This scientific letter analyses the efficacy, safety and tolerability of urea treatment in hospitalised patients with hyponatraemia secondary to the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Hyponatraemia is the most common electrolyte disorder, with SIADH being a major cause.1 Current treatments for hyponatraemia in SIADH have limitations and the search is on for more effective and affordable therapy, with urea being one of the most promising, a low-cost oral osmotic diuretic that increases the urinary excretion of water.2–5
The study was observational and retrospective, conducted at the Ribera-Povisa Hospital in Vigo, between January 2016 and June 2023. Patients with a confirmed diagnosis of SIADH defined as sodium (Na) <135 mmol/L, urinary osmolarity >300 Osm/kg, low plasma osmolarity (<280 Osm/kg), high urinary Na excretion (>20 mmol/L), clinical euvolemia, normal adrenal and thyroid function were included. Cases with specific conditions, such as severe liver disease or severe renal failure, were excluded.
The aim of the study was to return Na+ levels to normal with oral urea. Clinical and socio-demographic data of the patient group, mean urea dose, as well as changes in the parameters related to the effect of urea (Na, serum potassium and plasma urea) were included as variables. Adverse effects and mortality at 60 days were assessed. The study was approved by the Galician Research and Ethics Committee on 30/05/2023 with Registration Code 2023/146. The investigators followed the applicable ethical and legal regulations.
A descriptive analysis of the data collected was performed using the Statistical Package for the Social Sciences (SPSS) 19.0 software. To analyse the changes in the variables before and after urea treatment, a Student’s t-test was used for related data or the Wilcoxon test depending on whether the data followed a normal distribution or not.
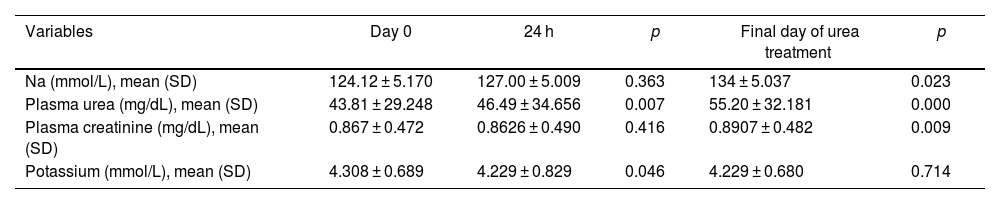
Eighty-two patients were included: mean age 75.9, sex ratio (F:M) 45:37. The aetiology of SIADH was related to drugs 42 (51.2%), tumours 24 (29.3%), central nervous system (CNS) disorders 4 (4.9%), non-malignant pulmonary disorders 1 (1.2%) and idiopathic 11 (13.4%). Treatment was initiated with urea doses of 15 g/24 h, sometimes requiring gradual increases to reach normal Na levels. The mean urea dose used in our study was 22.5 ± 7.61 g (between 15 and 30 g per day) with a mean of six days. After the addition of urea, the mean plasma Na concentration at baseline was 124.12 ± 5.170 mmol/L, at 24 h was 127.00 ± 5.009 mmol/L (p = 0.363) and at the end was 134 ± 5.037 mmol/L (p = 0.023). The proportion of urea-treated patients achieving normal sodium levels was 44.6%. The uraemia at the start of urea treatment was 43.81 ± 29.248 mg/dL and the mean on the day of completion was 55.20 ± 32.181 mg/dL (p < 0.001). There was a slight decrease in serum potassium 24 h after the start of treatment (p = 0.046) and a slight increase in creatinine at the end (p = 0.009), both without clinical significance (Table 1).
Changes in laboratory parameters from urea therapy initiation.
| Variables | Day 0 | 24 h | p | Final day of urea treatment | p |
|---|---|---|---|---|---|
| Na (mmol/L), mean (SD) | 124.12 ± 5.170 | 127.00 ± 5.009 | 0.363 | 134 ± 5.037 | 0.023 |
| Plasma urea (mg/dL), mean (SD) | 43.81 ± 29.248 | 46.49 ± 34.656 | 0.007 | 55.20 ± 32.181 | 0.000 |
| Plasma creatinine (mg/dL), mean (SD) | 0.867 ± 0.472 | 0.8626 ± 0.490 | 0.416 | 0.8907 ± 0.482 | 0.009 |
| Potassium (mmol/L), mean (SD) | 4.308 ± 0.689 | 4.229 ± 0.829 | 0.046 | 4.229 ± 0.680 | 0.714 |
SD: standard deviation; Na: sodium.
No serious adverse effects were observed (only mild gastrointestinal symptoms in 5% of patients), there were no cases of Na hypercorrection and the 60-day mortality was 21.7%.
The limitations of our study were the absence of a control group and a small sample size. Randomised controlled studies are needed to confirm the benefit of urea in SIADH.
The study concludes that oral urea treatment is effective, safe and well tolerated in correcting hyponatraemia caused by SIADH. We suggest that oral urea could be considered as a first-line drug in the treatment of this condition.
Ethical considerationsInformed consent was obtained.
Patients with SIADH and hyponatraemia (Na < 135 mmol/L) under follow-up in the Internal Medicine Department of the Hospital Ribera-Povisa were included in the study once they had been informed of the objectives of the study and had given their consent.
FundingThis study has not received any funding.
Conflict of interestThe authors declare that they have no conflicts of interest.