The classification of lymphomas by the World Health Organization (WHO) and its updates is widely known and adopted by hematopathologists. However, its application in clinical practice is poorly understood.
ObjectiveAnalyze and compare an initial evaluation by a general pathologist in relation to an evaluation by a hematopathologist.
Material and methodsRetrospective case series, which included patients with the novo lymphoma diagnosis, relapse and resistance/failure of treatment. An initial evaluation was issued by a general pathologist, which was then sent to a hematopathologist for a revision. Discrepancies were sorted by: “Major”, where the therapeutic approach was modified, “Minor”, where the diagnosis changed, but therapeutic approach remain the same, and “In Accordance”, meaning full concordance between initial evaluation and revision.
ResultsFourteen cases were analyzed (5 females and 9 males). Mean age was 44 years. In Accordance were found in 2 cases (14%), Major discrepancies were found in 11 (79%) and Minor Discrepancies were found in 1 (7%). Immunohistochemical studies were performed, presenting a variance between initial evaluation and revision of 5 (0–8) and 9.5 (6–18), respectively.
ConclusionThere is a great need to generate pathologists with the necessary training and experience to perform the diagnostics attached to the current WHO classification.
The World Health Organization's lymphoma classification and its different updates are widely-known, as well as adopted, by hematopathologists. However, its application in everyday practice by general pathologists is little-known. This constitutes a diagnostic challenge in countries where general pathologists are the main resource in the diagnosis of lymphoid pathologies, becoming one of the most difficult tasks where even the most experienced pathologists may find difficulties.1,2
Previous classifications of lymphoma -such as the Keil and Working Formulation-served as the cornerstone for diagnoses for years. However, the Keil classification of lymphoma is based on morphological features, and does not always correlate with clinical behavior, unlike the Working Formulation, which was designed to be a translation system amongst different classifications, and separates malignancies into low, intermediate and high histological subgroups.3 Both, with a 100% morphological approach, coexisted and were utilized indistinctively by pathologists.
In 1994, the Revised European-American Classification of Lymphoid Neoplasms (REAL) was created, which proposed the incorporation of objective criteria, like morphology, clinical picture and immunophenotype, in addition to genetic alterations and the proposal of a cell of origin in each of these neoplasms – especially B-cells. Subsequently, in the year 2000, the WHO made the effort to sort the necessary parameters to establish a proper and replicable histopathological diagnosis to diagnose the results of treatments on patients with lymphoproliferative neoplasms based on the REAL classification.4,5
Since the introduction of the classification of lymphoproliferative neoplasms by the WHO, the histological interpretation of lymph nodes has been complex for pathologists who do not have specific training in hematopathology,5 since this classification stresses the importance of adapting morphological, immunophenotypical, genetic and molecular characteristics for a proper diagnosis. Some of the diagnostic problems include confusion with the terminology used in the current classification, as well as the use of mixes with previous classifications.6
The proper medical management of patients with hematological neoplasms is based on the correct histopathological diagnosis, since therapeutic strategies vary depending on the different subtypes of malignancies.
In recent decades, hematopathology has been one of the fields of pathology which has shown some of the greatest progress in diagnostic techniques, with more specific and more sensitive immunohistochemistry-antibodies, in addition to molecular and genetic techniques which help the differential diagnosis of lymphomas with unusual morphology or those of aberrant immunophenotypes, which represent a diagnostic challenge, since this requires ancillary diagnostic assessments, such as studies to assess the presence of B-cell or T-cell clones.5
Cases of hematological neoplasms are particularly complex because they involve different systems and organs, and are capable of developing a wide variety of signs and symptoms, which sometimes require interdisciplinary management for a diagnosis. In previous researches, there has been a history of a relatively high rate of medical malpractice linked to mistakes in the diagnosis of lymphoma.2,6,7 Discrepancy ranges between the initial diagnosis issued by a general pathologist and the reviewed diagnosis by a hematopathologist, both evaluating the same material, are reported to be from 5.8% to 60%.2,8
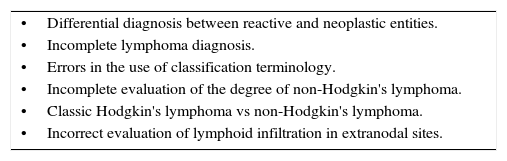
Authors conclude that the approach of the lymphoma classification by the WHO is less replicable in countries where general pathologists are not accustomed to the extensive use of support techniques; hence, under these conditions, a team of consultant hematopathologists for integral revision is important in order to minimize mistakes in interpretations. Moreover, evidence shows that the greater discrepancies with ambiguous diagnoses reflects the lack of familiarization of the general pathologist with the WHO classification for lymphomas.2 Critical mistakes in diagnosis and common situations which make diagnostic errors more common are mentioned in Tables 1 and 2.1
Common situations that favor a diagnostic error in lymphoproliferative neoplasias.
| •Differential diagnosis between reactive and neoplastic entities. |
| •Incomplete lymphoma diagnosis. |
| •Errors in the use of classification terminology. |
| •Incomplete evaluation of the degree of non-Hodgkin's lymphoma. |
| •Classic Hodgkin's lymphoma vs non-Hodgkin's lymphoma. |
| •Incorrect evaluation of lymphoid infiltration in extranodal sites. |
There is a rising tendency in Mexico in the frequency of lymphomas; the Globocan 2012 reported 6175 cases, of which 76% were from patients at a productive age.
The objective of the present study is to conduct a comparative analysis of the initial reports of general pathologists in relation to a reassessment by a hematopathologist.
Material and methodsA set of cases was conducted and reviewed in retrospective between the periods of March, 2014 and January, 2016. The biopsies previously analyzed by a general pathologist were sent to a hematopathologist for a second opinion. The origin of the patients varied; we obtained cases from the public as well as the private sector. All patients were cared for in the metropolitan area of Guadalajara, Jalisco, Mexico. Patient selection was by convenience, the study included patients with a first time lymphoma diagnosis, as well as relapsed and/or refractory patients. A total of 15 cases were analyzed with the initial report of the general pathologist, denominated “Initial Assessment”, and the assessment by a hematopathologist, denominated “Revision”. A total of 14 cases were included for full analysis. The diagnostic material was the same for the initial assessment, as well as the revision. Demographic and medical data was obtained, such as age, gender, date of initial assessment and revision, amount of immunohistochemistry markers performed and final diagnosis issued by both pathologists. Discrepancies were classified as “Major” if therapeutic conduct was modified according to the National Comprehensive Cancer Network® (NCCN) treatment guidelines, “Minor” if there was a change in the diagnosis but not in the therapeutic conduct, and “In Accordance” when there was concordance between initial diagnosis by the general pathologist and the reviewed diagnosis by the hematopathologist.2,6
For data processing and statistical analysis, we used the program Microsoft Excel 2014 (Redmond, WA, USA). The data are presented in raw numbers and proportions.
ResultsA total of 14 cases were analyzed. Nine were male and 5 were female, with a mean age of 44 years old, and an age range from 19 to 73 years old. Only 2 patients (14%) obtained an “In Accordance” diagnosis, while 11 (79%) presented a “Major” discrepancy. The types of mistakes observed within the major discrepancies consisted of: a) ambiguity or lack of complete diagnosis (7/11), b) change of lesions from malignant to benign (2/11), and c) change in the type of hematologic neoplasia (2/11). Only one patient (7%) obtained a Minor discrepancy (case 7).
It is important to mention that, in 4 patients of the cases denominated as “major discrepancy” there was no change in therapeutic conduct, since the review occurred prior to the beginning of treatment; despite this, they were included in this group because the information of the initial diagnosis was ambiguous or incomplete, and did not provide the necessary information to generate a recommendation of treatment based on the NCCN's clinical guidelines utilized in patients with hematological neoplasms.6
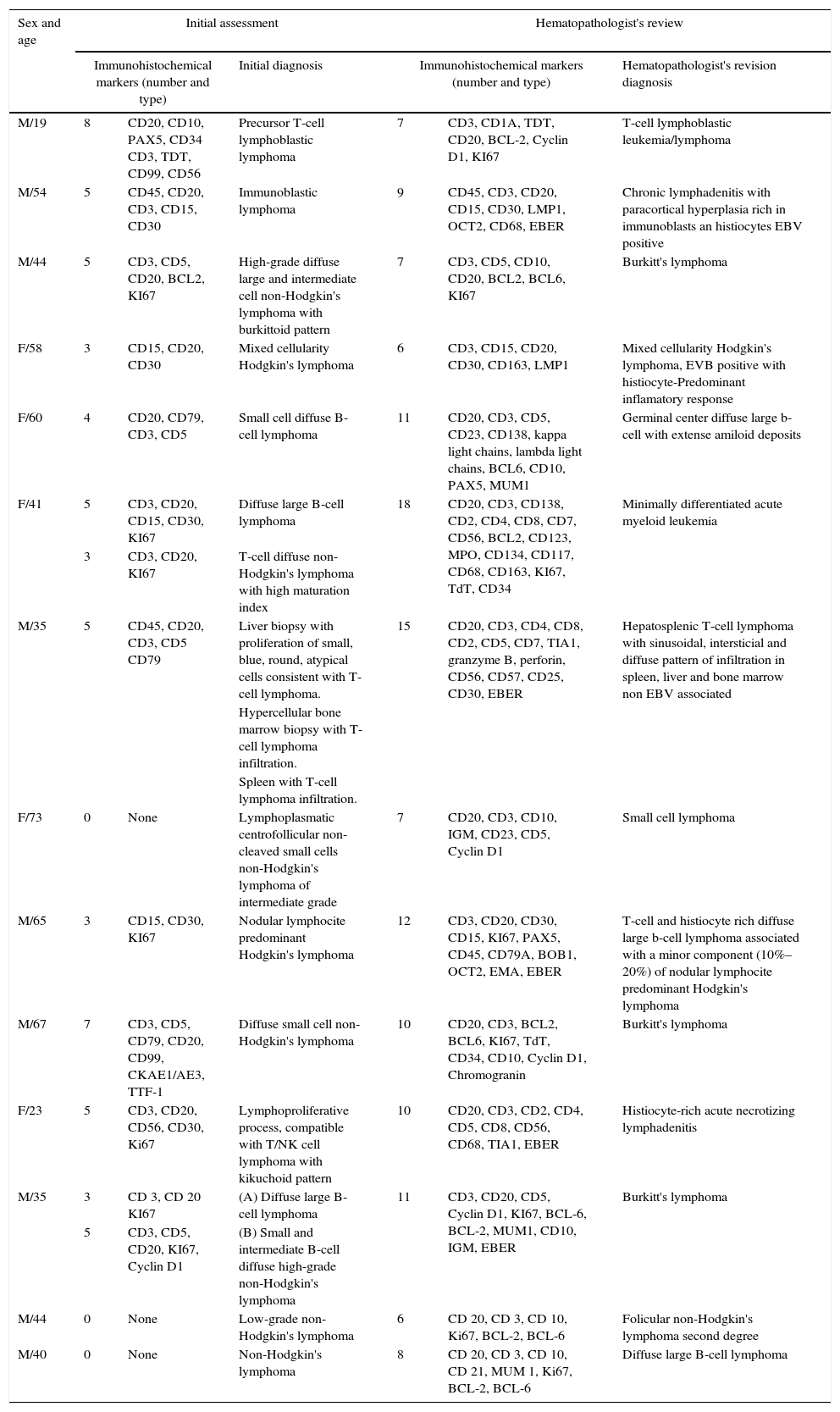
The immunohistochemical studies conducted on the samples presented a significant variation between the initial assessment and the review, the details are shown in Table 3. The average number of markers utilized in the initial assessment was 5 markers (0–8) versus the average of 9.5 (6–18) by the reviewer.
Initial diagnostic assessment and review.
| Sex and age | Initial assessment | Hematopathologist's review | ||||
|---|---|---|---|---|---|---|
| Immunohistochemical markers (number and type) | Initial diagnosis | Immunohistochemical markers (number and type) | Hematopathologist's revision diagnosis | |||
| M/19 | 8 | CD20, CD10, PAX5, CD34 CD3, TDT, CD99, CD56 | Precursor T-cell lymphoblastic lymphoma | 7 | CD3, CD1A, TDT, CD20, BCL-2, Cyclin D1, KI67 | T-cell lymphoblastic leukemia/lymphoma |
| M/54 | 5 | CD45, CD20, CD3, CD15, CD30 | Immunoblastic lymphoma | 9 | CD45, CD3, CD20, CD15, CD30, LMP1, OCT2, CD68, EBER | Chronic lymphadenitis with paracortical hyperplasia rich in immunoblasts an histiocytes EBV positive |
| M/44 | 5 | CD3, CD5, CD20, BCL2, KI67 | High-grade diffuse large and intermediate cell non-Hodgkin's lymphoma with burkittoid pattern | 7 | CD3, CD5, CD10, CD20, BCL2, BCL6, KI67 | Burkitt's lymphoma |
| F/58 | 3 | CD15, CD20, CD30 | Mixed cellularity Hodgkin's lymphoma | 6 | CD3, CD15, CD20, CD30, CD163, LMP1 | Mixed cellularity Hodgkin's lymphoma, EVB positive with histiocyte-Predominant inflamatory response |
| F/60 | 4 | CD20, CD79, CD3, CD5 | Small cell diffuse B-cell lymphoma | 11 | CD20, CD3, CD5, CD23, CD138, kappa light chains, lambda light chains, BCL6, CD10, PAX5, MUM1 | Germinal center diffuse large b-cell with extense amiloid deposits |
| F/41 | 5 | CD3, CD20, CD15, CD30, KI67 | Diffuse large B-cell lymphoma | 18 | CD20, CD3, CD138, CD2, CD4, CD8, CD7, CD56, BCL2, CD123, MPO, CD134, CD117, CD68, CD163, KI67, TdT, CD34 | Minimally differentiated acute myeloid leukemia |
| 3 | CD3, CD20, KI67 | T-cell diffuse non-Hodgkin's lymphoma with high maturation index | ||||
| M/35 | 5 | CD45, CD20, CD3, CD5 CD79 | Liver biopsy with proliferation of small, blue, round, atypical cells consistent with T-cell lymphoma. | 15 | CD20, CD3, CD4, CD8, CD2, CD5, CD7, TIA1, granzyme B, perforin, CD56, CD57, CD25, CD30, EBER | Hepatosplenic T-cell lymphoma with sinusoidal, intersticial and diffuse pattern of infiltration in spleen, liver and bone marrow non EBV associated |
| Hypercellular bone marrow biopsy with T-cell lymphoma infiltration. | ||||||
| Spleen with T-cell lymphoma infiltration. | ||||||
| F/73 | 0 | None | Lymphoplasmatic centrofollicular non-cleaved small cells non-Hodgkin's lymphoma of intermediate grade | 7 | CD20, CD3, CD10, IGM, CD23, CD5, Cyclin D1 | Small cell lymphoma |
| M/65 | 3 | CD15, CD30, KI67 | Nodular lymphocite predominant Hodgkin's lymphoma | 12 | CD3, CD20, CD30, CD15, KI67, PAX5, CD45, CD79A, BOB1, OCT2, EMA, EBER | T-cell and histiocyte rich diffuse large b-cell lymphoma associated with a minor component (10%–20%) of nodular lymphocite predominant Hodgkin's lymphoma |
| M/67 | 7 | CD3, CD5, CD79, CD20, CD99, CKAE1/AE3, TTF-1 | Diffuse small cell non-Hodgkin's lymphoma | 10 | CD20, CD3, BCL2, BCL6, KI67, TdT, CD34, CD10, Cyclin D1, Chromogranin | Burkitt's lymphoma |
| F/23 | 5 | CD3, CD20, CD56, CD30, Ki67 | Lymphoproliferative process, compatible with T/NK cell lymphoma with kikuchoid pattern | 10 | CD20, CD3, CD2, CD4, CD5, CD8, CD56, CD68, TIA1, EBER | Histiocyte-rich acute necrotizing lymphadenitis |
| M/35 | 3 | CD 3, CD 20 KI67 | (A) Diffuse large B-cell lymphoma | 11 | CD3, CD20, CD5, Cyclin D1, KI67, BCL-6, BCL-2, MUM1, CD10, IGM, EBER | Burkitt's lymphoma |
| 5 | CD3, CD5, CD20, KI67, Cyclin D1 | (B) Small and intermediate B-cell diffuse high-grade non-Hodgkin's lymphoma | ||||
| M/44 | 0 | None | Low-grade non-Hodgkin's lymphoma | 6 | CD 20, CD 3, CD 10, Ki67, BCL-2, BCL-6 | Folicular non-Hodgkin's lymphoma second degree |
| M/40 | 0 | None | Non-Hodgkin's lymphoma | 8 | CD 20, CD 3, CD 10, CD 21, MUM 1, Ki67, BCL-2, BCL-6 | Diffuse large B-cell lymphoma |
The World Health Organization's classification of lymphoproliferative neoplasms combines cytomorphological, immunohistochemical, cytogenetic and molecular aspects, which has resulted in the diagnosis of said neoplasms being a real challenge for pathologists, even those in reference centers.5
The introduction of targeted cancer therapies in clinical practice, in which patients are selected for new treatments based on the results of molecular tests, has created new challenges for the accurate diagnosis of these patients. This information is vital for formulating an accurate treatment plan for patients, since the different varieties of lymphoma require treatments that change in the type of drugs used, as well as the doses and the frequency of application; an error in the diagnosis can lead to sub- or over-treating a patient, leading to increased toxicity or poor tumor response to treatment that in both cases may lead to an adverse outcome.1,9,10
Lack of access to a wider range of immunohistochemical markers outside the reference centers, as well as the lack of repeated exposure of local pathologists to a larger number of lymphoma cases are a major factor against them.11
In the current review, we observed that the diagnoses were “In Accordance” in 14% of cases, a Major Discrepancy was present in 79% of cases and a Minor Discrepancy was present in only 7% of cases. These results seem in proportion to what was observed in Asia by Chang et al.,2 with Major Discrepancies in 55%, Minor Discrepancies in 5% and In Accord in 40%. This in turn contrasts sharply with the data reported by Wilkins, Matasar and LaCasce,6,8,9 reported Major Discrepancies in 5–18% and global discrepancies in 6–26%. It is important to mention that these studies were mainly carried out in developed countries. Among the Major Discrepancies found, the 3 main causes of these were ambiguous or non-diagnostic reports (64%), a change in type of hematologic neoplasia (18%), and a change from a malignant to a benign lesion (18%), which is similar to that observed by Chang, et al., who observed 52%, 23% and 14%, respectively.2
We should mention that the reports that were generated in the Initial Evaluations did not adhere to the nomenclature provided by the WHO to classify lymphoproliferative neoplasias, and the descriptions of the diagnoses was similar to the nomenclature used in previous classifications, mainly the Working Formulation dating back to the 1980s and were even mixed in some cases, which creates a great deal of confusion for the doctors in charge of decision making in the clinical field. These same problems had been previously observed, and were classified as ambiguous diagnoses, where the information that the assessment generates does not allow a therapeutic recommendation based on international consensus, and in addition poses a great danger to patients.2,6
In addition to the previously reported implications, it is important to note that, with the emergence of the new therapeutic weapons known as targeted therapies, such as monoclonal antibodies or kinase inhibitors, the absence of a clear or complete diagnosis of lymphoproliferative neoplasms means that patients who can benefit from these drugs are excluded from these therapeutic options, since the use of these is restricted to very specific diagnoses or patients with certain immunohistochemical, cytogenetic and molecular characteristics expressed in their tumors.
Given the great need to standardize the diagnostic criteria, an international conference was held in 2011, with the objectives of developing a universally accepted, unambiguous classification to improve the staging and response criteria for Hodgkin's lymphoma and non-Hodgkin's lymphoma, from which arose the new Lugano criteria, which included new imaging techniques such as the PET-Scan.12,13 In the present study, only histopathological diagnostic modalities were used.
The main limitation of the study is its retrospective character, since the majority of the patients were evaluated in a second opinion manner, in cases refractory to treatment.
It will be important in the future to conduct clinical trials with a greater number of samples that compare the different diagnostic methods for lymphoproliferative diseases, seeking to standardize them in Mexico, since it is a pathology with an increasing incidence.4
In the case of patients with suspected or diagnosed hematological malignancies, mainly lymphoproliferative, tissue samples should be evaluated by trained pathologists and with adequate diagnostic armaments to generate diagnoses attached to the current classification and thus optimize the treatments they receive or will receive.14
ConclusionsIn this brief series of cases, we can verify that 79% of them showed Major Discrepancies in their diagnoses that lead to a change in their treatment. Similar to the reports in the literature, there is a great need to generate pathologists with training and experience to generate diagnoses adhering to the current classification of the WHO or, in their absence, to generate regional reference centers with the staff and material necessary for this.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.