Abdominal tuberculosis is the sixth most common form of extra-pulmonary tuberculosis. It manifests predominantly in four forms: tuberculous lymphadenopathy, peritoneal tuberculosis, intestinal tuberculosis and visceral tuberculosis. The perforation of the colon in intestinal tuberculosis is a complication which should be recognized, and treated surgically, as quickly as possible.
Clinical caseA 17-year-old female who was admitted with low back pain, limiting flexion of the thigh, fever, vaginal discharge, abdominal pain and weight loss. The CT scan showed a left tubo-ovarian abscess, pelvic cavity collection, reactive retroperitoneal lymph nodes, left psoas abscess and spondylodiscitis from L2 to L3. An exploratory laparotomy was performed, and we found a perforation in the cecum and ascending colon, left tubo-ovarian abscess, and a collection of fecal matter in the pelvic gap. We debrided and drained the left psoas collection, and performed a right hemicolectomy, an ileostomy, a transverse colon mucocutaneus fistula and a left salpingo-oophorectomy. The trans-operatory biopsy of the retroperitoneal adenopathy revealed a chronic granulomatous process compatible with tuberculosis. The Gram stain showed abundant polymorphonuclears and moderate Gram bacilli (−), and the BAAR stain was negative. The cultures showed Escherichia coli ESBL (Extended-spectrum beta-lactamases) sensitive to imipenem. Pathology reported chronic granulomatous salpingitis, chronic granulomatous peritonitis, and ulcerated and perforated cronic granulomatous colitis.
The patient was treated with broad-spectrum antibiotic therapy for tuberculosis.
ConclusionWith patients that present a perforation in an intestine with tuberculosis, their general condition should be evaluated, as well as their nutritional state and intestinal viability, before performing primary repair, resection and anastomosis or resection and bowel diversion surgery.
Abdominal tuberculosis is the sixth most frequent form of extra-pulmonary tuberculosis. It is an entity which manifests predominantly in 4 different forms: lymphadenopathy tuberculosis, peritoneal tuberculosis, intestinal tuberculosis and visceral tuberculosis, which affects solid organs. Extra-pulmonary tuberculosis is known to be difficult to diagnose, since the signs and symptoms of the disease imitate many other diseases. Intestinal tuberculosis in particular may imitate malignancy, bacterial infectious disease and inflammatory intestinal diseases such as Crohn's disease.1
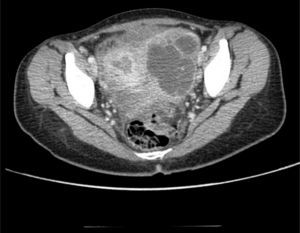
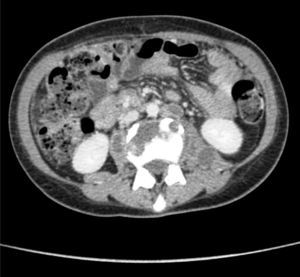
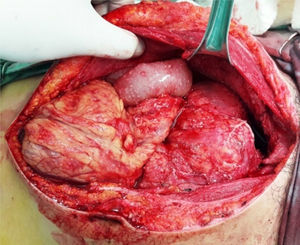
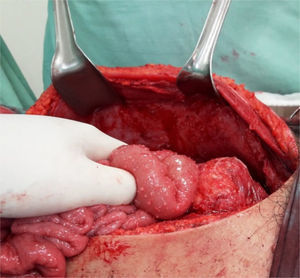
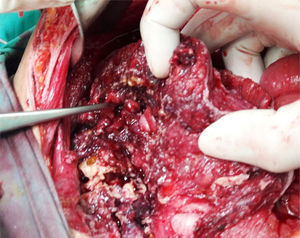
Clinical caseThe patient was a 17-year-old female without any relevant medical background. Six months prior to her admittance she presented backache and limitation in the flexion of the thigh, which was treated with NSAIDs without any improvement. She presented intermittent fever and leucorrhea in the last couple of months and abdominal pain in the last two days. She refers to a 12-Kgs weight loss. During physical examination, her temperature was 38°C, with a heart rate of 120 beats per minute, a respiratory frequency of 26 breaths per minute, blood pressure of 100/60mmHg, abdominal pain in lower quadrants of the abdomen, and data of peritoneal irritation. The labs report 12g/dl of hemoglobin, 16K/μl of leukocytes and 2.1g/dl of albumin. A pelvic ultrasound is conducted, reporting a left tubo-ovarian abscess and pelvic cavity collection. A CT scan is performed, reporting a left tubo-ovarian abscess and pelvic cavity collection as previously described in the ultrasound (Fig. 1), reactive retroperitoneal lymph nodes, abscess in the left psoas muscle (Fig. 2) and L2–L3 spondylodiscitis. She is moved to the operating room in order to conduct an exploratory laparotomy and granulomatosis lesions are found in the peritoneum, colon and small intestine (Figs. 3 and 4), the omentum migrated to the pelvic cavity, and a perforation of the cecum and the proximal part of the ascending colon (Fig. 5) was found, as well as a left ovarian-tube abscess, 50cc of a purulent aspect liquid and fecal matter in pelvic cavity. Samples are sent to be studied; the left psoas muscle is exposed, 60cc of purulent material is debrided and drained and sent for culturing; a right hemicolectomy, ileostomy, transverse colon mucocutaneus fistula and a left salpingo-oophorectomy were performed. Retroperitoneal adenopathy transoperative biopsy is sent, reporting a chronic granulomatous process compatible with tuberculosis. Drainages are placed in the pelvic cavity and retroperitoneum. Gram staining results report abundant polymorphonuclears and moderate Gram bacilli (−) and negative BAAR staining. Cultures reveal Escherichia coli ESBL (extended spectrum beta lactamas) resistant to all 2nd, 3rd and 4th generation cephalosporins, including cefepime and aztreonam, but sensitive to imipenem. Pathology results show a granulomatous chronic salpingitis, granulomatous chronic peritonitis, ulcerated and perforated cronic granulomatous colitis. A sputum smear microscopy and culture are performed, reporting negative BAAR staining and a culture positive for Mycobacterium tuberculosis. The viral panel was negative. The patient was administered a broad-spectrum antibiotic therapy (imipenem) as well as an anti-tuberculosis drug. A lumbar magnetic resonance is conducted, confirming the L2–L3 spondylodiscitis diagnosis. The Orthopedic Department decides on a conservative management. Postoperative evolution is positive, and the drainages are removed on postoperative day 7 after presenting a little serous exudate. The intravenous scheme is completed and hospital discharge is decided to continue with an ambulatory anti-tuberculous treatment and a follow-up consultation in order to program the ileostomy closure.
Tuberculosis (TB) has become a public health problem worldwide. Its rates continue to rise, causing a mortality rate of 6%.2 Peritoneal tuberculosis is found in up to 5% of patients with pulmonary tuberculosis and makes up 25–60% of abdominal tuberculosis cases.3 The most common symptoms are abdominal distension, weight loss, fever, abdominal pain and abdominal mass. The tuberculin skin test is positive in 85% of patients.4 The CT scan plays an important role in the detection and characterization of intraperitoneal abscesses caused by tuberculosis. Even though qualitative diagnosis requires positive pathological findings, certain CT characteristics (i.e. multiple septum, peripheral enhancement, lymphatic with enhanced border and hypodense mass, changes in the peritoneum, mesentery and omentum) are necessary for diagnosis.5
Tuberculosis peritonitis constitutes up to 1% of all ascites causes. Abdominal tuberculosis may be diagnosed by a M. tuberculosis culture grown in ascites fluid, or the presence of caseificant granulomas, with or without positive cytology for acid-alcohol resistant bacilli, in biopsy samples obtained laparoscopically.6 Gram's staining sensitivity is 0–6% and most tuberculosis ascites staining results are negative.7 Ascites culture sensitivity is around 30%, but it requires a long time, delaying diagnosis.8
Laparoscopy with a directed biopsy is currently the best way to make a quick and specific diagnosis. PCR analysis for M. tuberculosis in ascites fluid is a quick test to obtain a diagnosis, but it may not be very accurate. PCR sensitivity reaches up to 95% in patients with positive sputum smear microscopy, yet sensitivity is low (48%) in patients with negative sputum smear microscopy.8 Adenosine deaminase activity (ADA) in ascites fluid has been proposed as a diagnostic test useful for abdominal tuberculosis, with good precision.6
The only treatment for peritoneal tuberculosis is pharmacological. The efficacy of the therapy is determined by the resolution of the symptoms and the disappearance of ascites. A delay in the beginning of therapy has been linked to greater mortality rates.6 A greater incidence of perforation has been reported in patients with intestinal tuberculosis who were in treatment for tuberculosis. This ought to be recognized timely and surgical intervention should be considered in order to prevent mortality secondary to perforation.9
Surgery is reserved for complicated cases, such as obstruction, hemorrhage, perforation, abscesses and fistulae formation. The most efficient surgical treatment in perforation cases is the removal of the affected segment with termino-terminal anastomosis.10 The selection of a surgical procedure depends on the site and extension of the disease, the status of the remaining intestine, the general health of the patient, and the surgeon's experience and individual preference. In complicated cases with fecal peritonitis and intraabdominal sepsis, a two-stage procedure is preferred with the creation of a stoma (ileostomy) to the primary anastomosis.11
A psoas abscess can be classified as primary or secondary, depending on the presence or absence of an underlying illness.12 Primary psoas abscess is originated by an infection in a distant site which spreads via lymphatic or hematogenous, while secondary psoas abscess is caused by the direct extension of an infection of adjacent structures.13
Staphylococcus aureus (88%) is considered to be the most common cause of primary psoas abscess, followed by streptococci and E. coli. The etiology of primary psoas abscess is not clear. However, lymphatic and haematogenous dissemination of an infectious process as a result of a hidden source in the body has often been linked to immunosuppression. A major cause of psoas abscess in developing countries can be Mycobacterium tuberculosis, by haematogenous dissemination or directly from a vertebral osteomyelitis.12
Clinical presentation of a psoas abscess is usually variable and non-specific. The clinical triad consists of fever, backache and a limp, and it is present in only 30% of patients with a psoas abscess.14 Mortality rates in patients with a primary psoas abscess is relatively low (2.4%). On the other hand, the mortality rate rises to 18.9% for secondary psoas abscess and it nears 100% in patients who are not treated.13
In our clinical case, we present a patient with significant weight loss, hypoalbuminemia and bad conditions in general, in addition to presenting a psoas abscess, an tubo-ovarian abscess, a collection of purulent material with fecal matter in the pelvic cavity and a perforation in colon with an intestine non-viable for primary repair or performance of an anastomosis. Consequently, we decided to perform a hemicolectomy with ileostomy and transverse colon mucocutaneus fistula.
ConclusionIn patients who present a perforation in an intestinal tuberculosis, their general conditions ought to be assessed, their nutritional status, intestinal viability to perform a primary repair, resection and anastomosis or resection and bowel diversion surgery. The surgical decision is at the surgeon's discretion and the correct assessment of the procedure will avoid complications like a leakage of the primary repair or the anastomosis. When this occurs in conjunction with a psoas abscess, it must be drained during the same surgical time. In these patients an anti-tuberculosis treatment ought to be continued until completing the scheme in an ambulatory manner posterior to surgical treatment.
Conflict of interestThe authors have no conflicts of interest to declare.