This study analyzed the impact of patients’ age, sex, vaccination, immunosuppressive treatment, and previous comorbidities on the risk of developing persistent COVID-19 or SARS-CoV-2 virus reinfection.
MethodPopulation-based observational retrospective study of a cohort of 110,726 patients aged 12 years or older, who were diagnosed with COVID-19 between June 1st, 2021 and February 28th, 2022 in the island of Gran Canaria.
Results340 patients suffered reinfection. The combination of advanced age, female sex and lack of complete or incomplete vaccination against COVID-19 was strongly predictive of reinfection (p<0.05). In the 188 patients who developed persistent COVID-19, the persistence of symptoms was more frequent in adult patients, women, and patients with a diagnosis of asthma. Complete vaccination was associated with a lower risk of reinfection ([OR] 0.05, 95%CI 0.04–0.07; p<0.05) and of developing persistent COVID-19 ([OR] 0.07, 95%CI 0.05–0.10; p<0.05). None of the patients with reinfection or persistent COVID-19 died during the period of the study.
ConclusionsThis study confirmed the link between age, sex, asthma and risk of persistent COVID-19. It was not possible to define the patient's comorbidities as a factor that influences the development of reinfection, but its association with age, sex, type of vaccine and hypertension was demonstrated. Higher vaccination coverage was associated with a lower risk of persistent COVID-19 or SARS-CoV-2 reinfection.
Se analizó el impacto de la edad, el sexo, la vacunación, el tratamiento inmunosupresor y las comorbilidades previas del paciente sobre la condición de riesgo de desarrollar COVID-19 persistente o reinfección por el virus del SARS-CoV-2.
MétodoEstudio retrospectivo observacional de base poblacional en una cohorte de 110.726 pacientes de 12 o más años de edad diagnosticados de COVID-19 entre el 1 de junio de 2021 y el 28 de febrero de 2022 en la isla de Gran Canaria.
ResultadosTrescientos cuarenta pacientes sufrieron reinfección por COVID-19. La combinación de edad avanzada, sexo femenino y falta de vacunación completa o incompleta contra la COVID-19 fue fuertemente predictiva de reinfección (p<0,05). En los 188 pacientes que desarrollaron COVID-19 persistente, la persistencia de síntomas fue más frecuente en pacientes en edad adulta, mujeres y pacientes con diagnóstico de asma. La vacunación completa se asoció con un menor riesgo de reinfección ([OR] 0,05, IC 95% 0,04-0,07; p <0,05) y de desarrollar COVID-19 persistente ([OR] 0,07, IC 95% 0,05-0,10; p <0,05). Ninguno de los pacientes con reinfección o COVID-19 persistente falleció durante el período del estudio.
ConclusionesEste estudio confirmó el vínculo entre la edad, el sexo, el asma y el riesgo de COVID-19 persistente. No se pudo definir las comorbilidades del paciente como factor que influye en el desarrollo de reinfección, pero sí se demostró su asociación con edad, sexo e hipertensión arterial. Una mayor cobertura de vacunación se asoció a un menor riesgo de COVID-19 persistente o reinfección por SARS-CoV-2.
The respiratory infection caused by SARS-CoV-2 was first documented by the end of December, 2019 in Wuhan,1 from where it spread globally, and caused a pandemic with unprecedented consequences.2 As of November 22nd, 2022, there have been more than 600 million cases and more than 6.6 million deaths worldwide.3
Initial studies suggest that COVID-19 vaccines protect against severe forms of the disease. However, post-vaccination SARS-CoV-2 infection may occur because the COVID-19 vaccines do not offer 100% protection.4 There are limited data on the occurrence of reinfections and on the impact of vaccination in terms of reducing the transmission of the disease.5 In this regard, clinical trials conducted with the first three vaccines that were licensed for use (BNT162b2 mRNA or Pfizer-BioNTech, ChAdOx1 nCoV-19 or AstraZeneca-University of Oxford, and mRNA-1273 or Moderna) showed high efficacy for the three of them. Pfizer-BioNTech showed 94.6% efficacy (95%CI: 89.9%-97.3%) in patients with or without evidence of a previous SARS-CoV-2 infection. The efficacy 14 days after a second dose of Moderna was 93.6% (95%CI: 88.5–96.4%) in patients with or without evidence of a previous SARS-CoV-2 infection. The efficacy in participants without evidence of a previous SARS-CoV-2 infection and a 10–12-week interval between the first and the second dose (preferably 12 weeks) was around 80% for AstraZeneca. A 66.9% efficacy (95%CI: 59–73.4) was found from day 14 post-administration of Janssen on, in subjects without evidence of a previous SARS-CoV-2 infection.6
Confirmed reinfection has important implications. If reinfection is not uncommon, herd immunity might not be enough to eliminate SARS-CoV-2; although subsequent infections might be milder than the first one.7 It is likely that COVID-19 continues to exist in the human population just like other human coronaviruses. Reinfection is common for “seasonal” coronaviruses 229E, OC43, NL63, and HKU1. In some cases, reinfection occurs despite patient's stable levels of specific antibodies. It is thus possible that vaccines do not provide lifelong protection against COVID-19.8
Reinfection may occur 4–5 months after a first episode of symptomatic infection. Vaccination should also be considered for subjects with a known history of COVID-19.9–14 Moreover, during the current COVID-19 pandemic, groups of patients have emerged who continue to present symptoms after the acute phase of the disease, a condition commonly known as “persistent COVID-19”.8,9
The National Institute for Health and Care Excellence (NICE) established a definition for persistent COVID-19, including signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks, and are not explained by an alternative diagnosis.10 Common symptoms in people with persistent COVID-19 are sensory (loss of taste and anosmia), neurological (problems with concentration and “brain fog”), and cardiorespiratory (fatigue, dyspnea, reduced exercise capacity) problems. In patients who experience persistent COVID-19, 1 or more symptoms may be present.11
Risk factors identified for persistent COVID-19 include: severity of the disease (need for hospital admission), need for ventilatory support during the acute phase,12 age (over 50 years), sex (female), and comorbidities (asthma or previous respiratory disease, obesity, and higher body mass index).13
The characteristics of patients with persistent COVID-19 or reinfection are not well known. Further studies on reinfection are essential for research and development of a more effective vaccine. In this study, the epidemiological and clinical characteristics of persistent COVID-19 and reinfection cases in Gran Canaria were analyzed.
MethodDesignPopulation-based observational retrospective study conducted on a cohort of 110,726 patients aged 12 years or more, who were diagnosed with COVID-19 between June 1st, 2021 and February 28th, 2022, in the island of Gran Canaria.
Inclusion criteriaConfirmed COVID-19 was considered for patients who met the clinical criteria for suspected COVID-19 and showed positive results in an AIDT (active infection diagnostic test), or asymptomatic patients with positive AIDT plus negative or not undertaken IgG-test. Suspected COVID-19 was considered for patients with acute respiratory infection of sudden onset of any degree of severity, who presented with fever, cough or shortness of breath, among other signs. Further signs or symptoms like odynophagia, anosmia, ageusia, muscle pain, diarrhea, chest pain, headache and others were also considered symptoms of suspected SARS-CoV-2 infection, depending on the doctor's criterion.
Eligibility criteriaThe inclusion criteria were patients aged 12 years or more who were diagnosed with COVID-19 between 1 June 2021 and 28 February 2022 on the island of Gran Canaria. The exclusion criteria were as follows: age<12 years.
Definition of persistent COVID-19Persistent COVID-19 was defined as “signs and symptoms that develop during or after an infection consistent with COVID-19, continue for more than 12 weeks and are not explained by an alternative diagnosis”.14,15 Asthenia, tiredness, fatigue, anosmia, cognitive impairment, malaise, and disorientation were the symptoms present in patients with persistent COVID-19.
Complete vaccination schedulePatients were considered to have received complete vaccination if (1) they had received 2 doses of the vaccine separated by a minimum of: 19 days if the first dose was BNT162b2 mRNA (Pfizer-BioNTech), 21 days if it was ChAdOx1 nCoV-19 (AstraZeneca-University of Oxford), or 25 days if it was mRNA-1273 (Moderna); and (2) if the minimum time elapsed since the last dose was: 7 days if the last dose was Pfizer, or 14 days if it was AstraZeneca or Moderna. Patients were also considered to be fully vaccinated if they had received one dose of Ad26.COV2.S (Janssen) more than 14 days before. Patients up to 65 years old, were also considered as fully vaccinated if they had passed the disease and subsequently received a dose of any of the vaccines, after the corresponding mentioned period for the second dose. Subjects vaccinated with a heterologous schedule consisting of a first dose of AstraZeneca and a second dose of an mRNA vaccine were considered fully vaccinated after 7 days, if the second dose was Pfizer or 14 days if it was Moderna.6
VariablesAge, sex, personal history issues (asthma, cancer, dementia, diabetes, coronary heart disease, chronic obstructive pulmonary disease or COPD, high blood pressure, congestive heart failure or CHF, obesity, obstructive sleep apnea syndrome or OSAS), body mass index (BMI), date of first, second and booster doses of a COVID-19 vaccine, type of COVID-19 vaccine (Pfizer, Moderna, Astrazeneca, Janssen), death, immunosuppressive treatment, diagnosis of persistent COVID-19, and diagnostic test results (polymerase chain reaction with reverse transcription (RT-PCR), antigen test or serology).
Data source and collectionThe identification data of all patients who were vaccinated against COVID-19 in Gran Canaria (from December 28th, 2020 to February 28th, 2022) were obtained from REGVACU (the registry of vaccination against COVID-19 in Spain). The identification data of all COVID-19 cases in Gran Canaria that were notified to the Epidemiological Surveillance Network of the Canary Islands (REVECA), were obtained from the General Directorate of Public Health (DGSP) (period: June 1st, 2021 to February 28th, 2022). Post-vaccination COVID-19 cases reported to the DGSP were identified by combining both databases. The clinical information of patients diagnosed with COVID-19 was obtained from their Primary Care electronic medical records (DRAGO AP). DRAGO is the healthcare management system of the Canary Islands.
DefinitionsParticipants were classified as suffering from diabetes if they had basal glycemia≥126mg/dl or reported to be under antidiabetic treatment. They were considered to present obesity for BMI≥30kg/m2; and hypertension for systolic blood pressure≥140mmHg and/or diastolic blood pressure≥90mmHg, or if they were under antihypertensive treatment.
Suspected reinfectionReinfection was suspected in patients with symptoms compatible with COVID-19, who presented SARS-CoV-2 infection, confirmed through AIDT, more than 90 days before. Such patients were investigated with a PCR analysis and were considered to be reinfected if the result was positive.16 Asymptomatic patients with an AIDT-confirmed SARS-CoV-2 infection more than 90 days before plus a positive result (obtained for example in contact follow-up or screening procedures, etc.) were considered to be reinfected if the result was from a PCR or were subjected to a PCR if the result was from a rapid antigen test (and classified accordingly).
In patients, who presented negative PCR results but were strongly suspected of suffering the disease, according to clinical-epidemiological criteria, the PCR was repeated. The 90-day period used to consider that a patient has acquired a second infection was arbitrarily agreed, to avoid confusion with first infections that present prolonged positive PCR results. However, it does not mean that the cause of reinfection is a loss of immunity after three months or that reinfection cannot occur before three months (although the later is much less frequent than prolonged excretion of viral genetic material).17,18
Statistical analysisA descriptive analysis of the results was carried out using frequency and percentages for categorical variables; and mean and standard deviation (SD) for analytical determinations and quantitative variables. The cumulative incidence of persistent COVID-19 or COVID-19 reinfection was calculated as the reason between the number of cases of persistent COVID-19 or reinfection in SARS-CoV-2 infected people in Gran Canaria (numerator) and the total number of SARS-CoV-2 infected people (denominator), during the studied period. Bivariate analysis of qualitative variables was carried out with the χ2 test, using the Likelihood Ratio when necessary. In addition to the bivariate analysis, a logistic regression analysis was conducted to adjust for main confounders. Statistical significance was established at 5% (p<0.05) and the level of confidence, at 95%. Data were analyzed with the Statistical Package for the Social Sciences (SPSS) v20 and Microsoft® Excel (2010).
This study was approved by the Ethics Committee for Research of the University Hospital of Gran Canaria Dr. Negrín (registration number 2021-355-1 COVID19) on September 24th, 2021. It was conducted in accordance with the local laws and regulations, the Declaration of Helsinki, and the Good Clinical Practices.
ResultsDuring the period of the study, June 1st, 2021 to February 28th, 2022, 340 patients presented reinfection and 188 presented persistent COVID-19. The cumulative incidence was 0.17% (95%CI 0.15–0.20) for persistent COVID-19 and 0.31% (95%CI 0.28–0.34) for reinfection.
The study included 110,726 patients diagnosed with COVID-19; 347 of them died (0.31%). The mean age was 41 years (SD 16.8), with the predominant age group in the sample being 18 to 49 years; 61.5% of patients with reinfection and 67.6% patients with persistent COVID-19 were women. Reinfection predominantly affected the 18–49 years age group, while persistent COVID-19 mainly affected patients over 70 years.
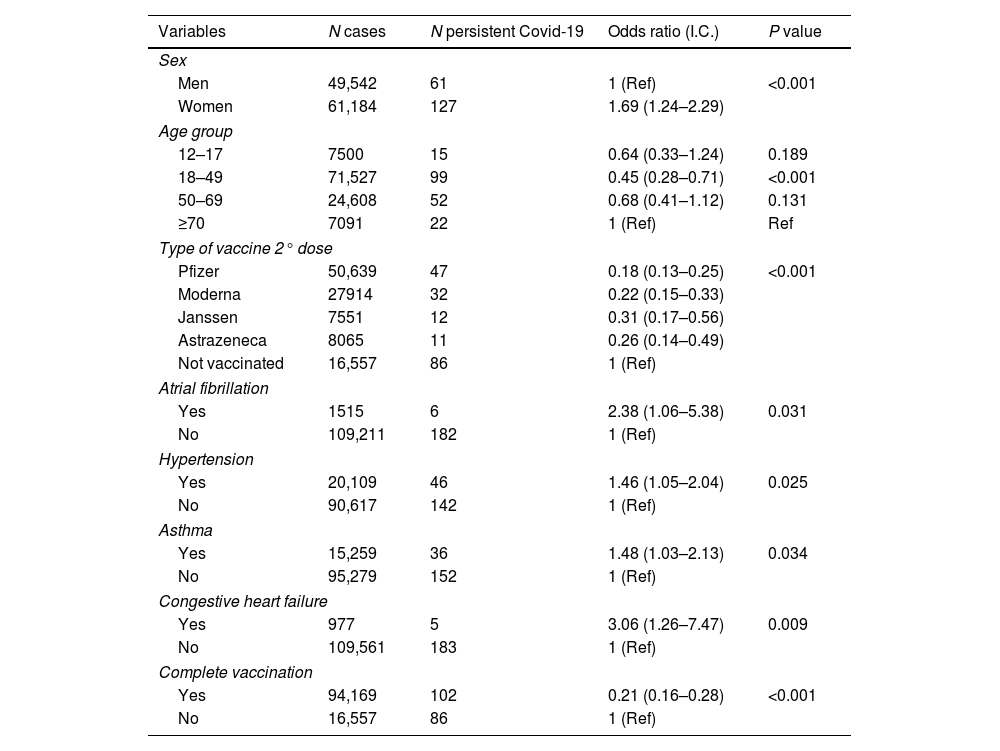
The 188 (0.17%) patients who presented persistent COVID-19 reported the following symptoms in order of frequency: asthenia (168; 89.4%), tiredness (54; 28.7%), fatigue (39; 20.7%), anosmia (31; 16.5%), cognitive impairment (18; 9.6%), malaise (9; 4.8%) and disorientation (6; 3.2%).
In the bivariate analysis, all the vaccines considered in the study significantly reduced the risk of reinfection or persistent COVID-19 as compared with unvaccinated subjects (p<0.05), (Tables 1 and 2). The bivariate analysis also showed that pre-existing asthma (OR 1.48, 95%CI 1.03–2.13; p<0.05), hypertension (OR 1.46, 95%CI 1.05–2.04; p<0.05), AF (OR 2.38, 95%CI 1.06–5.38; p<0.05) and CHF (OR 3.06, 95%CI 1.26–7.47; p=0.002) were risk factors for the development of persistent COVID-19 (Table 2).
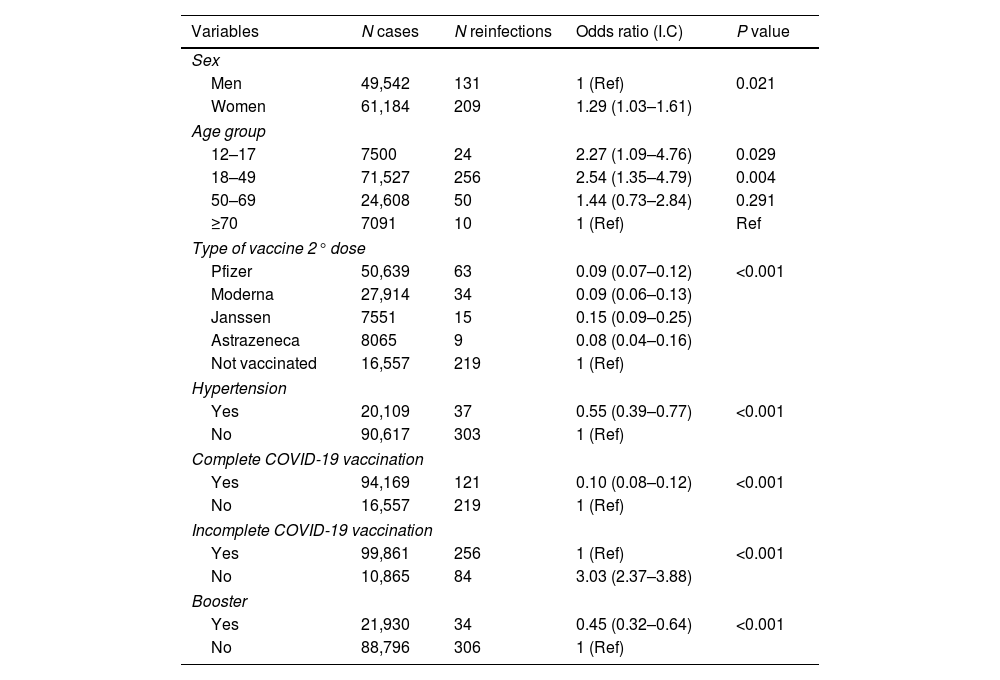
Bivariate analysis. Associations in the 340 patients with reinfection in the 110,726 patient population.
| Variables | N cases | N reinfections | Odds ratio (I.C) | P value |
|---|---|---|---|---|
| Sex | ||||
| Men | 49,542 | 131 | 1 (Ref) | 0.021 |
| Women | 61,184 | 209 | 1.29 (1.03–1.61) | |
| Age group | ||||
| 12–17 | 7500 | 24 | 2.27 (1.09–4.76) | 0.029 |
| 18–49 | 71,527 | 256 | 2.54 (1.35–4.79) | 0.004 |
| 50–69 | 24,608 | 50 | 1.44 (0.73–2.84) | 0.291 |
| ≥70 | 7091 | 10 | 1 (Ref) | Ref |
| Type of vaccine 2° dose | ||||
| Pfizer | 50,639 | 63 | 0.09 (0.07–0.12) | <0.001 |
| Moderna | 27,914 | 34 | 0.09 (0.06–0.13) | |
| Janssen | 7551 | 15 | 0.15 (0.09–0.25) | |
| Astrazeneca | 8065 | 9 | 0.08 (0.04–0.16) | |
| Not vaccinated | 16,557 | 219 | 1 (Ref) | |
| Hypertension | ||||
| Yes | 20,109 | 37 | 0.55 (0.39–0.77) | <0.001 |
| No | 90,617 | 303 | 1 (Ref) | |
| Complete COVID-19 vaccination | ||||
| Yes | 94,169 | 121 | 0.10 (0.08–0.12) | <0.001 |
| No | 16,557 | 219 | 1 (Ref) | |
| Incomplete COVID-19 vaccination | ||||
| Yes | 99,861 | 256 | 1 (Ref) | <0.001 |
| No | 10,865 | 84 | 3.03 (2.37–3.88) | |
| Booster | ||||
| Yes | 21,930 | 34 | 0.45 (0.32–0.64) | <0.001 |
| No | 88,796 | 306 | 1 (Ref) | |
Bivariate analysis. Associations in the 188 cases of persistent COVID-19 in the 110,726 patient population.
| Variables | N cases | N persistent Covid-19 | Odds ratio (I.C.) | P value |
|---|---|---|---|---|
| Sex | ||||
| Men | 49,542 | 61 | 1 (Ref) | <0.001 |
| Women | 61,184 | 127 | 1.69 (1.24–2.29) | |
| Age group | ||||
| 12–17 | 7500 | 15 | 0.64 (0.33–1.24) | 0.189 |
| 18–49 | 71,527 | 99 | 0.45 (0.28–0.71) | <0.001 |
| 50–69 | 24,608 | 52 | 0.68 (0.41–1.12) | 0.131 |
| ≥70 | 7091 | 22 | 1 (Ref) | Ref |
| Type of vaccine 2° dose | ||||
| Pfizer | 50,639 | 47 | 0.18 (0.13–0.25) | <0.001 |
| Moderna | 27914 | 32 | 0.22 (0.15–0.33) | |
| Janssen | 7551 | 12 | 0.31 (0.17–0.56) | |
| Astrazeneca | 8065 | 11 | 0.26 (0.14–0.49) | |
| Not vaccinated | 16,557 | 86 | 1 (Ref) | |
| Atrial fibrillation | ||||
| Yes | 1515 | 6 | 2.38 (1.06–5.38) | 0.031 |
| No | 109,211 | 182 | 1 (Ref) | |
| Hypertension | ||||
| Yes | 20,109 | 46 | 1.46 (1.05–2.04) | 0.025 |
| No | 90,617 | 142 | 1 (Ref) | |
| Asthma | ||||
| Yes | 15,259 | 36 | 1.48 (1.03–2.13) | 0.034 |
| No | 95,279 | 152 | 1 (Ref) | |
| Congestive heart failure | ||||
| Yes | 977 | 5 | 3.06 (1.26–7.47) | 0.009 |
| No | 109,561 | 183 | 1 (Ref) | |
| Complete vaccination | ||||
| Yes | 94,169 | 102 | 0.21 (0.16–0.28) | <0.001 |
| No | 16,557 | 86 | 1 (Ref) | |
Most subjects in the sample (94,169; 85%) had received complete COVID-19 vaccination by the time of the study. The percentage of subjects with at least incomplete vaccination was higher (99,861; 90.2%). However, the percentage of patients who had received a booster dose was not so high (21,930; 19.8%). Pfizer was the most frequently applied vaccine as a first dose (62,714; 56.6%), followed by Moderna 20,972 (18.9%), AstraZeneca 8626 (7.8%) and Janssen 7549 (6.8%). For the second dose, Pfizer was again the most frequently used vaccine 50,639 (45.7%), followed by Moderna 27,914 (25.2%), AstraZeneca 8065 (7.3%) and subjects without a second dose 16,557 (15%). The third dose was mostly Moderna 11,269 (10.2%) followed by Pfizer 10,661 (9.6%); 88,796 subjects (80.2%) had not received a booster dose. A total of 10,865 (9.8%) patients had not received any dose of any vaccine.
Complete vaccination was associated with a lower risk of reinfection ([OR] 0.05, 95%CI 0.04–0.07, p<0.05) or persistent COVID-19 ([OR] 0.07, 95%CI 0.05–0.10, p<0.05). None of the patients with reinfection or persistent COVID-19 died during the period of the study.
Diabetes, coronary heart disease, atrial fibrillation, chronic obstructive pulmonary disease, immunosuppressive treatment, asthma, congestive heart failure, cancer and obesity did not show a statistically significant association related to reinfection (p<0.005).
Diabetes, coronary heart disease, atrial fibrillation, chronic obstructive pulmonary disease, immunosuppressive treatment, asthma, congestive heart failure, cancer, obesity, incomplete vaccination and booster did not show a statistically significant association related to persistent COVID-19 (p<0.005).
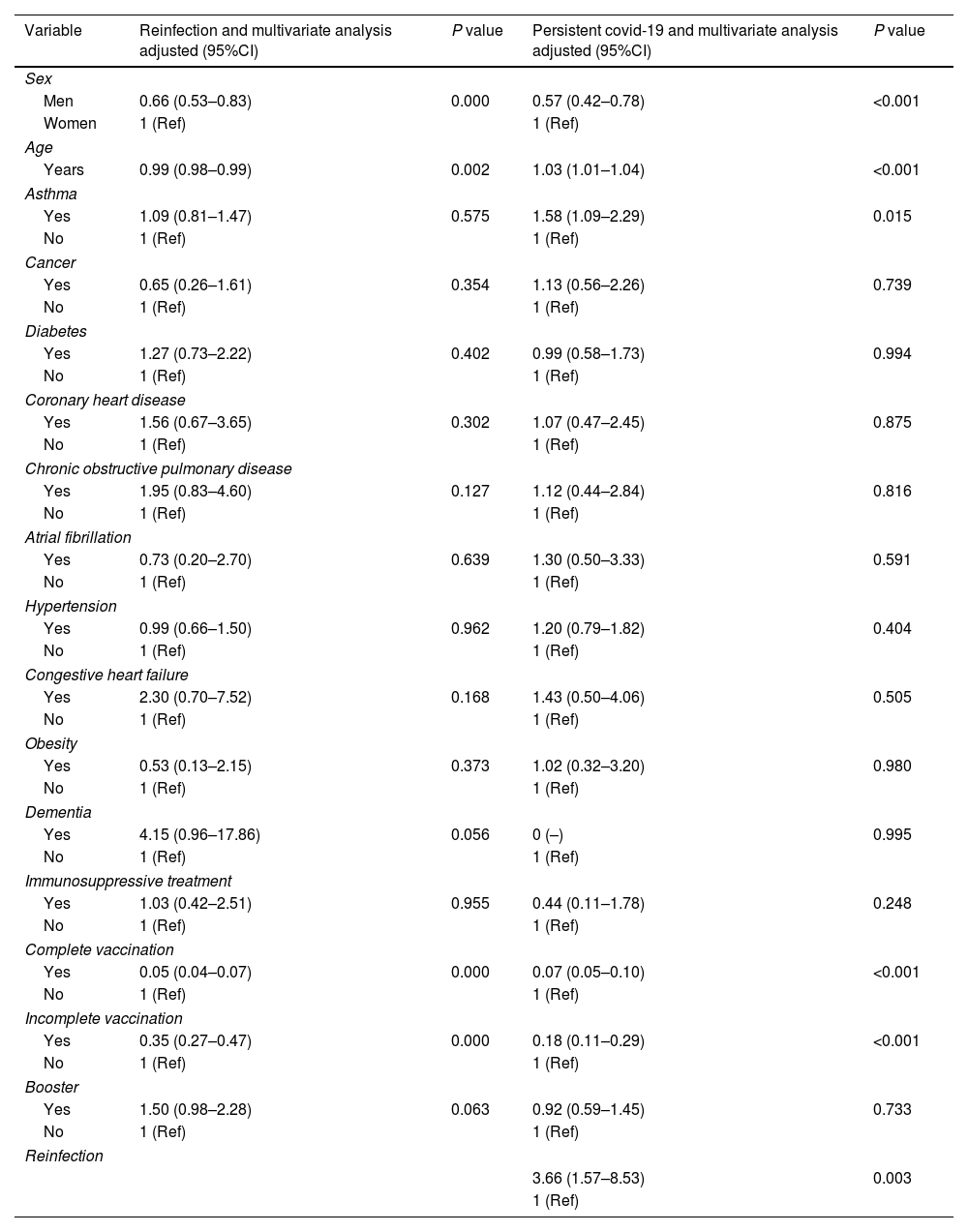
The combination of advanced age, female sex and lack of complete or incomplete COVID-19 vaccination was strongly predictive of reinfection (p<0.05) in the multivariate analysis (Table 3). The combination of adult age, female sex, diagnosed asthma, reinfection and lack of complete or incomplete COVID-19 vaccination was strongly predictive of persistent COVID-19 (p<0.05) in the multivariate analysis (Table 3).
Reinfection and persistent COVID-19 according to gender, age and association with obesity, diabetes, hypertension, cancer, coronary heart disease, COPD, CHF and dementia in the 110,726 inpatients positive for SARS-CoV-2.
| Variable | Reinfection and multivariate analysis adjusted (95%CI) | P value | Persistent covid-19 and multivariate analysis adjusted (95%CI) | P value |
|---|---|---|---|---|
| Sex | ||||
| Men | 0.66 (0.53–0.83) | 0.000 | 0.57 (0.42–0.78) | <0.001 |
| Women | 1 (Ref) | 1 (Ref) | ||
| Age | ||||
| Years | 0.99 (0.98–0.99) | 0.002 | 1.03 (1.01–1.04) | <0.001 |
| Asthma | ||||
| Yes | 1.09 (0.81–1.47) | 0.575 | 1.58 (1.09–2.29) | 0.015 |
| No | 1 (Ref) | 1 (Ref) | ||
| Cancer | ||||
| Yes | 0.65 (0.26–1.61) | 0.354 | 1.13 (0.56–2.26) | 0.739 |
| No | 1 (Ref) | 1 (Ref) | ||
| Diabetes | ||||
| Yes | 1.27 (0.73–2.22) | 0.402 | 0.99 (0.58–1.73) | 0.994 |
| No | 1 (Ref) | 1 (Ref) | ||
| Coronary heart disease | ||||
| Yes | 1.56 (0.67–3.65) | 0.302 | 1.07 (0.47–2.45) | 0.875 |
| No | 1 (Ref) | 1 (Ref) | ||
| Chronic obstructive pulmonary disease | ||||
| Yes | 1.95 (0.83–4.60) | 0.127 | 1.12 (0.44–2.84) | 0.816 |
| No | 1 (Ref) | 1 (Ref) | ||
| Atrial fibrillation | ||||
| Yes | 0.73 (0.20–2.70) | 0.639 | 1.30 (0.50–3.33) | 0.591 |
| No | 1 (Ref) | 1 (Ref) | ||
| Hypertension | ||||
| Yes | 0.99 (0.66–1.50) | 0.962 | 1.20 (0.79–1.82) | 0.404 |
| No | 1 (Ref) | 1 (Ref) | ||
| Congestive heart failure | ||||
| Yes | 2.30 (0.70–7.52) | 0.168 | 1.43 (0.50–4.06) | 0.505 |
| No | 1 (Ref) | 1 (Ref) | ||
| Obesity | ||||
| Yes | 0.53 (0.13–2.15) | 0.373 | 1.02 (0.32–3.20) | 0.980 |
| No | 1 (Ref) | 1 (Ref) | ||
| Dementia | ||||
| Yes | 4.15 (0.96–17.86) | 0.056 | 0 (–) | 0.995 |
| No | 1 (Ref) | 1 (Ref) | ||
| Immunosuppressive treatment | ||||
| Yes | 1.03 (0.42–2.51) | 0.955 | 0.44 (0.11–1.78) | 0.248 |
| No | 1 (Ref) | 1 (Ref) | ||
| Complete vaccination | ||||
| Yes | 0.05 (0.04–0.07) | 0.000 | 0.07 (0.05–0.10) | <0.001 |
| No | 1 (Ref) | 1 (Ref) | ||
| Incomplete vaccination | ||||
| Yes | 0.35 (0.27–0.47) | 0.000 | 0.18 (0.11–0.29) | <0.001 |
| No | 1 (Ref) | 1 (Ref) | ||
| Booster | ||||
| Yes | 1.50 (0.98–2.28) | 0.063 | 0.92 (0.59–1.45) | 0.733 |
| No | 1 (Ref) | 1 (Ref) | ||
| Reinfection | ||||
| 3.66 (1.57–8.53) | 0.003 | |||
| 1 (Ref) | ||||
This is the study with the largest number of subjects up to date, that describes the clinical and epidemiological characteristics of patients diagnosed with persistent COVID-19 or COVID-19 reinfection in Gran Canaria. The data, corresponding to the last 9 months (June 2021–February 2022) illustrate the new reality of the disease in a population with high vaccination rates.
Age and comorbidities were strong predictors of persistent COVID-19 and, to a lesser extent, of COVID-19 reinfection. Female sex was associated to a higher risk of persistent
COVID-19 (OR 1.69, 95%IC 1.24–2.29; p<0.05). These findings are in agreement with Asadi et al. (OR 1.42, 95%CI 1.10–1.90; p<0.02).19,20
Besides the above demographic factors, suffering from asthma was significantly associated with persistent COVID-19 diagnosis (OR 1.58, 95%CI 1.09–2.29; p<0.05). These findings are in agreement with Loosen et al., who also identified asthma as a risk factor for the development of persistent COVID-19 (OR 1.33, 95%IC 1.20–1.47; p<0.001).21
A study conducted in the United Kingdom showed that the probability of presenting symptoms beyond day 28 post-infection was reduced by 50% in subjects with complete vaccination. Similarly, complete vaccination reduced the probability of suffering more than five symptoms in the first week post-infection by 31% and the probability of hospitalization by 71%, which resulted in a decreased probability of persistent COVID-19 since both risk factors for its development were reduced.22,23
These findings suggest that complete vaccination provides protection against reinfection in subjects with a history of SARS-CoV-2 infection. In previously infected people in Gran Canaria, the probability of reinfection was ten times higher in those who were not vaccinated than in those with complete vaccination.
All eligible subjects should be offered vaccination, including those with a prior SARS-CoV-2 infection, so as to reduce their risk of future infection.7 Furthermore, the likelihood of reinfection should be evaluated in patients who are on immunosuppressive therapy.
Limitations. First, reinfection was not confirmed through genome sequencing, which is necessary to demonstrate that reinfection was caused by a virus variant different from that causing the first infection. Although positive diagnostic test results were repeatedly obtained, this could indicate prolonged viral dissemination or failure to clear the initial viral infection.24 Given the time elapsed between the initial and the subsequent positive molecular tests, reinfection was the most probable cause for such results. Second, the definition of reinfection has been interpreted differently in different studies. Finally, this was a retrospective study, based on data from a single population, recorded over a 9-month period; thus, the results cannot be used to infer causality. Further prospective studies with larger populations are needed to support these findings.
The main strength of this research was its large sample size, with a number of participants much higher than most of the studies on the subject conducted in Spain.
In conclusion, this study confirmed a link between age, sex, asthma, reinfection and vaccination and the risk of persistent COVID-19. Patients’ comorbidities were not demonstrated to be a factor influencing the occurrence of reinfection. However, COVID-19 reinfection was found to be associated with age, sex and vaccination. Vaccination against COVID-19 may help control the pandemic. Two doses of the SARS-CoV-2 vaccine were highly effective in preventing COVID-19 reinfection in all age groups. More complete vaccination was associated with lower risk of persistent COVID-19. These findings suggest that COVID-19 vaccination can help in bringing the pandemic under control.
Authors’ contributionAll the authors participated in the design of the study, data collection and preparation of the manuscript, and they declare that they approve its final version and are publicly responsible for its content.
Informed consent statementThis study was approved by the Ethics Committee for Research of the University Hospital of Gran Canaria Dr. Negrín (registration number 2021-355-1 COVID19) and it was compliant with the local laws and regulations, the Declaration of Helsinki, and the Good Clinical Practices. Patient consent was waived due to anonymization/dissociation of patient data and the results did not affect the clinical management of patients.
Ethical aspectsThe confidentiality of the data is guaranteed. Informed consent of the patients is not required due to the anonymization/dissociation of patient data and the results did not affect the clinical management of the patients.
Data availability statementThe data are not publicly available due to privacy or ethical reasons. Data are available from the management of Primary Care of Gran Canaria, Spain, for researchers who meet the criteria for access to confidential data.
Sources of supportWe would like to thank the following entities for collaboration and funding: Fundación Española de Calidad Asistencial (G74295718), without whose contribution this study would not have been carried out.
Conflicts of interestThe authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.