The nirmatrelvir/ritonavir is an oral combination of antiviral drugs used to treat the COVID-19. In this study we evaluated the risk of hospitalization and death, comparing COVID-19 patients who received and did not receive ambulatory nirmatrelvir/ritonavir prescription.
Material and methodsA two-group comparative study was carried out using data from the Mexican Institute of Social Security medical information systems. We analyzed of 83,348 ambulatory patients aged 18 years old and over and with laboratory confirmed SARS-CoV-2 infection during the period from September 12th, 2022, to September 30th, 2023. Of them, 27,437 received nirmatrelvir/ritonavir prescription (32.9%) and 55,911 did not (67.1%). We compared the 60-day COVID-19 related hospitalization and all-cause death risk among groups using a multivariate Poisson regression model.
ResultsThe 60-day COVID-19 related hospitalization and all-cause death combined incidence was 0.13% in the patients who received nirmatrelvir/ritonavir and 0.26% among the cases who did not receive the prescription. In the multivariate model, after adjusting for age, sex, and previous medical conditions such as chronic kidney disease, chronic obstructive pulmonary disease and cardiovascular disease, the nirmatrelvir/ritonavir prescription was associated with a decrease in 60-day hospitalization and death, with an adjusted risk ratio of 0.52 (95% confidence interval from 0.36 to 0.75).
ConclusionsOur data supports that nirmatrelvir/ritonavir prescription is associated with a risk reduction of 60-day COVID-19 related hospitalization and death, in outpatients with COVID-19.
La combinación oral de fármacos antivirales nirmatrelvir/ritonavir se utiliza para tratar la COVID-19. El objetivo de este estudio fue evaluar el riesgo de hospitalización y muerte, comparando pacientes con COVID-19 que recibieron y no recibieron prescripción ambulatoria de nirmatrelvir/ritonavir.
Material y métodosSe realizó un estudio comparativo de 2 grupos utilizando los datos de los sistemas de información médica del Instituto Mexicano del Seguro Social. Se analizaron 83.348 pacientes ambulatorios de 18 años o más con infección por SARS-CoV-2 confirmada por laboratorio durante el período comprendido entre el 12 de septiembre de 2022 al 30 de septiembre de 2023. De los sujetos estudiados 27.437 recibieron prescripción de nirmatrelvir/ritonavir (32,9%) y 55.911 no (67,1%). Mediante un modelo de regresión de Poisson multivariante se evaluó el riesgo de hospitalización relacionada con la COVID-19 y muerte por cualquier causa a los 60 días entre los grupos.
ResultadosLa incidencia combinada de hospitalización relacionada con la COVID-19 y muerte por cualquier causa a los 60 días fue del 0,13% en los pacientes que recibieron nirmatrelvir/ritonavir y del 0,26% entre los casos que no recibieron la prescripción. En el modelo multivariante, después de ajustar por edad, sexo y condiciones médicas previas como enfermedad renal crónica, enfermedad pulmonar obstructiva crónica y enfermedad cardiovascular, la prescripción de nirmatrelvir/ritonavir se asoció con una disminución en la hospitalización y muerte a 60 días, con un cociente de riesgo ajustado de 0,52 (intervalo de confianza del 95% de 0,36 a 0,75).
ConclusionesNuestros datos respaldan que la prescripción de nirmatrelvir/ritonavir se asocia con una reducción del riesgo de hospitalización y muerte relacionada con la COVID-19 a 60 días en pacientes ambulatorios con dicha enfermedad.
The nirmatrelvir/ritonavir is an oral combination of antiviral drugs used to treat the SARS-CoV-2 infection.1 In the one hand nirmatrelvir blocks the activity of the viral replication enzyme SARS-CoV-2-3CLpro protease, while on the other hand, ritonavir is a CYP3A4 inhibitor that is co-administered at low dose to slow down the nirmatrelvir degradation.2,3
Currently, international guidelines recommend the use of nirmatrelvir/ritonavir as treatment for adult outpatients with mild to moderate COVID-19 at high risk of disease progression.4–6 In Mexico, the nirmatrelvir/ritonavir approbation by Federal Agency took place during 20227 and their prescription at the Mexican Institute of Social Security (IMSS) started during the last trimester of that year. The IMSS is the main health institution in Mexico who provides health care, economic assistance, and social services to about 74 million affiliates, that is about 58% of the Mexican population.8
Many studies have shown that nirmatrelvir/ritonavir is associated with lower frequency of hospitalization and death.9–11 However, in our knowledge, there is no previous report of this association in Mexico. Furthermore, the conduction of observational studies also allows to explore the use of nirmatrelvir–ritonavir and their outcomes in real-world situations. So, in this study, we aimed to compare the risk of hospitalization and death in ambulatory patients who received and did not receive nirmatrelvir/ritonavir prescription at the IMSS.
Materials and methodsStudy design and populationA two-group comparative cohort retrospective study was carried out using the national-wide data from the IMSS medical information systems. Our secondary analysis was based in the use of data from the Online Epidemiological Surveillance System (SINOLAVE) and from the Institutional Supply System (SAI). In the one hand, the SINOLAVE collects information regarding COVID-19 cases, underlying medical conditions, COVID-19 vaccination, and laboratory test results. On the other hand, SAI contains data from medical prescriptions including nirmatrelvir/ritonavir. Both systems are used in all the IMSS health care facilities encompassed across the Mexican territory.
For each subject we acquired information regarding sex, age, medical previous conditions (such as obesity, diabetes, and hypertension among others) and occupation from SINOLAVE. We considered three geographical regions according where the first attention took place in SINOLAVE (Supplementary Fig. 1). Data on emergency room attention, hospitalization and death were complemented with information from Virtual Center in Emergencies and Disasters (CVOED) platform and from the Hospital Discharges System (SUI13). The CVOED system data derived from the COVID-19 IMSS hospitals while SUI13 included information from the 251 second care level and 25 tertiary health care hospitals.
We selected ambulatory patients aged 18 years old and over, with laboratory confirmed SARS-CoV-2 by polymerase chain reaction or rapid antigen test and that were attended at primary health care facilities during the period from September 12th, 2022, to September 30th, 2023. Subjects without indication for receiving nirmatrelvir/ritonavir and those with nirmatrelvir/ritonavir prescription after 5 days from starting symptoms were excluded. Thereby, we analyzed a total of 83,348 ambulatory patients, of which, 27,437 (32.9%) received nirmatrelvir/ritonavir prescription and 55,911 (67.1%) did not.
The onset of symptoms was considered as the study starting time and the analyzed three main outcomes were the following: (1) COVID-19 related hospitalization at 60 days, (2) all-cause death at 60 days and (3) COVID-19 related hospitalization and all-cause death at 60 days. Follow-up was computed from symptoms onset until 60 days or until the above-mentioned outcomes occurrence, whichever occurred first.
Statistical analysisTo compare the patient's characteristics among nirmatrelvir/ritonavir prescription groups, we estimated proportion differences with 95% confidence intervals (95% CI).12 After that, we calculated the incidence of the three previously mentioned main outcomes.
In the bivariate analysis, crude relative risk (RR) with 95% CI were estimated to evaluate the association between patient's variables and the above-mentioned outcomes. In this step, Kaplan–Meier failure curves were also constructed, and log-rank test p values were calculated. Finally, we conducted multivariate Poisson regression models to adjust the RR of nirmatrelvir/ritonavir prescription with COVID-19 related hospitalization and all-cause death at 60 days. In these models only variables that were significant in the comparative or in the bivariate analysis were included.
Statistical analysis was carried out using Stata version 14.
Ethical statementThe procedures were followed according to the national and institutional laws, to ethical the standards and to the 1964 Helsinki declaration and its later amendments.
In this study patients were not involved, so neither personal patient follow-up nor subject interviews were performed. Given that the research was based only in the use of anonymized data from institutional medical information systems, this research was determined as exempt of reviewing by the Institutional Review Board and formal informed consent was not required.
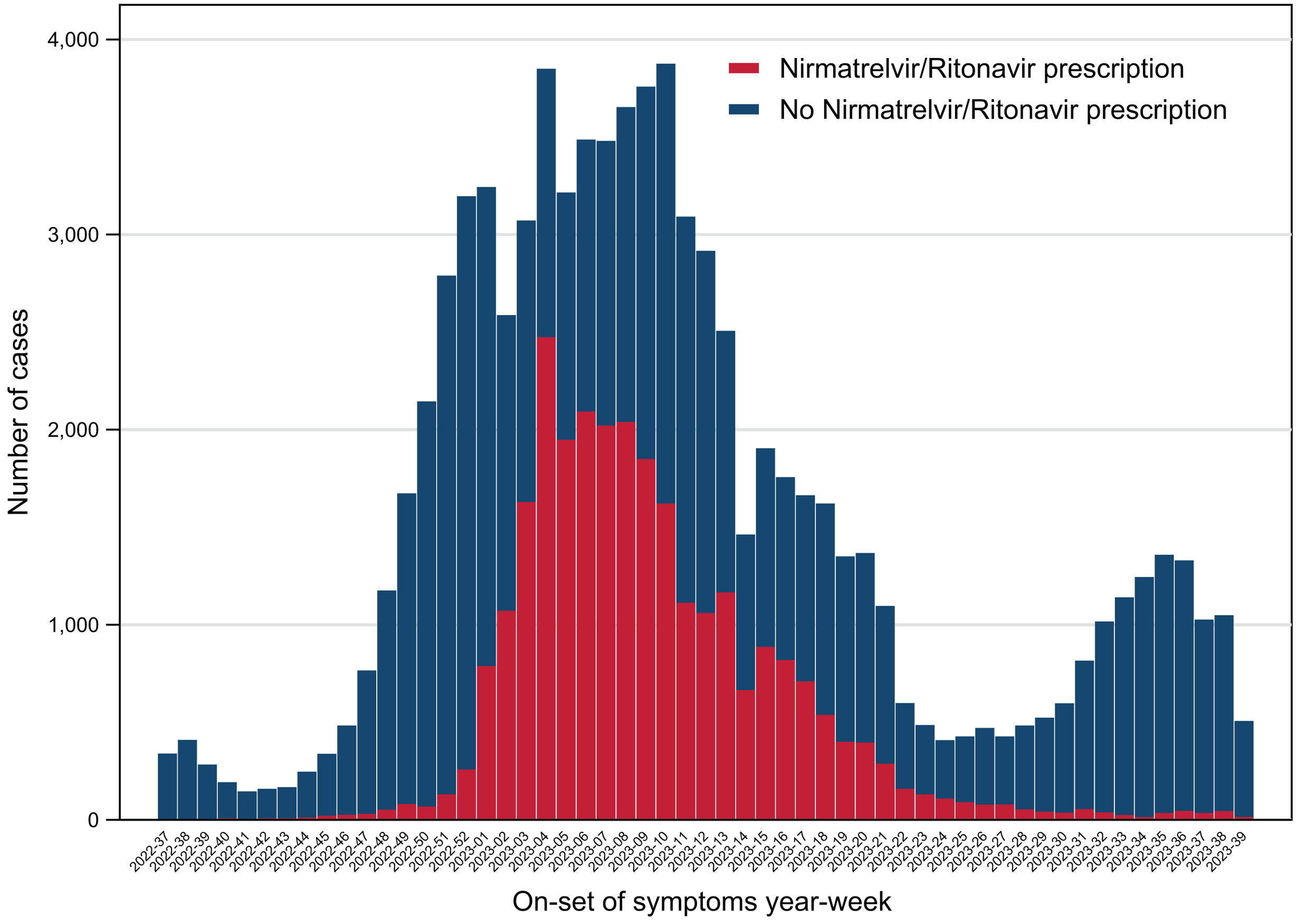
ResultsAs previously described, we analyzed a total of 83,348 ambulatory patients during the period from September 12th, 2022, to September 30th, 2023. Of them, 27,437 (32.9%) received nirmatrelvir/ritonavir prescription (32.9%) and 55,911 did not (67.1%). Most of the cases occurred during the winter season at the end of 2022 and the beginning of 2023 (Fig. 1).
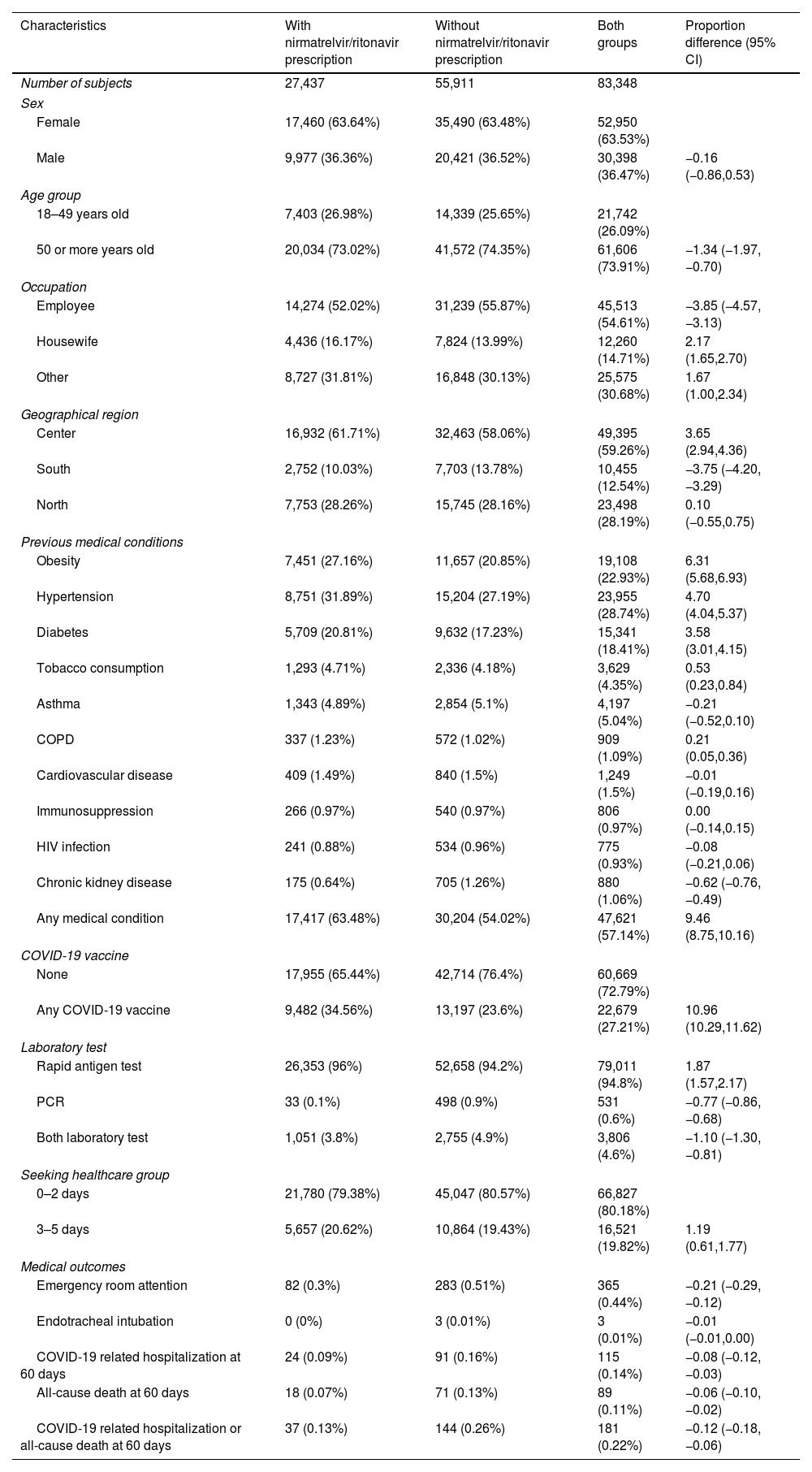
When we compared study groups, nirmatrelvir/ritonavir prescription subjects showed higher proportion of COVID-19 vaccination and previous medical conditions such as obesity, hypertension, diabetes, tobacco consumption and chronic obstructive pulmonary disease. The frequency emergency room attention and the three main outcomes were lower in the nirmatrelvir/ritonavir prescription group. The 60-day COVID-19 related hospitalization and all-cause death combined incidence was 0.13% in the patients who received nirmatrelvir/ritonavir and 0.26% among the cases who did not receive the prescription (Table 1).
Characteristics of 83,348 COVID-19 patients, according to the nirmatrelvir/ritonavir prescription groups.
| Characteristics | With nirmatrelvir/ritonavir prescription | Without nirmatrelvir/ritonavir prescription | Both groups | Proportion difference (95% CI) |
|---|---|---|---|---|
| Number of subjects | 27,437 | 55,911 | 83,348 | |
| Sex | ||||
| Female | 17,460 (63.64%) | 35,490 (63.48%) | 52,950 (63.53%) | |
| Male | 9,977 (36.36%) | 20,421 (36.52%) | 30,398 (36.47%) | −0.16 (−0.86,0.53) |
| Age group | ||||
| 18–49 years old | 7,403 (26.98%) | 14,339 (25.65%) | 21,742 (26.09%) | |
| 50 or more years old | 20,034 (73.02%) | 41,572 (74.35%) | 61,606 (73.91%) | −1.34 (−1.97,−0.70) |
| Occupation | ||||
| Employee | 14,274 (52.02%) | 31,239 (55.87%) | 45,513 (54.61%) | −3.85 (−4.57,−3.13) |
| Housewife | 4,436 (16.17%) | 7,824 (13.99%) | 12,260 (14.71%) | 2.17 (1.65,2.70) |
| Other | 8,727 (31.81%) | 16,848 (30.13%) | 25,575 (30.68%) | 1.67 (1.00,2.34) |
| Geographical region | ||||
| Center | 16,932 (61.71%) | 32,463 (58.06%) | 49,395 (59.26%) | 3.65 (2.94,4.36) |
| South | 2,752 (10.03%) | 7,703 (13.78%) | 10,455 (12.54%) | −3.75 (−4.20,−3.29) |
| North | 7,753 (28.26%) | 15,745 (28.16%) | 23,498 (28.19%) | 0.10 (−0.55,0.75) |
| Previous medical conditions | ||||
| Obesity | 7,451 (27.16%) | 11,657 (20.85%) | 19,108 (22.93%) | 6.31 (5.68,6.93) |
| Hypertension | 8,751 (31.89%) | 15,204 (27.19%) | 23,955 (28.74%) | 4.70 (4.04,5.37) |
| Diabetes | 5,709 (20.81%) | 9,632 (17.23%) | 15,341 (18.41%) | 3.58 (3.01,4.15) |
| Tobacco consumption | 1,293 (4.71%) | 2,336 (4.18%) | 3,629 (4.35%) | 0.53 (0.23,0.84) |
| Asthma | 1,343 (4.89%) | 2,854 (5.1%) | 4,197 (5.04%) | −0.21 (−0.52,0.10) |
| COPD | 337 (1.23%) | 572 (1.02%) | 909 (1.09%) | 0.21 (0.05,0.36) |
| Cardiovascular disease | 409 (1.49%) | 840 (1.5%) | 1,249 (1.5%) | −0.01 (−0.19,0.16) |
| Immunosuppression | 266 (0.97%) | 540 (0.97%) | 806 (0.97%) | 0.00 (−0.14,0.15) |
| HIV infection | 241 (0.88%) | 534 (0.96%) | 775 (0.93%) | −0.08 (−0.21,0.06) |
| Chronic kidney disease | 175 (0.64%) | 705 (1.26%) | 880 (1.06%) | −0.62 (−0.76,−0.49) |
| Any medical condition | 17,417 (63.48%) | 30,204 (54.02%) | 47,621 (57.14%) | 9.46 (8.75,10.16) |
| COVID-19 vaccine | ||||
| None | 17,955 (65.44%) | 42,714 (76.4%) | 60,669 (72.79%) | |
| Any COVID-19 vaccine | 9,482 (34.56%) | 13,197 (23.6%) | 22,679 (27.21%) | 10.96 (10.29,11.62) |
| Laboratory test | ||||
| Rapid antigen test | 26,353 (96%) | 52,658 (94.2%) | 79,011 (94.8%) | 1.87 (1.57,2.17) |
| PCR | 33 (0.1%) | 498 (0.9%) | 531 (0.6%) | −0.77 (−0.86,−0.68) |
| Both laboratory test | 1,051 (3.8%) | 2,755 (4.9%) | 3,806 (4.6%) | −1.10 (−1.30,−0.81) |
| Seeking healthcare group | ||||
| 0–2 days | 21,780 (79.38%) | 45,047 (80.57%) | 66,827 (80.18%) | |
| 3–5 days | 5,657 (20.62%) | 10,864 (19.43%) | 16,521 (19.82%) | 1.19 (0.61,1.77) |
| Medical outcomes | ||||
| Emergency room attention | 82 (0.3%) | 283 (0.51%) | 365 (0.44%) | −0.21 (−0.29,−0.12) |
| Endotracheal intubation | 0 (0%) | 3 (0.01%) | 3 (0.01%) | −0.01 (−0.01,0.00) |
| COVID-19 related hospitalization at 60 days | 24 (0.09%) | 91 (0.16%) | 115 (0.14%) | −0.08 (−0.12,−0.03) |
| All-cause death at 60 days | 18 (0.07%) | 71 (0.13%) | 89 (0.11%) | −0.06 (−0.10,−0.02) |
| COVID-19 related hospitalization or all-cause death at 60 days | 37 (0.13%) | 144 (0.26%) | 181 (0.22%) | −0.12 (−0.18,−0.06) |
Data are presented as number (%).
Proportion differences among patients with and without nirmatrelvir/ritonavir prescription.
Abbreviations: CI, confidence interval; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; PCR, polymerase chain reaction.
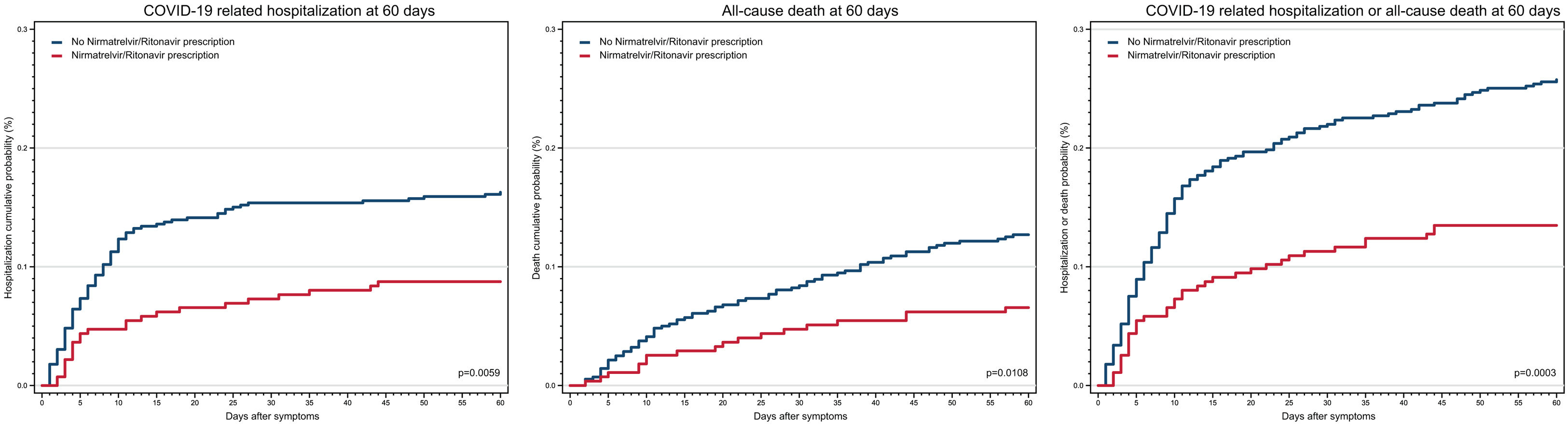
In the bivariate step analysis, nirmatrelvir/ritonavir prescription showed lower risk for the three outcomes (Fig. 2). Additionally, chronic obstructive pulmonary disease, chronic kidney disease, cardiovascular disease, and age 50 years old and over were the most important variables associated with 60-day COVID-19 related hospitalization and all-cause death, while obesity was inversely associated with 60-day COVID-19 related hospitalization alone and combined with all-cause death outcomes. Only asthma, immunosuppression and HIV infection were no statistical associated during this examination (Supplementary Table 1 and Supplementary Fig. 2).
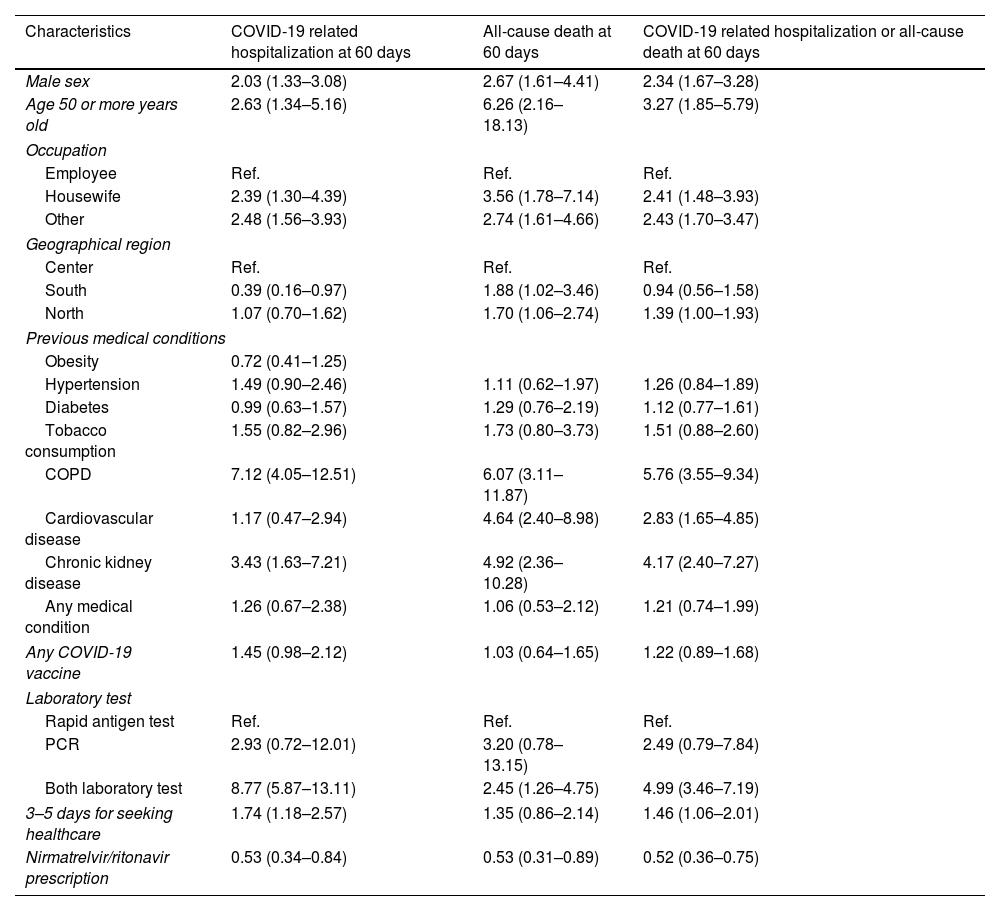
In the final analysis, Poisson regression models were computed to adjust the RR of nirmatrelvir/ritonavir prescription by relevant variables. After adjusting for age, sex, occupation, geographical region, previous medical conditions, COVID-19 vaccination, laboratory test and days for seeking healthcare, nirmatrelvir/ritonavir prescription was associated with a decrease in 60-day COVID-19 related hospitalization (adjusted RR 0.53, 95% CI 0.34–0.84), 60-day all-cause death (adjusted RR 0.53, 95% CI 0.31–0.89) and 60-day COVID-19 related hospitalization/all-cause death combined (adjusted RR 0.52, 95% CI 0.36–0.75) (Table 2).
Association between the patient's characteristics and COVID-19 related hospitalization and all-cause death at 60 days. Adjusted relative risks in 83,348 COVID-19 patients.
| Characteristics | COVID-19 related hospitalization at 60 days | All-cause death at 60 days | COVID-19 related hospitalization or all-cause death at 60 days |
|---|---|---|---|
| Male sex | 2.03 (1.33–3.08) | 2.67 (1.61–4.41) | 2.34 (1.67–3.28) |
| Age 50 or more years old | 2.63 (1.34–5.16) | 6.26 (2.16–18.13) | 3.27 (1.85–5.79) |
| Occupation | |||
| Employee | Ref. | Ref. | Ref. |
| Housewife | 2.39 (1.30–4.39) | 3.56 (1.78–7.14) | 2.41 (1.48–3.93) |
| Other | 2.48 (1.56–3.93) | 2.74 (1.61–4.66) | 2.43 (1.70–3.47) |
| Geographical region | |||
| Center | Ref. | Ref. | Ref. |
| South | 0.39 (0.16–0.97) | 1.88 (1.02–3.46) | 0.94 (0.56–1.58) |
| North | 1.07 (0.70–1.62) | 1.70 (1.06–2.74) | 1.39 (1.00–1.93) |
| Previous medical conditions | |||
| Obesity | 0.72 (0.41–1.25) | ||
| Hypertension | 1.49 (0.90–2.46) | 1.11 (0.62–1.97) | 1.26 (0.84–1.89) |
| Diabetes | 0.99 (0.63–1.57) | 1.29 (0.76–2.19) | 1.12 (0.77–1.61) |
| Tobacco consumption | 1.55 (0.82–2.96) | 1.73 (0.80–3.73) | 1.51 (0.88–2.60) |
| COPD | 7.12 (4.05–12.51) | 6.07 (3.11–11.87) | 5.76 (3.55–9.34) |
| Cardiovascular disease | 1.17 (0.47–2.94) | 4.64 (2.40–8.98) | 2.83 (1.65–4.85) |
| Chronic kidney disease | 3.43 (1.63–7.21) | 4.92 (2.36–10.28) | 4.17 (2.40–7.27) |
| Any medical condition | 1.26 (0.67–2.38) | 1.06 (0.53–2.12) | 1.21 (0.74–1.99) |
| Any COVID-19 vaccine | 1.45 (0.98–2.12) | 1.03 (0.64–1.65) | 1.22 (0.89–1.68) |
| Laboratory test | |||
| Rapid antigen test | Ref. | Ref. | Ref. |
| PCR | 2.93 (0.72–12.01) | 3.20 (0.78–13.15) | 2.49 (0.79–7.84) |
| Both laboratory test | 8.77 (5.87–13.11) | 2.45 (1.26–4.75) | 4.99 (3.46–7.19) |
| 3–5 days for seeking healthcare | 1.74 (1.18–2.57) | 1.35 (0.86–2.14) | 1.46 (1.06–2.01) |
| Nirmatrelvir/ritonavir prescription | 0.53 (0.34–0.84) | 0.53 (0.31–0.89) | 0.52 (0.36–0.75) |
Adjusted relative risk and 95% confidence interval are shown.
Results of a multivariate Poisson regression model.
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; Ref., reference group.
In this study, we conducted a secondary database analysis in 83,348 COVID-19 ambulatory patients and we found that nirmatrelvir/ritonavir prescription within 5 days from starting symptoms was associated with a reduction in COVID-19 related hospitalizations and all-cause death.
Our results are in line with reports from previous observational studies. Our association between nirmatrelvir/ritonavir and hospitalization-death combined (RR 0.52) were very similar to those stated by Dryden-Peterson13 and Schwartz14 (RR 0.56). However, given that those two studies included only subject aged 50 years old and older, lower proportion of unvaccinated patients and higher proportions of previous medical conditions and main outcomes were observed when compared with our results. It is important to note that our sample size for nirmatrelvir/ritonavir was the biggest in this comparison (n=8876 for Schwartz, n=12,450 for Dryden-Peterson and n=27,450 for this study).
In several studies conducted by Wai in Hong Kong,15 by Evans in Wales16 and by Lewnard in Unites States,17 a decrease in hospitalization and death was observed in outpatients receiving nirmatrelvir/ritonavir. Compared with our results, the observed associations in those studies were significant lower (RR 0.26 for all-cause hospitalization or death within 28 days in Evans study; RR 0.19 for death in Wai study; and 53 6% effectiveness for all-cause hospital admission or death within 30 days of positive SARS-CoV-2 test in the Lewnard study). While our study had similar age distribution among comparison groups, the higher age in control group and consequently the higher incidence in outcomes could explain the differences in the observed RR for Wai and Evans studies. Again, our sample size for nirmatrelvir/ritonavir group was the biggest in this other comparison (n=7247 for Lewnard study, n=4442 for Wai study and n=206 for Evans study).
A risk increase for hospitalization and death among COVID-19 vaccinated subjects was an interesting finding in our study. This association was not significant during the multivariate analysis but was only statistically significant for hospitalization at 60 days in bivariate step. Our relationship was not consistent with other studies that have shown that vaccination has contributed to reducing hospitalizations in COVID-19 patients.18,19 After examining the COVID-19 vaccinated group, we observed higher frequencies of previous medical conditions compared with unvaccinated subjects (Supplementary Table 2). Moreover, we also found that days from last vaccine until the onset of symptoms was very long (median 394 days) (Supplementary Fig. 3). Given that vaccine effectiveness decreased by the time,20 this long time since vaccine together with a more prone to be hospitalized comorbid population in vaccinated group may explain our incidental finding.
Our study has some limitations to be mentioned. First, large institutional databases data, which have been not designed for research purposes, was used to construct our comparative analysis. This situation impeded to obtain events occurred outside our institution and further detailed clinical information such as medication adherence, clinical status, patient quality of life, adverse event information and other medications received as well. Second, we conducted an observational study design which, compared with clinical controlled trials, has lower validity degree. And third, we found imbalances among comparison groups, especially in previous medical conditions. Despite these limitations, the considerable sample size, the country-wide extension, the real-life strategy study, and the multivariate analysis performed are strengths to be noted and that supports our conclusions, highlighting the importance of implementing the treatment with nirmatrelvir/ritonavir in COVID-19 outpatients at high risk of disease progression.
ConclusionsOur data suggest that nirmatrelvir/ritonavir prescription is associated with a risk reduction of 60-day COVID-19 related hospitalization and all-cause death, in adult outpatients with COVID-19 in the context of primary health care settings.
Ethical considerationsThe procedures were followed according to the national and institutional laws, to ethical the standards and to the 1964 Helsinki declaration and its later amendments.
In this study patients were not involved, so neither personal patient follow-up nor subject interviews were performed. Given that the research was based only in the use of anonymized data from institutional medical information systems, this research was determined as exempt of reviewing by the Institutional Review Board and formal informed consent was not required.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestNone declared.
We gratefully acknowledge to Dirección de Administración of the IMSS for their support to this project.