Acute kidney injury (AKI) is a widely-seen pathology in the hospital environment. Today, prevention is the best available treatment. Nowadays, one of the most common causes is sepsis-induced AKI, which is seen in critical care patients. Sepsis-induced AKI includes in its pathophysiology injury associated with low kidney perfusion and toxicity caused by inflammatory biomarkers but has a more complex treatment.
There is not yet a consensus of when to initiate renal replacement therapy, but it seems that early initiation confers a better prognosis, as well as that continuous renal therapy, could have an impact on life expectancy and early renal recovery. We analyze epidemiology, pathophysiology, and treatment in the following article, particularly the early initiation of renal replacement therapy in sepsis-induced AKI.
Definition, epidemiology, and prognosis of acute kidney injury.
Acute kidney injury (AKI) is defined as a sudden and sustained loss of renal function, causing the accumulation of products and toxins, both nitrogenated and non-nitrogenated, accompanied by disorders in the equilibrium of liquids, electrolytes, and acid-base.1
AKI incidence has increased, while mortality rates linked to its diagnosis have not had significant changes.2 Estimations suggest that between 1 and 30% of hospitalized patients will present AKI. This percentage is even higher (70%)3 in the intensive care unit (ICU).4 Also, 20% of patients with this diagnosis will require renal replacement therapy (RRT).5
Additionally, AKI diagnosis and treatment have an impact on hospital costs by increasing hospital stay and the use of resources.6 AKI is linked to 2.8 more days of hospitalization, in addition to an increase of 7082 dollars in hospitalization costs, combined with a more significant morbidity and mortality rate.7,8 It also has long-term consequences, since 8% of patients which suffered from AKI with an RRT requirement, will present chronic RRT dependency, versus 0.1% of patients who did not have AKI.7 A mortality rate of 28% up to 80% has been reported.8,9 The highest percentage is linked to when there is involvement of multiple organ failure and/or requirement of renal replacement therapy.
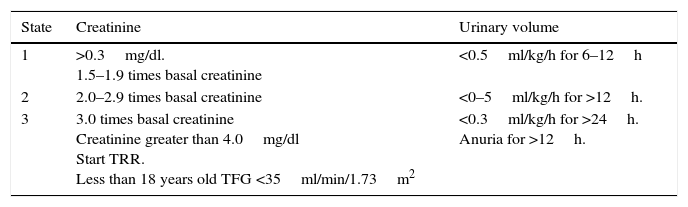
Since 2012, the “Kidney Disease Improvement Global Outcome” workgroup (KDIGO),10 published the criteria for the diagnosis of acute kidney injury. Today, this classification is the most widely utilized. This classification includes 3 degrees, depending on the severity (Table 1). The most outstanding aspect of these criteria is the increase in serum creatinine of just 0.3mg/dl within 48h of the injury, or a decrease of diuresis at 0.5ml/kg/h for 6h.10
Classification of LRA KDIGO 2012.10
| State | Creatinine | Urinary volume |
|---|---|---|
| 1 | >0.3mg/dl. 1.5–1.9 times basal creatinine | <0.5ml/kg/h for 6–12h |
| 2 | 2.0–2.9 times basal creatinine | <0–5ml/kg/h for >12h. |
| 3 | 3.0 times basal creatinine Creatinine greater than 4.0mg/dl Start TRR. Less than 18 years old TFG <35ml/min/1.73m2 | <0.3ml/kg/h for >24h. Anuria for >12h. |
KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012;2(1):1–138.
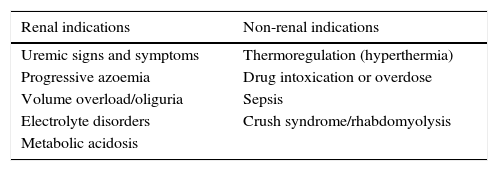
For many years there has been controversy about when to begin RRT. Nowadays, current criteria to begin RRT are the following: having a third-degree KDIGO, or one of the following criteria: acute hyperkalemia (greater than 6meq/L), acute metabolic acidosis (pH less than 7.15). Also hydric overload (weight gain greater than 10%, or acute pulmonary edema) or ureic nitrogen over 100mg/dL (Table 2).11 Likewise, there is still controversy over whether beginning RRT with these criteria is too late, and whether or not the use of creatinine as the only marker is enough.12 Thus, non-conventional RRT indicators have been explored, including non-renal ones (Table 2).
11RRT indications.
| Renal indications | Non-renal indications |
|---|---|
| Uremic signs and symptoms | Thermoregulation (hyperthermia) |
| Progressive azoemia | Drug intoxication or overdose |
| Volume overload/oliguria | Sepsis |
| Electrolyte disorders | Crush syndrome/rhabdomyolysis |
| Metabolic acidosis |
Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med. 2016;375(2):122–133.
Different types of RRT may be used in patients with AKI, including peritoneal dialysis (PD) and extracorporeal therapies like hemodialysis (HD) and continuous renal replacement therapy (CRRT).13 Peritoneal dialysis was the first replacement therapy used in AKI in 1970.14 It offers some advantages over extracorporeal therapies, some of the most significant ones are the fact that it does not require the use of anticoagulation, and thus there is less risk of hypotension during treatment and a lower cost. Some of the disadvantages are the requirement of an abdomen without recent surgeries, a higher risk of infection and the difficult control over ultrafiltration.15 Despite the fact that results are similar to those of hemodialysis, the use of PD has decreased over the last few years.16
The currently utilized therapy is intermittent hemodialysis (IHD). This therapy utilizes a venous catheter and, through diffusion and osmosis mechanisms, conducts an exchange of small molecular weight particles. Among the advantages of IHD are the fact that this therapy is the most efficient of all, accomplishing a faster electrolyte and acid/base control compared to the other two, a lower cost compared to CRRT and a lower anticoagulation dose. Some of the disadvantages are the risks of bleeding caused by the use of heparin, hypertension during treatment, and the need for a new venous access.
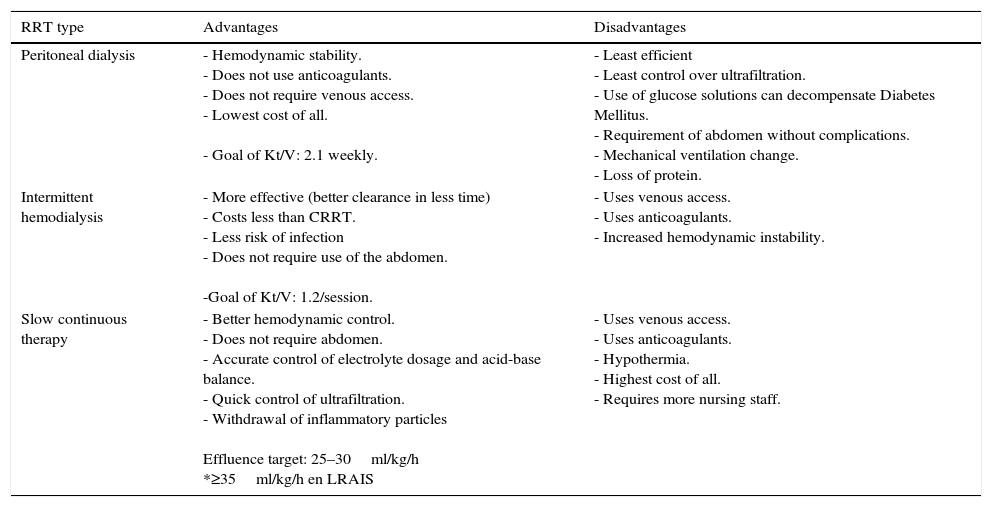
Advantages and disadvantages of the different RRTs are summarized in Table 3.
18Advantags and disadvantages of different types of RRT.
| RRT type | Advantages | Disadvantages |
|---|---|---|
| Peritoneal dialysis | - Hemodynamic stability. - Does not use anticoagulants. - Does not require venous access. - Lowest cost of all. - Goal of Kt/V: 2.1 weekly. | - Least efficient - Least control over ultrafiltration. - Use of glucose solutions can decompensate Diabetes Mellitus. - Requirement of abdomen without complications. - Mechanical ventilation change. - Loss of protein. |
| Intermittent hemodialysis | - More effective (better clearance in less time) - Costs less than CRRT. - Less risk of infection - Does not require use of the abdomen. -Goal of Kt/V: 1.2/session. | - Uses venous access. - Uses anticoagulants. - Increased hemodynamic instability. |
| Slow continuous therapy | - Better hemodynamic control. - Does not require abdomen. - Accurate control of electrolyte dosage and acid-base balance. - Quick control of ultrafiltration. - Withdrawal of inflammatory particles Effluence target: 25–30ml/kg/h *≥35ml/kg/h en LRAIS | - Uses venous access. - Uses anticoagulants. - Hypothermia. - Highest cost of all. - Requires more nursing staff. |
Ricci Z, Romagnoli S, Ronco C. Renal replacement therapy. F1000Research. 2016;5(F1000 Faculty Rev):103. doi:10.12688/f1000research.6935.1.
Continuous renal replacement therapy (CRRT) is defined as an extracorporeal method of purifying blood over an extended period, looking to replace renal function. First used by Kramer in 197717 for patients with acute kidney injuries in a critical state using continuous arteriovenous hemofiltration (CAVH).18 CAVH uses blood pressure to create a flow and a transmembrane pressure gradient, thus accomplishing spontaneous ultrafiltration. However, this pressure gradient depends on the patient's mean blood pressure. Today, the same venous lumen is utilized with extracorporeal pumps to achieve transmembrane pressure gradient regardless of the patient's mean blood pressure, thus preventing many of the complications that an arterial catheter causes (i.e., ischemia, atheroemboli, bleeding, pseudoaneurysm).19 Compared with intermittent renal replacement therapies, CRRT has not shown to be superior regarding survival rate; however, it has shown to be superior in the management of liquid volumes, as well as in renal recovery rate.20,21
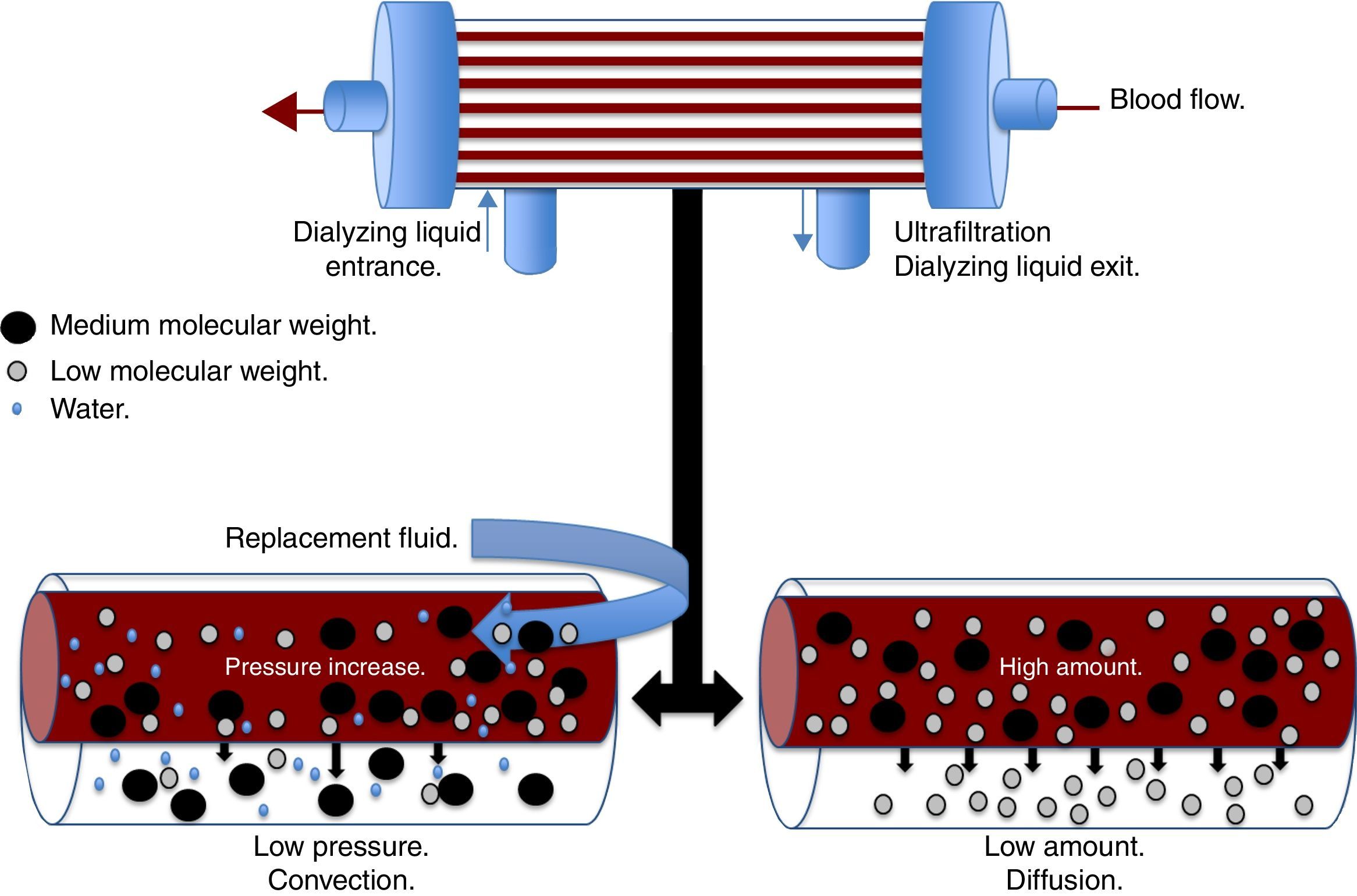
There are different physical and chemical principles involved in the different modalities of CRRT, which are responsible for the depuration of toxins and water usually eliminated by the kidney. The main principles are ultrafiltration, diffusion, convection, and adsorption. Ultrafiltration is the process by which the water in the plasma is forced through a semipermeable membrane by a hydrostatic pressure gradient.21 The resulting amount of ultrafiltration will depend on the product between the coefficient of the ultrafiltration membrane and the pressure gradient of the transmembrane.21 The diffusion or dialysis is the movement of a solute from an area of greater concentration to an area of lesser concentration through a semipermeable membrane.18 The diffusion speed is determined by the characteristics of the solute, the characteristics of the dialyzing membrane, both blood and dialyzing liquid flow, and the gradient of the solute between the blood and dialyzing liquid. Diffusion plays an important role in the clearance of low-molecular-weight substances, <20kDa, such as urea, creatinine, uric acid, ions, IL-6, heparin, and ammonia.22
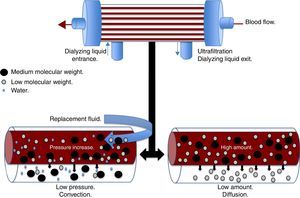
Convection (Fig. 1) occurs when a transmembrane pressure gradient moves water from the plasma through a semipermeable membrane, concomitantly dragging solutes, provided the porosity of the membrane allow it.18 This principle is independent to the solute concentration gradient through the filtration membrane. The transmembrane pressure gradient is generated by the difference of pressures in the behavior of blood, as well as the dialyzing solution.23 In the first, through a rise in hydrostatic pressure or through an increase in speed of blood flow, while in the latter through negative pressure. Convection has the ability to remove molecules of medium molecular weight (<60kDa) such as IL-8, TNF, IL-10, complements, eicosanoids, platelet activating factors and other mediators in sepsis.22 Lastly, adsorption confers the ability to retain high-molecular-weight molecules through different types of synthetic membranes; however, the adsorption ability of the filter is saturated during the first hours of treatment.24
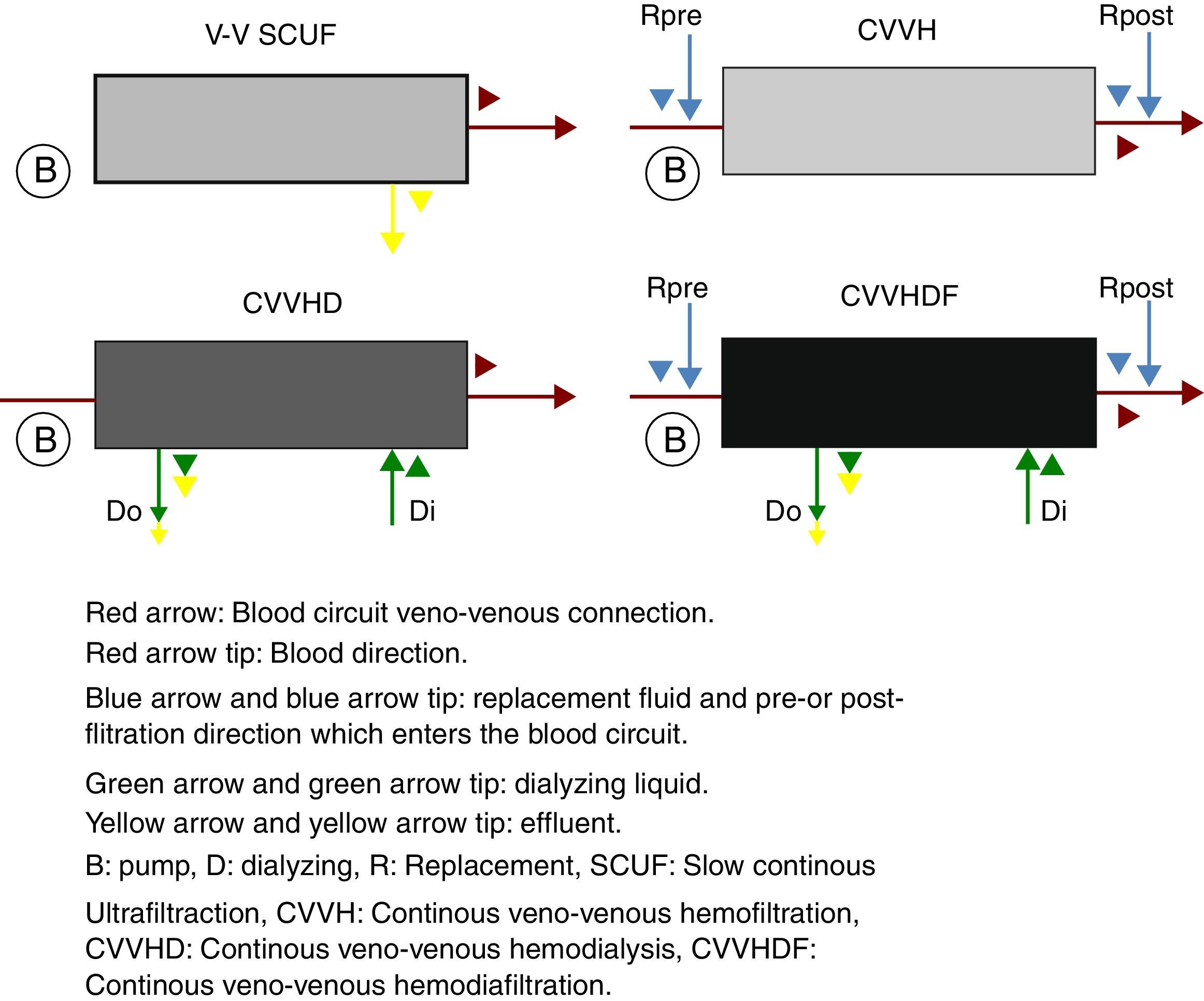
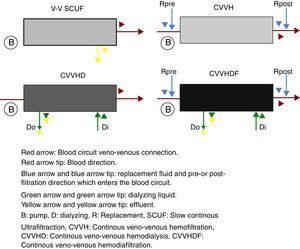
The continuous renal replacement therapies spectrum consists mainly of four modalities (Fig. 2), described hereafter. (1) Slow continuous ultrafiltration (SCUF), which utilizes the ultrafiltration principle to remove excess water in plasma. It is used for low ultrafiltration volumes of 100–500mL/h and does not use dialyzing substances or replacement liquid.22 (2) Continuous veno-venous hemofiltration (CVVH), which through convection is capable of removing both the water in plasma, as well as inflammatory toxins through dragging.18 In this type of therapy, it is necessary to replace the filtered fluid with replacement fluid, which may be infused before (pre-dilutional hemofiltration) or after (post-dilutional) filtering.21 There are advantages and disadvantages to pre- or post-dilutional administration. By infusing the liquid before filtering, blood hemoconcentration is reduced, making the clearing of the different solutes more inefficient, nevertheless prolonging the life of the filtration circuit due to a lower protein agglomeration on the fibers of the filter. On the other hand, by infusing the liquid after filtering, solute clearing is more efficient, yet shortening the circuits life. (3) Continuous veno-venous hemodialysis, which through diffusion accomplishes solute exchange between blood and the dialyzing solution.22 The removal of solutes is proportional to the dialyzing solution flow and from the blood flow in counter flow. This therapy does not require replacement liquid since it does not require liquid removal. (4) Continuous veno-venous hemofiltration, which combines hemofiltration and hemodialysis to accomplish blood purification.18 Among the main advantages of this technique are its hemodynamic stability and high efficiency in toxin clearing. Thus, it is preferred for patients with acute renal injury in a critical state.25
Adapting the dosage in kidney replacement therapyRenal replacement therapy dosage is defined as the quantitative measurement of purified blood, waste and toxins, through some type of renal replacement therapy.24 The amount or dosage of dialysis is measured comparing the initial and end concentration of a particular solute in the patient's blood. The greater the reduction of the solute, the greater the efficiency of the dialysis. However, the clearing of a specific solute does not represent the clearing of all solutes accumulated during the acute kidney injury, since the distribution volume, as well as the clearing kinetic of each particle, are different. Due to its simplicity regarding its clinical measurement and significant accumulation in AKI, creatinine and urea are the reference solutes used to measure clearing in renal replacement therapy.20
Dialysis doses can be described through different aspects: efficiency, intensity, frequency, and efficacy.18,24 The efficiency of renal replacement therapy represents the concept of clearing (K) and is expressed in volume over time (mL/min, mL/h). Clearing of a specific solute depends on several factors, like the molecular size of said solute, the clearing modality utilized (by convection or diffusion.) Also the operational characteristics of the dialysis equipment [blood flow speed (Qs), replacement fluid flow (Qf), dialysis liquid flow (Qd) and size of the hemo-dialyzing]. Blood flow speed depends on the type of vascular access the patient may have. Clearing modality by convection depends on the replacement liquid flow (Qf) and the solute's sieving coefficient (SC). Sieving coefficient is the concentration index of the solute in the filtrated liquid and the water fraction in blood plasma. A 1.0 SC reflects complete permeability, while a value of 0 reflects complete rejection.24 On the other hand, clearing by diffusion is proportional to the speed of the dialysis liquid (Qd) as long as the Qd/Qs index does not exceed 0.3, whenever this index is exceeded, linear relation is lost.
Regarding the intensity of the dialysis dose, this can be defined as the clearing product by time (mL/min*24h, *4h), and is represented by Kt.18 However; it does not consider the amount of solute required to be cleared. Lastly, the efficacy of renal replacement therapy is the result of the effective elimination of a solute as a result of the administration of a dialysis dose.18 Represented by Kt/V, where V is the distribution volume of a specific molecule in the body, in this case, urea, which is equivalent to the total body water of the person (approximately 60% of their weight). Kt/V is an established measurement of dialysis dosage correlated to the survival mean rate in patients with chronic hemodialysis.24 The goal of Kt/V varies depending on the type of renal replacement therapy to accomplish a greater efficiency.
CRRT in septic patientsPathophysiology of AKI in sepsisWe ought to take into consideration the importance of sepsis in AKI in the critical patient, since this is the primary etiological cause of AKI in this group of patients.26 As accurately mentioned by Gomez et al.,27 there have been attempts to establish the bases to understand the complex existing interaction between AKI and sepsis. While it is true that the thing that catches the eye at first is the fact that most AKI cases occur with shock (including sepsis), there is growing evidence showing that AKI may occur in the absence of direct hypoperfusion.27 In addition to other determinants which may trigger AKI, so it occurs as an entity of a multifactorial origin rather than as a result of an isolated insult. In patients with sepsis-induced AKI, we can see a decrease in glomerular filtration rate (GFR) followed by tubular dysfunction, even though this GFR decrease may not occur in all patients.28 The principal mechanisms described as responsible for the manifestation of sepsis-induced AKI include inflammation,29 alterations of micro- and macro-circulation (at a tubular and glomerular level),26 mitochondrial alterations and cellular arrest.30 According to this evidence, Gomez et al.27 proposed a unified vision of sepsis-induced AKI mechanisms, where they highlight the fact that early clinical as well as biochemical manifestations are adaptive responses of tubular cells to damage, and are amplified by the interrelation between inflammation and microvascular dysfunction. Hence, the mitochondria of the tubular cell must adapt and reorganize its structure and function to favor cellular survival in an attempt to avoid apoptosis, triggering the reduction of renal function (at the expense of decreasing tubular absorption and the secretion of solutes).27
When sepsis-induced AKI is already in place, we have precious time on our hands to try to restitute renal function; as mentioned before, there are non-conventional criteria for the start of RRT (Table 2). These include patients with a sepsis-induced AKI diagnosis; additionally, to these criteria, we ought to take into consideration the kinetics of creatinine,31 balance of fluid32 and creatinine adjusted to balance of fluid,33,34 to make a more accurate decision.
Epidemiology of sepsis-induced AKIIn 2007, a study was published where Bagshaw et al.35 included critical patients with AKI and sepsis-induced AKI, reporting high-value data, i.e., the fact that the origin of the sepsis, which later triggers sepsis-induced AKI, is mainly of thoracic and intraabdominal origin in around 54.3% of cases.35 He also described some clinical and laboratory characteristics which differ from non-septic origin AKI, such as normal renal function before the injury, a greater oligoanuria, higher lactate and a longer hospital stay; a lower pH, TAM bicarbonate, PaO2/FiO2 and survival. Some relevant data were the facts that patients with sepsis-induced AKI were patients whose condition was more severe, having higher severity scores in SOFA and SAPS II, as well as the use of vasoactive medications and invasive mechanic ventilation.35
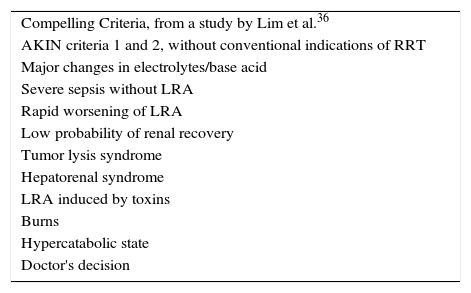
Early RRT in sepsis-induced AKIFor years, there has been a debate on whether or not to use early RRT in patients with sepsis-induced AKI, and if this gives any prognostic advantage amongst these patients. Recently, Lim et al.36 conducted a prospective study in a single center, where they included critical patients with AKI, dividing them into patients with conventional criteria (Table 2) and patients with AKI with “convincing” criteria (Table 4), including patients with sepsis-induced AKI. An interesting observation is that sepsis and ischemia were the main causes of AKI in both groups without a significant difference. The most widely used modality in this study was CRRT among both conventional and non-conventional criteria, with an effluent dose of 35.3–35.6mL/kg/h. They reported that RRT began on day one after being admitted to the ICU, and observed that there was no significant difference in the decrease of mortality in the ICU, as well as in the hospital, in those patients with non-conventional indications. Another interesting observation was the fact that they concluded that patients with RRT with conventional indications had a worse outcome, compared to those patients with an absence of conventional indications.36
Compelling criteria.
| Compelling Criteria, from a study by Lim et al.36 |
| AKIN criteria 1 and 2, without conventional indications of RRT |
| Major changes in electrolytes/base acid |
| Severe sepsis without LRA |
| Rapid worsening of LRA |
| Low probability of renal recovery |
| Tumor lysis syndrome |
| Hepatorenal syndrome |
| LRA induced by toxins |
| Burns |
| Hypercatabolic state |
| Doctor's decision |
Lim CC, Tan CS, Kaushik M, Tan HK. Initiating acute dialysis at earlier Acute Kidney Injury Network stage in critically ill patients without traditional indications does not improve outcome: a prospective cohort study. Nephrology. 2015;20(3):148–154. doi:10.1111/nep.12364.
More recently, a review was published whose objective was to determine the impact of early RRT versus late RRT in critical patients.37 They focused on high-impact studies, including nine studies with 1042 patients. The main problem was the use of the term “early,” since it was defined based on the criteria of the original authors. Thus, the definition was very broad, and therefore, was not exempt of heterogeneity. The main objective was mortality at one month, and the secondary objectives were ICU stay and hospital stay. They concluded that there was no benefit in survival in patients with early RRT. In the analysis of the high-impact studies, there was a trend of a lower mortality in patients where early RRT was conducted, with a mortality rate of 34% (192 out of 555 patients) versus 40.2% (196 out of 487 patients) for early and late RTT, respectively. Hence, we ought to have reservations regarding the conclusions of this review.37 Moreover, they did not include the more recent AKIKI11 and ELAIN studies in their study.38
In a study by Chon et al.39 a hypothesis was proposed suggesting that an early CRRT (<24h) in patients with sepsis-induced AKI may influence the survival rate of said patients. They included 55 patients with sepsis-induced AKI who required CRRT. The most common infection site was the lung (58.2%), followed by abdomen (23.65%), with an APACHE II average of 28.6 and a SOFA score of 13 at the moment of initiating CRRT. An interesting observation was that the “early” group was more likely to suffer from renal dysfunction. They reported a decrease in mortality at 28 days in those patients with an early initiation of CRRT, as well as more ventilator-free days. Another observation worth mentioning is that there was no difference in the mortality between patients with sepsis-induced AKI when comparing AKI severity (RIFLE-I vs. RIFLE-F).39
Very recently, two articles were published which focused on the early use of RRT: the AKIKI Study Group11 and the ELAIN study.38 The first was an open, multicenter, prospective, randomized study. A total of 619 patients were randomized; 311 patients were in the early treatment group, and 308 patients were in the late treatment group, without a significant difference in basal characteristics between both groups. The beginning of RRT for the early treatment group was approximately 2h after randomization, versus 57h in the late treatment group. Sepsis was the main cause of AKI in 80% of patients, and exposure to nephrotoxic drugs was present in 63% of patients. In this study, no significant differences were found between both groups in mortality at 28 days and 60 days, in ICU stay or their hospital stay. Something to take into consideration is that the study only included patients with a KDIGO 3 classification and an ATN diagnosis (in the context of ischemic or toxic injury), therefore, several days passed from the moment the patient was admitted until all the diagnostic requirements were met and RRT was initiated. Additionally, these patients may have had non-conventional indications since their admittance, which were not taken into account. Therefore, it is possible that some patients may have been benefited from RRT and did not receive treatment. Moreover, a post hoc analysis was conducted, comparing patients who never received RRT with those who received either early or late RRT. It found a lower mortality at 60 days in patients who never received RRT (37.1%), yet the higher mortality was found in patients in the late group (61.8%). An intermediate mortality for early RRT (48.5%) was also reported.11
On the other hand, the ELAIN38 study was also suggested as a main objective to determine whether or not early RRT in critical patients reduces mortality at 90 days. The study included 604 patients, of which 231 were included in the randomized groups (112 in the “early” RRT group and 119 in the “late” RRT group). All patients received CRRT with the CVVHDF modality. There were no differences in basal characteristics, nor in the criteria to begin RRT. The mean time of beginning RRT was 6h for the early group and 25.5h for the late group (P<0.0001). At the moment of beginning RRT, there were differences in the levels of creatinine and BUN, which were greater for the late initiation group. Another interesting observation was the mean time to receive RRT in patients with KDIGO2, which was 6h and 25.5h for the early group and the late group, respectively. The most outstanding part of this study was the decrease in mortality at 90 days in the early RRT group (39.3% versus 54.7% in the late RRT group) for a P=0.03, statistically significant. Additionally, a decrease in RRT days was observed (9 days versus 25 days) along with a greater renal recovery rate (53.6% versus 38.7%, P=0.02), less time spent on mechanical ventilation (125.5h versus 181h, P=0.002), and a decrease in the number of days of hospital stay (51 days versus 82 days, P<0.001) between the early and late groups, respectively.38
CRRT doseAnother topic of controversy is the dose utilized for sepsis-induced AKI; there is still no consensus on this matter. Currently, the tendency is the use of high doses of CRRT for sepsis-induced AKI management. The dose used in CRRT management in patients with sepsis-induced AKI has not been established yet. However, there are some studies which may give us some information regarding this problem. The current trend is, to begin with high volumes of effluent in CRRT, particularly at the expense of convective mechanism. This method is preferred since, as explained above, it allows us the depuration of small and moderate molecules. Thus, we expect a cleaning of inflammatory cytokines, improving the condition of the patient.
Back in 2000, Ronco et al.40 conducted a study where they assessed the effects caused by the CRRT dose, where they randomized three groups with three different prescriptions based on the patients’ body weight (20, 35, and 45mL/kg/h). They reported a significant improvement in survival in the groups with the higher doses compared to the smaller dose group. Concluding that according to these results the minimum required dose prescribed to a patient with sepsis-induced AKI should be 35mL/kg/h.40
Unfortunately, after this paper, the same improvement in mortality has not been seen when using doses higher than 35mL/kg/h. Bouman et al.41 conducted a study in acutely ill ventilated patients who had aggressive fluid resuscitation and remained oliguric despite the high doses of diuretics. The mean dose was 48.2mL/kg/h in the high-volume group versus 20.1mL/kg/h in the low-volume group, without finding an impact in the decrease of mortality in neither group. Hence, they concluded that a high initial volume did not improve the survival rate, nor did it improve renal recovery. The time of beginning RRT was when they met all the inclusion requirements. Therefore, the mean time was within the first 12h; moreover, it is possible that the diuretic dose may delay the beginning of RRT, knowing that a furosemide stress test could have been applied42,43 in an attempt to try to predict renal function behavior in these patients and begin early RRT.
In 2005, Ratanarat et al.44 proposed the use of high volume HDF (HVHDF) of 6 to 8 continuous hours, followed by CVVHDF with a conventional dose of 85mL/kg/h for 6h a day. They included five patients. They conducted HVHDF with a dose of 85mL/kg/h for 6h a day, followed by CVVHDF at 35mL/kg/h for 18h, which at the end of the day resulted in an accumulated dose of 48mL/kg/h. The interesting part of this article was the hemodynamic benefit of the high-volume pulses since they reported a decrease in the requirements of pressors, as well as an improvement of TAM, a benefit that lasted for up to 12h after treatment.44 These same hemodynamic benefits were reported by other studies.45–48
In more recent years, some studies have been published that explore the use of higher doses of CVVHDF in septic patients. Like Clark et al.,49 who performed a systematic review of the literature, limiting the search to studies that included effluents greater than 50ml/kg/h against low doses in patients with sepsis and septic shock. The primary endpoint was mortality at 28 days. They concluded that there is still insufficient evidence to recommend routine high-volume use in patients with sepsis-induced AKI. Tamme et al.48 had as main objective to evaluate if the method of RRT influenced the profile of cytokines in critically ill patients with septic shock. They evaluated hemodynamic variables and cytokine concentrations before and after CVVHDF using mainly a prediluted mode and maintaining a blood flow of 200ml/min. They used doses of 123ml/kg/h. and maintaining the dose for 10h. They did not report differences in cytokine washing before and after the use of RRT, although they found improvements in the hemodynamic profiles of the patients, which allowed them to significantly reduce the requirements of norepinephrine.48
In more recent years, some studies have been published exploring the use of higher doses of CVVHDF in septic patients. Like Clark et al.49 who undertook a systematic review of the literature, limiting the search to studies that included only effluents greater than 50ml/kg/h against low doses in patients with sepsis and septic shock. The primary endpoint was mortality at 28 days. The conclusion reached was that there is still insufficient evidence to recommend routine high-volume use in patients with sepsis-induced AKI. Likewise, Tamme et al.,48 had as main objective to evaluate if the RRT method influences the profile of cytokines in critical patients with septic shock. They evaluated hemodynamic variables and cytokine concentrations before and after CVVHDF using mainly in prediluted mode and maintaining blood flow of 200mL/min. They used doses of 123ml/kg/h and maintaining it for l0h there is still insufficient evidence to recommend routine high-volume use in patients with sepsis-induced AKI. Likewise, Tamme et al.,48 had as main objective to evaluate if the RRT method influences the profile of cytokines in critical patients with a prescription for 10h. They did not report differences in the cytokine wash before and after the use of RRT, although they found an improvement in the hemodynamic profile of the patients, which allowed a significant decrease in the requirements of norepinephrine.48
ConclusionsGiven the complexity of a patient with sepsis and AKI, the optimization of medical management is a real challenge. Thus, the use of early CRRT ought to be considered from the start in these patients in search of returning to basal homeostasis. Therefore, we can conclude that in the use of CRRT, the standard dose in sepsis-induced AKI should be no less than 35mL/kg/h. Moreover, in special cases of patients with a great inflammatory response and requirement of pressors in the context of septic shock, pulses of CVVHDF in very high volumes could be used (>60mL/kg/h). Also, we ought to consider the accumulated positive balance in the decision to begin RRT without waiting for conventional indications. All of this being ideal during the first hours of AKI KDIGO2.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.