Immunization -whether from polio, typhoid, flu or whooping cough- is never absolute. A shot in the arm may save your life -but you can't always rely on it... Nor is any immunization absolutely safe.1
Influenza is a major cause of morbidity and mortality; current estimates by the World Health Organization (WHO) are 3 to 5 million cases and 250,000 to 500,000 deaths worldwide every year.2 Most deaths associated with it occur among people age 65 or older, as well as among persons suffering a chronic debilitating disease regardless of age. The recent 2009 pandemic served to foster interest in this disease.3
An inactivated virus vaccine has been available since the late 1940´s but it only began to be used extensively when the influenza virus antigenic variability was taken into account. Aside from such variability, influenza viruses are capable of infecting a wide variety of vertebrates,4 including many avian species, both wild and domestic, thus it is essential to monitor the antigenic characteristics of influenza virus strains currently circulating, and so the vaccine formula has to be evaluated and modified accordingly every year.
Vaccine indications
The efficacy of influenza vaccine is relatively low (70%-90%)5 and vaccinated persons could have insufficient protection even to homologous virus strains, not to mention those viruses that have undergone antigenic changes, either drift or shift. Furthermore, other respiratory viruses such as parainfluenza, adenoviruses or respiratory syncytial virus could cause a similar illness, frequent anecdotal comments of acute respiratory illness (ARI) coincident with vaccine application is therefore not too surprising.
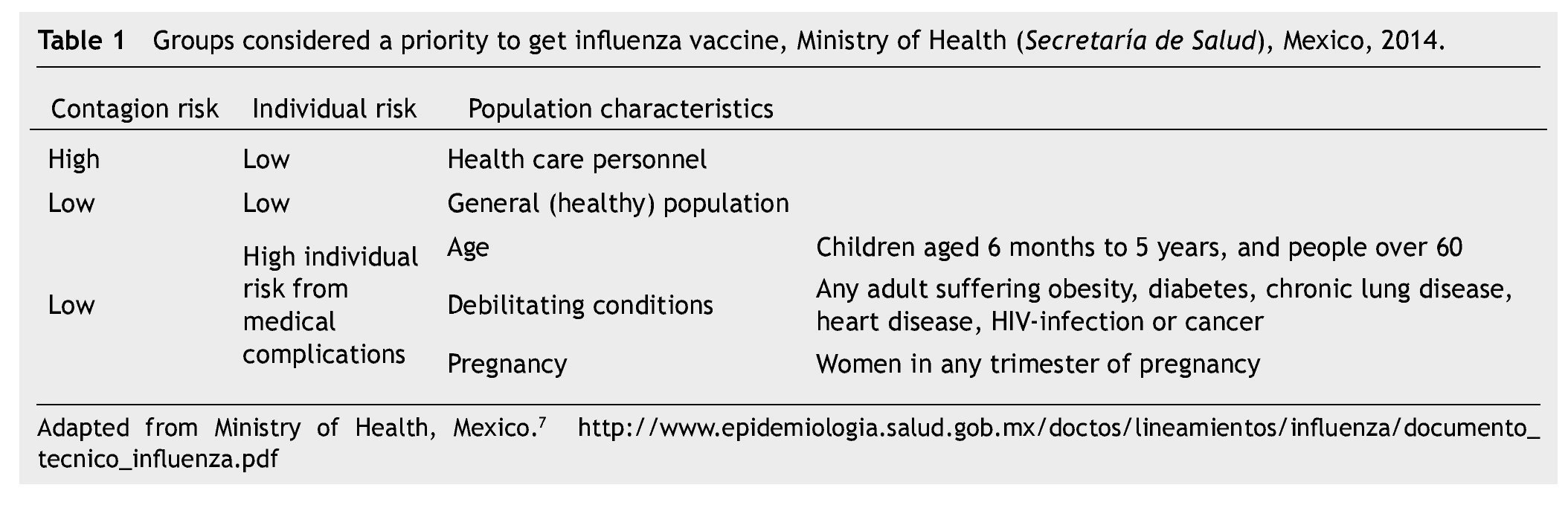
The risk of complications during an influenza episode, leading to hospitalization and death is higher in older people (≥ 65 years) and in those patients undergoing any of a well-known list of chronic debilitating diseases,6 yet the benefit of the influenza vaccine should be weighted in different situations. In Mexico, the Ministry of Health (Secretaría de Salud) recommends vaccine application to people belonging to certain groups7 (Table 1).
Additional information to make better particular recommendations for influenza vaccine use is available from WHO,8 as well as from the Advisory Committee on Immunization Practices9 in the United States of America:
• Healthy individuals: vaccination may be recommended from age 50 onwards.
• Adults and children with health conditions such as chronic pulmonary disease (including asthma) or cardiovascular (except isolated hypertension), renal, hepatic, neurological, hematologic, or metabolic disorders (including diabetes mellitus).
• Persons who have immunosuppression, including compromised immune systems caused by medications or human immunodeficiency virus (HIV) infection.
• Women who are or will be pregnant during the influenza season.
• Children and adolescents (aged 6 months-18 years) who are receiving long-term aspirin therapy and who might be at risk for experiencing Reye's syndrome after influenza virus infection.
• Residents of nursing homes and other long-term care facilities.
• Persons who are morbidly obese, with a body mass index (BMI) over 39.
Vaccine use and its public perception
Annual vaccination should take place ideally before the "flu season" starts, that is, the months of September-October of each year; if uptake in this period is missed however, later vaccination is always encouraged, especially for persons at risk.
Although influenza vaccine is recommended by the WHO and is firmly established worldwide as an effective measure for influenza control, the number of persons who receive influenza vaccine each year is very low even in countries with good health systems.6,9 Fear of adverse effects has discouraged public vaccine acceptance ever since Edward Jenner first proposed systematic smallpox prevention through cowpox immunization. The roots of this universal phenomenon are myths and misinformation such as beliefs of vaccine being the cause of disease, lack of vaccine efficacy, refusal to get medical care, or plain mistrust of the health care system. A combination of these factors results in deficient vaccine coverage.
Data on factors influencing vaccine uptake, such as age, gender, co-morbidity, educational level, income and area of residence are important. However, recent research provides an insight on the reasons for vaccination acceptance or rejection; an improvement on vaccine acceptance requires a significant level of knowledge and understanding of health beliefs, attitudes, perceptions and subjective experiences both on individual and collective levels. This is particularly evident in older people, who decide to be vaccinated based on the interpretation and evaluation of beliefs about whether it could cause or prevent colds and influenza, and the importance of side effects. Older people's subjective assessment of their own health is often incongruent with objective assessment.10
A group of police, fire fighters and prison workers in Spain, regarded as essential community workers, surveyed by Caballero et al.11 showed that the vaccine was better accepted by those who never had doubts about vaccine safety.
In 2009, the Ministry of Health in France purchased 94 million vaccine doses to ensure the vaccination of 65 million citizens. Yet, there was a low uptake of the vaccine that could have been related to a lack of high quality advice about the benefits of getting vaccinated; the same study also postulated that media and social networks may have contributed to raise undue concerns in the population. Participation of general practitioners may help to improve vaccine perception by providing face-to-face professional advice and information.12
Considerations for improvement
Many countries show vaccine uptake rates less than 50% in health care workers (HCW). Livni et al. found the overall vaccination rate among a group of pediatric HCW in Israel was 46.8%. Their data show that knowledge about the influenza vaccine by health care personnel leads to better vaccination rates.13
Blasi et al. suggest improving communication between health authorities, scientific societies, HCW and general population through simple, clear, honest and straightforward messages to ensure unbiased information about the vaccine is the basis for a person to accept it.14
Septimus et al. established a mandatory vaccination program for HCW aimed to foster patient safety, including categories for professional employees with patient care (clinical) duties as well as any other person who could be in the premises. The basis to establish these categories are: 1) access to clinical areas, and 2) work area within a 2 meter distance from the patient.15
Influenza vaccination rates are particularly low among marginalized, hard-to-reach urban populations, so intervention activities are to be designed with a high degree of inter-institutional cooperation, taking into account neighborhood particularities, strong community organization, and individual orientation.16
Probabilistic models have the power to handle large amounts of data; these models are also suited to analyze factors such as weather (low temperature, humidity, and rainfall), which has been widely anecdotically considered as associated with seasonal variation of ARI and influenza, and to enable better decision making, vaccination campaign planning and resource allocation during epidemics.17
American Indians and Alaskan natives are also groups targeted for influenza vaccination in the United States of America, so we propose to study the benefits of preventing influenza in Mexican and other Meso-American ethnic groups.
As we know from the past, fear and concern about vaccine safety have been present from the beginning of vaccination during the 19th Century (Fig. 1). With an ever-increasing amount of internet and social network users, anti-vaccination messages lacking scientific foundation may keep the public at large ill-informed and scared. HCW should be the best-informed group, with a solid knowledge of vaccination benefits and side effects. Vaccine perception should not be a black and white picture, but rather a balance between the many benefits obtained contrasted with a number of known and expected adverse effects. We have long postulated that a sound application of any vaccine has to be a carefully crafted benefit vs. risk evaluation, in other words, adverse reactions are to be considered the lesser evil18 given the higher hospitalization and death rates among high-risk groups.
Figure 1 Graphic depiction of fear elicited by smallpox vaccination in 1802. Painting by James Gillray (1756-1815). Image downloaded from the United States library of Congress's prints and Photographs division under the digital ID cph.3g03147. This artistic work belongs in the public Domain according to World Trade Organization Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS), 1994.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No financial support was provided.
Received: March 2014;
Accepted: April 2014
• Corresponding author:
Centro de Investigación y Desarrollo en Ciencias de la Salud,
(CIDICS) Universidad Autónoma de Nuevo León.
Carlos Canseco s/n and Av. Gonzalitos, Mitras Centro, C.P.
64460, Monterrey, N. L., Mexico. Telephone: 1340 4370 (J. G. Velasco-Castañón).