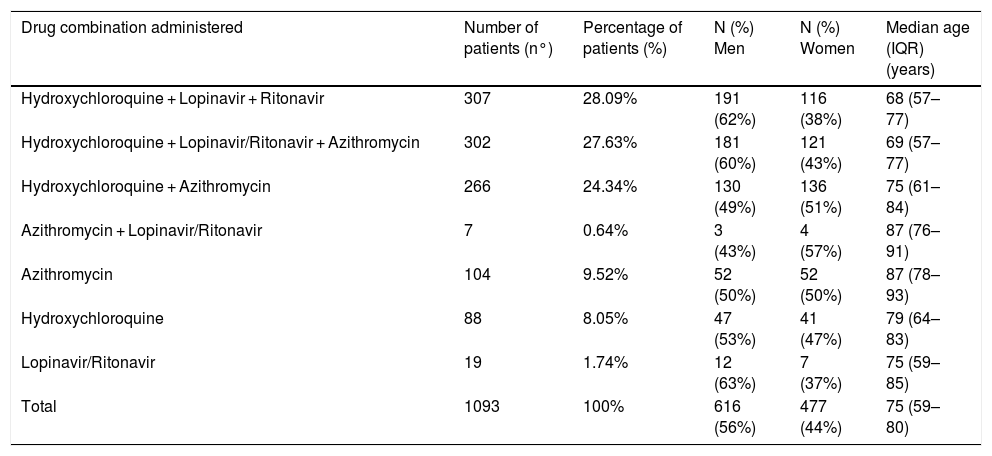
A total of 456 different drug substances were prescribed, with a median of 13 drug substances per patient (IQR 9–19). 73% (1,093) of patients received hydroxychloroquine, lopinavir/ritonavir, or azithromycin. 81% in combination (Table 1).
Profile of the most common antibiotic, antiviral and immunomodulatory treatments prescribed (combined or alone) during the study period.
| Drug combination administered | Number of patients (n°) | Percentage of patients (%) | N (%) Men | N (%) Women | Median age (IQR) (years) |
|---|---|---|---|---|---|
| Hydroxychloroquine + Lopinavir + Ritonavir | 307 | 28.09% | 191 (62%) | 116 (38%) | 68 (57–77) |
| Hydroxychloroquine + Lopinavir/Ritonavir + Azithromycin | 302 | 27.63% | 181 (60%) | 121 (43%) | 69 (57–77) |
| Hydroxychloroquine + Azithromycin | 266 | 24.34% | 130 (49%) | 136 (51%) | 75 (61–84) |
| Azithromycin + Lopinavir/Ritonavir | 7 | 0.64% | 3 (43%) | 4 (57%) | 87 (76–91) |
| Azithromycin | 104 | 9.52% | 52 (50%) | 52 (50%) | 87 (78–93) |
| Hydroxychloroquine | 88 | 8.05% | 47 (53%) | 41 (47%) | 79 (64–83) |
| Lopinavir/Ritonavir | 19 | 1.74% | 12 (63%) | 7 (37%) | 75 (59–85) |
| Total | 1093 | 100% | 616 (56%) | 477 (44%) | 75 (59–80) |
Hydroxychloroquine and lopinavir/ritonavir were among the options recommended in the protocol, alone or in combination, while the lack of results of the hydroxychloroquine and azithromycin combination, and the risk of QT prolongation of both drugs, were reported.4 The recommendation to use combination therapies is based on the possible synergistic action of their different mechanisms of action. However, there is no evidence about their benefit, and they present a risk of cardiac complications, as the three drugs increase the QT interval.1
The prescription profiles against the virus used in clinical practice are unknown. In a recent systematic review,5 the drug most frequently administered was lopinavir/ritonavir (21.9%), with hydroxychloroquine (1.2%) and azithromycin (1.4%) with a much lower frequency. These data contrast with those observed in the study sample.
The immunosuppressive treatments recommended to act on the progression of the disease1 were prescribed in 48% of the patients, with corticosteroids being the most widely used: 84% methylprednisolone (with a more powerful immunosuppression profile), 8.7% dexamethasone, 3.8% associated both corticosteroids, and 3.5% associated with tocilizumab (methylprednisolone 84%), in line with the recommendations of some studies.5
There is currently insufficient quality evidence to recommend any treatment, and safety alerts are issued regarding the use of combinations that put patients at risk without obtaining any benefit. More randomised and controlled clinical studies are needed to clarify the optimal treatment for SARS-CoV-2 infection.
FundingThis article has not received any type of funding.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Álvarez Nonay A, Cabia Fernández L, Bandrés Liso A. Perfil de prescripción de los pacientes con infección por SARS-CoV-2 hospitalizados en Aragón. Med Clin (Barc). 2021;156:88–89.