Askin tumor is an uncommon malignant neoplasm of a neuroectodermic origin that arises from the soft tissues of the thoracopulmonary wall. Defined histologically by Askin and Rosai in 1979 as a malignant small round cell tumor. It is described within a group of malignant neoplasms with an aggressive behavior. The lack of clinical guides that establish a standardized management contributes to its poor prognosis and short overall survival. Once a primitive neuroectodermal tumor has been diagnosed, treatment will consist of a multimodal management.
Askin tumor is an uncommon malignant neoplasm of a neuroectodermal origin that arises from the soft tissues of the thoraco-pulmonary wall.1 Histologically designated by Askin and Rosai in 1979 as a malignant small round cell tumor,2 it is described within a group of malignant neoplasms with an aggressive behavior, classified by the World Health Organization (WHO) in 2002 as Ewing's Sarcoma/Peripheral Primitive Neuroectodermal Tumor (SE/PPNETS), organizing tumors of neuroectodermal, bone and soft tissue origin as a single entity. Ewing's sarcoma of the bone, neuroepithelioma, neuroblastoma and peripheral primitive neuroectodermal tumor (PNET), are neoplasms included in this group, and within the PNETs, the Askin tumor.3
According to medical literature, there is a greater incidence in younger patients.1–6 It presents a non-specific clinical behavior, making its precise diagnosis difficult in its early stages.2–6 Its high rate of local recurrence and the lack of clinical guidelines which establish a standardized management contribute to a less than favorable prognosis and a short survival rate.4 Due to late diagnoses, large size masses are found, which compromise the tumor's surgical resection because of the presence of adjacent vital anatomic structures.1 There are, however; studies proving a higher survival rate in patients treated with neoadjuvant chemotherapy followed by a surgical approach and postoperative radiotherapy.5,6 The contribution made by Demir et al. on surgical treatment clarifies the need for a multimodal management, showing that induction with chemotherapy contributes to tumor-free resection margins.5
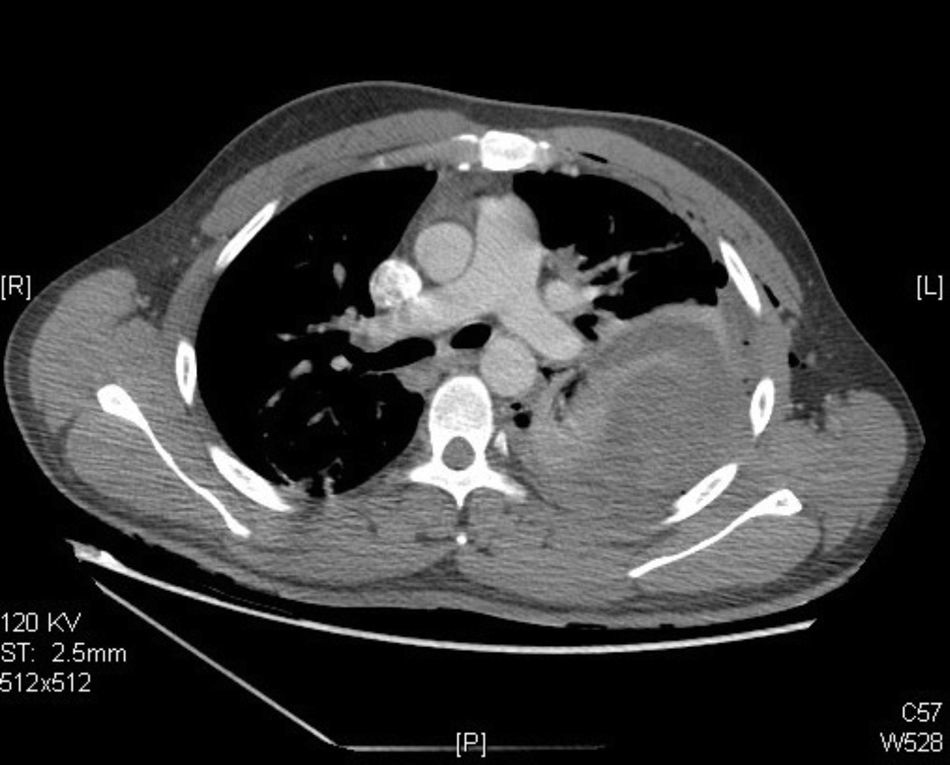
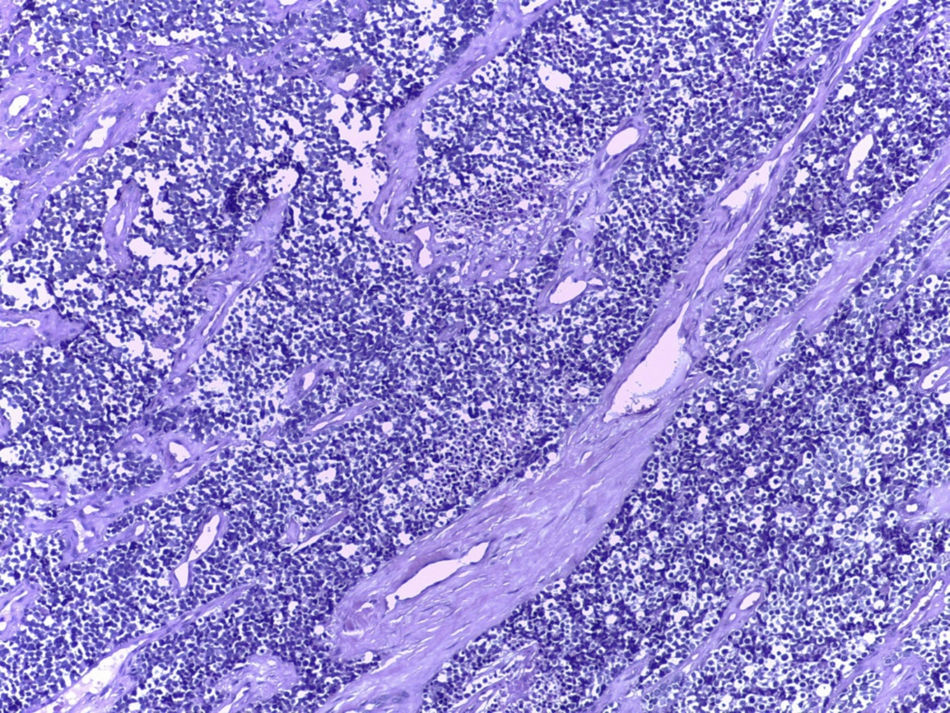
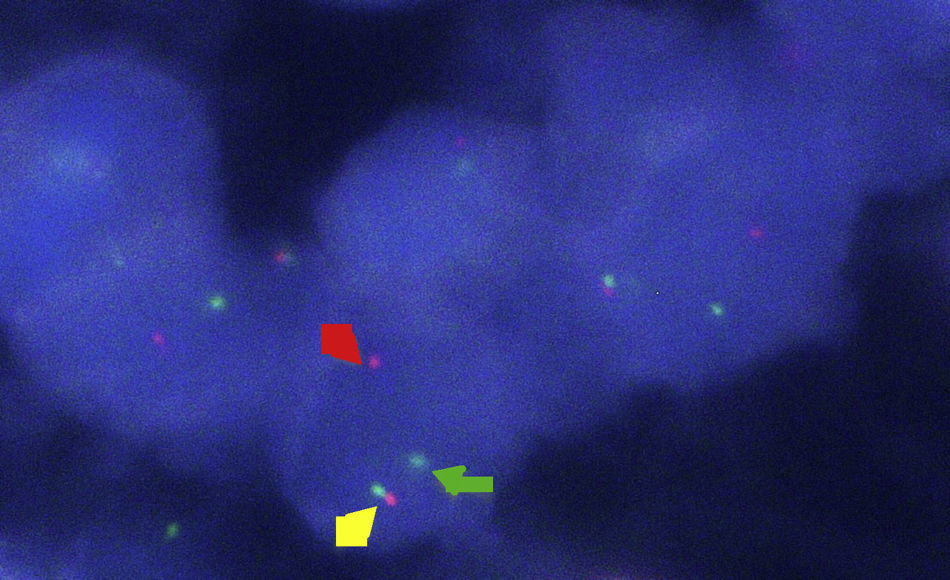
Clinical caseA 25-year-old male with a background of active smoking (12 packs/year), is admitted after presenting intermittent pleuritic pain in the left hemithorax, of variable intensity, of a two-month evolution; accompanied by a non-productive cough, non-quantified fever and a 10kg weight loss in a course of three months, without a history of occupational exposure hemoptysis or wheezing. Patient refers to have received a previous ambulatory treatment with sulfamethoxazole/trimethoprim, clindamycin and levofloxacin for 7 days without improvement. During physical examination, we observed in a central trachea, cylindrical thorax and symmetric respiratory movements without the use of accessory muscles and with no evident or palpable masses. At auscultation, a reduced vesicular murmur is found in the base of the left hemithorax, with no clinical data added. Blood studies were within normal parameters. Thoracic X-rays are performed, where a radio-opacity is found in the left hemithorax with effacement of the costophrenic angle (Fig. 1). Computed contrasted tomography of the thorax showed the presence of an extra pulmonary tumor (17.6cm×12.6cm×11.3cm), dependent on soft tissue of the left thoracic wall, extending from the fourth to the twelfth posterior costal arches, with a heterogeneous rise of the contrast material, without evidence of mediastinal adenopathies (Fig. 2). An incisional biopsy is performed through a left posterolateral thoracotomy with a trans-operatory histopathological report of a slightly differentiated malignant neoplasia. Our patient courses with a post-operative evolution with no negative eventualities. After performing a definite histopathological analysis, the presentation of a confirmed primitive neuroectodermal tumor through the presence of small round cells is described (Fig. 3), with immunohistochemical markers positive for FLI-1 and CD99, and negative for cytokeratin, vimentin, TdT, CD45, CD43 and CD56, with a positive flurosescence in situ hybridization (FISH) for rearrangement of the EWSR1 gene (Fig. 4). Oncological management with chemotherapy is started, under an alternate scheme using: vincristine 2mg/m2, doxorubicin 75mg/m2, cyclophosphamide 1200mg/m2 (Day 1) with ifosfamide 1800mg/m2 and etoposide 100mg/m2 (Day 1–5) every 3 weeks, dosage dependent on patient's tolerance under radiographic control of the tumor. At the time of the edition of this article, the patient is stable, receiving his first cytotoxic cycle, and expecting a reduction in the size of the tumor, thus making resection of the lesion possible.
Despite the advent of new diagnostic techniques and tools, Askin tumor has maintained a low incidence since it was first described, which makes us wonder about the factors associated with the onset of the responsible genetic mutation.
Tumors of the SE/PPNET family are categorized under the same entity because of the common histological conformations they show, differentiating from one another by their degree of neuroectodermal differentiation. They also present chromosomic translocation (11; 22) (q24; q12) which fusion expresses the gene EWS/ETS or EWS/FL1.3 This chimerical gene is observed in 85% of SE/PPNET group tumors, making its determination a crucial tool in the cytogenetic diagnosis.7
Its incidence is greater in males, and 80% of diagnosed patients are under 20 years of age; however, there are reported cases of elderly patients.1 The most common causes of death are associated with local recurrence, distant metastases and infiltration of the pulmonary parenchyma.1,4 Among the most recent reports, Contesso et al. showed a general survival rate of 14% at 6 years and a disease-free survival rate of 17% at 6 years.8
The clinical picture usually describes ambiguous symptoms including: a palpable mass in the thorax, either painful or painless (the most common finding),1,9 pleuritic pain, dyspnea, fever, cough and weight loss, generally of insidious onset 6 months prior to diagnosis, and, less frequently, Horner's syndrome.1,2,8,10 The lack of clinical suspicion often leads to an erroneous diagnostic impression, treating the patient initially as a pleural effusion.10 Unlike the reports of a classic presentation, in the case of our patient we did not observe a palpable mass as an initial symptom, despite the dimension of the tumor mass. Nevertheless, it presents the characteristic ambiguous symptomatology associated with the chronic evolution of the clinical picture. Furthermore, we point out the importance of considering tumors of the soft tissue of the thoracic wall within the differential diagnosis in those patients whose symptomatology is compatible with that of an unresolved infectious process. Few studies evaluating the radiological characteristics of this tumor have been conducted. However, the aid of a computed tomography (CT) scan or magnetic resonance imaging (MRI) is crucial to determine the extent, the possible pulmonary invasion of the tumor, and invasion of local and distal structures, as well as response to treatment.4 Findings by CT with contrast include: a soft-tissue-dependent mass, usually >5cm, with displacement of the adjacent structures, accompanied by a heterogeneous rise of the contrast material. The presence of calcifications, lymphadenopathies, necrotic areas and hemorrhages are less frequent findings. Regarding the MRI, masses of intermediate to hyperintense intensity are described, located in T1 and T2, with a heterogeneous rise when contrast dye was applied in the necrotic areas. Although Sabate et al. concluded that radiological findings in a CT and MRI are not specific and are indistinguishable from other malignancies of the thoracic wall.4 Regarding our patient's imaging assessment, performing a systemic review of the thoracic X-ray was highly beneficial, hence it presents an homogeneous radiopacity with clean edges and superior convexity, that in retrospection is of great importance when there is suspicion of a thoracic tumor.
The periosteum and soft tissues from the thoracic wall are typically involved, with extension to the pleura and pulmonary parenchyma.1 There are reports of cases with distant metastases to distal long bones, bone marrow, liver and the central nervous system (CNS). The neuroectodermal origin of the tumor explains the invasion of sympathetic chain ganglia, resulting in atypical presentations.10
Diagnostic precision is unavoidable, because the choice of therapeutic management depends on it. The tumors are solid, circumscribed, opaque brown with focal areas of necrosis and hemorrhage.3 Histologically, we are able to find the demonstration of round, small, slightly differentiated cells organized in strands.2 Also, it is possible to identify the presence of pseudorosettes as well as the absence of fixation or a weak fixation to periodic acid Schiff stains (PAS). With the use of electronic microscopy, we are able to observe the presence of neurosecretory granules, in addition to underdeveloped cytoplasmic organelles.11
The immunohistochemistry in this tumor in particular provides a definite base in the differential diagnosis in relation to the variety of malignancies within the group of tumors in round and small cells, such as: neuroblastomas, rhabdomyosarcoma and non-Hodgkin's lymphoma, chondrosarcoma and retinoblastoma.11
The neuroectodermal origin can be confirmed with the presence of immunohistochemical markers with a positive result for CD99, FLI1, NSE and vimentin. TdT, CD45, LCA, EMA, cytokeratin, desmin and actin markers must be included in the immunohistochemical panel in order to perform an exclusion of other round-cell malignancies. CD99 (mic-2) protein is found in the cellular membrane consistently in neoplasms of neuroectodermal SE/PPNETS origin. It is highly sensitive, but unspecific, thus it must be used as a complement with other immunohistochemical markers.12
Fluorescence in situ hybridization (FISH) and the amplification of genomic particles with a polymerase chain reaction (PCR) are necessary as complementary tools for chromosomic translocation detection (11;22) (q24; q12), which is present in 95% of the cases of these neoplasms.6 Despite the fact that there is no standardized study algorithm, in our patient, different diagnostic modalities, as well as multidisciplinary work, were included. However, similar to what has been described, the histopathological report was confirmatory, highlighting the presence of small round cells and immunohistochemical markers for CD99, with the corresponding stains, which allows us to make a diagnosis by exclusion, as well as the positive FISH for the rearrangement of the EWS/FL1 gene identified in this case.
At first, the treatment of this pathology was performed through a surgical approach.2 Even when different attempts to standardize a diagnostic and therapeutic algorithm have been made, trustworthy studies proposing a definite scheme are few. Treatments nowadays are based on findings which have marked a milestone in multimodal use, including neoadjuvant chemotherapy schemes as well as systemic treatment for every case, in conjunction with local therapy based on surgical resection and/or radiotherapy.1,6
The choice of these treatment modalities is based on each patient individually. Neoadjuvant chemotherapy reduces the possibility of microscopic residual disease, increases the possibility of a complete resection and increases the disease-free survival rate.5 Sirivella et al. place an emphasis on the necessity of making an early stage diagnosis through the use of a fine needle biopsy, before any surgical resection. They report an extension of survival free of disease of 10 years upon implementing the use of neoadjuvant chemotherapy, in up to 84% of patients whose tumors were found when the disease did not present metastatic spread, compared to those who presented metastatic spread and costovertebral compromise, who showed a survival rate of 33% at 10 years. Patients who showed a poor response to chemotherapy were benefitted by the use of local radiotherapy with the goal of reducing the size of the tumor.13
The surgical approach is essential in the management and removal of the tumor, and facilitating direct radiotherapy with less exposure. Veronesi et al. made a summary of the benefits of applying aggressive neoadjuvant chemotherapy treatment in comparison to postoperative chemotherapy, suggesting that there is a reduction of the intraoperative risk of tumor rupture and dissemination of the tumor cells, making a complete surgical resection possible.14 Surgical resection following neoadjuvant chemotherapy allows negative margins of 71%, compared to the 37% of patients who are subjected to surgery as an initial approach.12
Surgical strategy should consist of making a radical resection of the tumor mass, including the costal level involved, as well as the adjacent inferior or superior rib, circumscribed muscle and adherent soft tissue, procuring tumor-free margins of 3–4cm, and reconstructing the thoracic wall with flaps or muscular tissue.12 In cases where the pulmonary parenchyma is compromised, a lobectomy or pneumonectomy should be performed, if the case merits it. The importance of a complete surgical resection is that it prolongs disease-free survival, although development of local recurrences will remain constant.5
The chemotherapy outlines used have been tested in distinct studies with the intent of homogenizing an effective treatment. Outlines similar to those used to treat Ewing's Sarcoma have been used, which are performed through the use of vincristine at 1.5–2mg/m2, doxorubicin at 40mg/m2, cyclophosphamide at 1200mg/m2 and Actinomycin D at 1.25mg/m2 (VACA) or vincristine, adriamycin and ifosfamide at 1800mg/m2 and Etoposide at 100mg/m2, according to the protocol studied at EICESS 92, with cycles every 3 weeks.6,15
Radiotherapy is used as an aid in the local control of the primary lesion, posterior to surgical resection, with the aim of eliminating any fragment of the disease. Treatment should be directed to avoid a dose which would damage a near organ. In the CESS 81, CESS 86 and EICESS 92 studies, the use of postoperative radiotherapy, including its use as a local treatment, showed favorable results in relation to local recurrence. Currently, the dose used in radiotherapy is from 20 to 60Gy.6,15
Diagnostic age has been determined in multiple studies to be a factor in a negative prognosis, considering an age over 18 to be a risk. Poor response to initial chemotherapy and the presence of pleural effusion have been other factors associated with unfavorable prognoses.1 Demir et al. observed that the size of the tumor and the initial response to treatment, as well as the location of the tumor, negatively impacted survival rate. An osseous or pulmonary origin, or a tumor located in the costovertebral union, showed decreased survival rates.5
The importance of identifying these neoplasms resides in its aggressive behavior and in its high rate of recurrence. Even though there are numerous case reports, its clinical course, diagnosis and treatment are not very clear. Therefore, it is of great importance that a multidisciplinary team is familiar with these tumors, to make an adequate correlation correlation with the patient's history, which will bring us to a better understanding of its behavior and establish a treatment which will improve the prognosis and survival of the patient.
In conclusion, the Askin tumor should be considered within the differential diagnosis of thoracic neoplasms. Once the diagnosis of a primitive neuroectodermal tumor is made, optimal treatment will consist of a multimodal management, using neoadjuvant chemotherapy in association with surgical resection and/or postoperative radiotherapy, with which a decreased local recurrence and a greater disease-free period can be achieved. Due to its aggressive behavior, follow-up of these patients should be performed under strict adherence.
Conflict of interestThe authors have no conflicts of interest to declare.