Introduction
Allergic rhinitis (AR) is an inflammatory disease of the nose that is mediated by IgE after the repeated exposure to an allergen, mainly through inhalation which produces nose, eye and pharyngeal symptoms.1,2 Although it is generally not considered to be a severe disorder, the socioeconomic costs of AR are substantial. AR is a major problem of public health affecting up to 24% of the adult population and up to 42% of the pediatric population. Four hundred million persons worldwide have allergic rhinitis. In addition up to 70% of persons have reported having asthma have also reported having AR.3
In recent years, in addition to traditional subcutaneous immunotherapy (SCIT)4,5,6 sublingual immunotherapy (SLIT) has become a standard treatment for both children and adults.7,8 Its efficacy has been evaluated and the WHO (World Health Organization) has approved its clinical use9 SLIT has become popular in Europe, Asia and Australia and is becoming increasingly so in the United States.10 Nevertheless, despite its ever increasing use in Latin America, there have only been a few double-blind, placebo-controlled (DBPC) clinical studies regarding the efficacy of SLIT in Latin American populations.11 To date, there are still some uncertain issues with SLIT regarding the magnitude of its clinical efficacy on allergic rhinitis, the length of treatment and the standard dose to be used. Efficacy of SLIT is achieved a doses ranking between 2-500 times of those used for SCIT.12,13
The aim of this study was to assess the use of SLIT in adult, Mexican patients with AR due to house dust mite Dermatophagoides pteronyssinus, using a low doses scheme during a 6 month period (24 weeks). We evaluated efficacy in regard of symptom reduction, relief medication use, adverse reaction to therapy and compliance to this SLIT scheme.
Material and methods
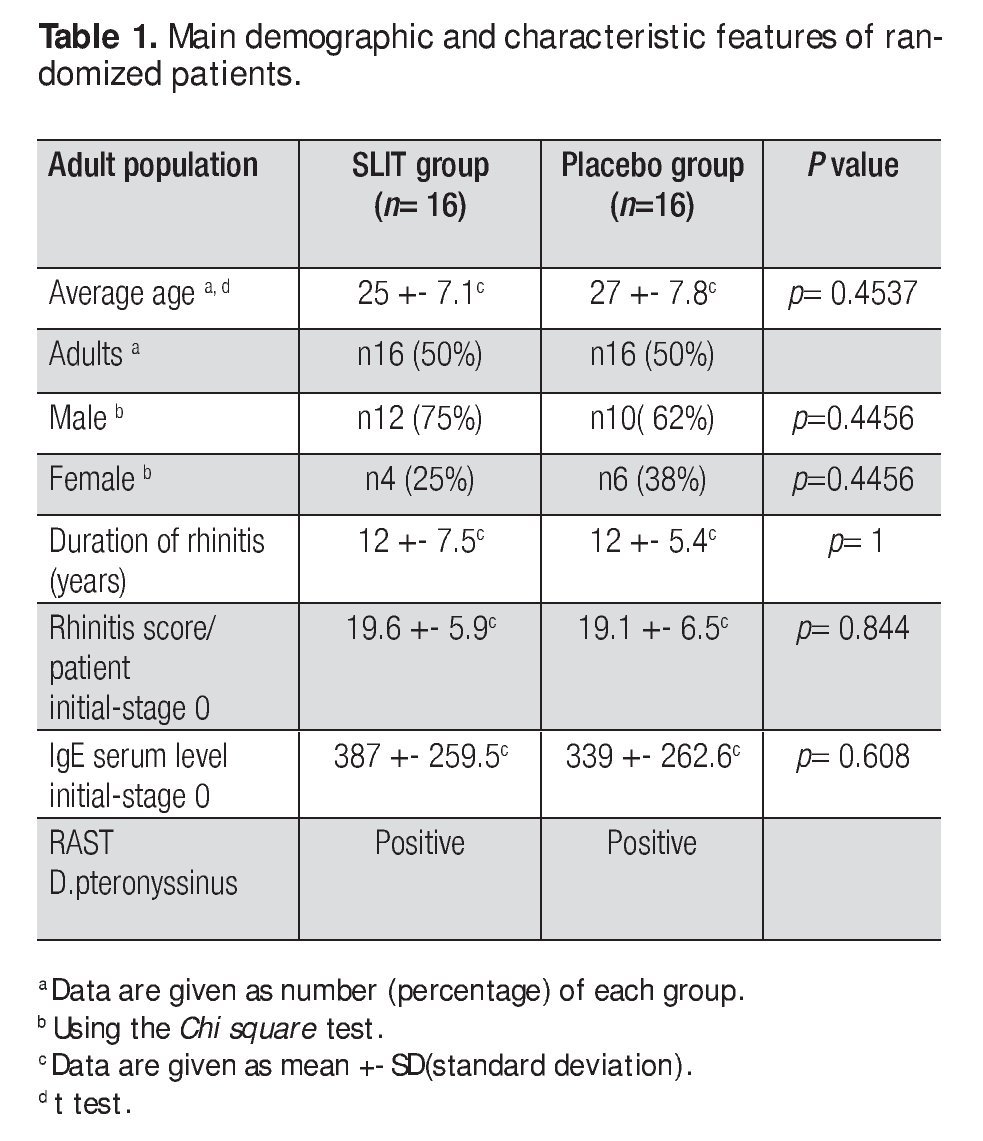
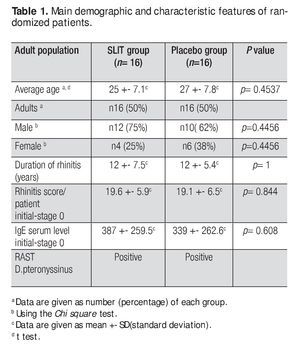
This study was double-blind, placebo-controlled (DBPC), and randomized and was approved by the ethics and research committee of the Faculty of Medicine and University Hospital of UANL in Monterrey, Mexico in 2001-2002. We included adult patients with diagnosis of chronic, moderate to severe AR: 22 male (69%) and 10 female (31%) patients aged from 18 to 40 years. The demographics of the patients are shown in Table 1.
All of the patients understood the study and signed the informed consent letter. The inclusion criteria were: 1) a history of moderate to severe AR without seasonal pattern and according to ARIA,2 2) a positive skin (++ or greater) test for Dermatophagoidespteronyssinus 3) a positive IgE test by immunoenzymatic assay at levels higher than 300 UI) specific to D. pteronyssinus. 4) eosinophilia higher than 5% in both nose mucosa and blood, 5) elevated serum, total IgE levels, 6) willingness to participate in the study and 7) a willingness and commitment to compliance.
Exclusion criteria were: 1) previous immunotherapy treatment up to two years prior to the study, 2) use of local or systemic steroids or, chromoglycate or antihistaminic medication up to one month before the start of study, 3) pregnancy and 4) lack of commitment to compliance.
Clinical evaluation
A complete clinical evaluation and clinical history was made at the start of the study at 30 days, and at weeks 6, 12 18 and 24. As previously mentioned all of the patients were selected according to ARIA2 and all of the patients required medication for the control of AR symptoms.
Symptom scores
The patients were instructed to complete a symptom diary, which was filled up once at the end of the day, on a daily basis throughout the study, for a record of nasal, pharyngeal and ocular symptoms, and the occurrence of any adverse symptoms and compliance of the doses.
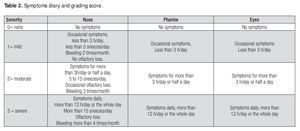
Twelve symptoms were evaluated: a) nasal (nasal obstruction, itching, rhinorrhea and sneezing), b) pharyngeal (pharyngeal itching, pharyngeal pain, sore throat and retronasal discharge) and c) ocular (eye redness, sore eye, ocular itching and tearing). The symptoms were recorded in a symptom-diary designed for this purpose and the symptoms varied from patient to patient accordingly the number and severity of symptoms; we added only the severity of symptoms to Table 6, the patients had variations of symptoms in the number and severity; we added only the severity of symptoms.
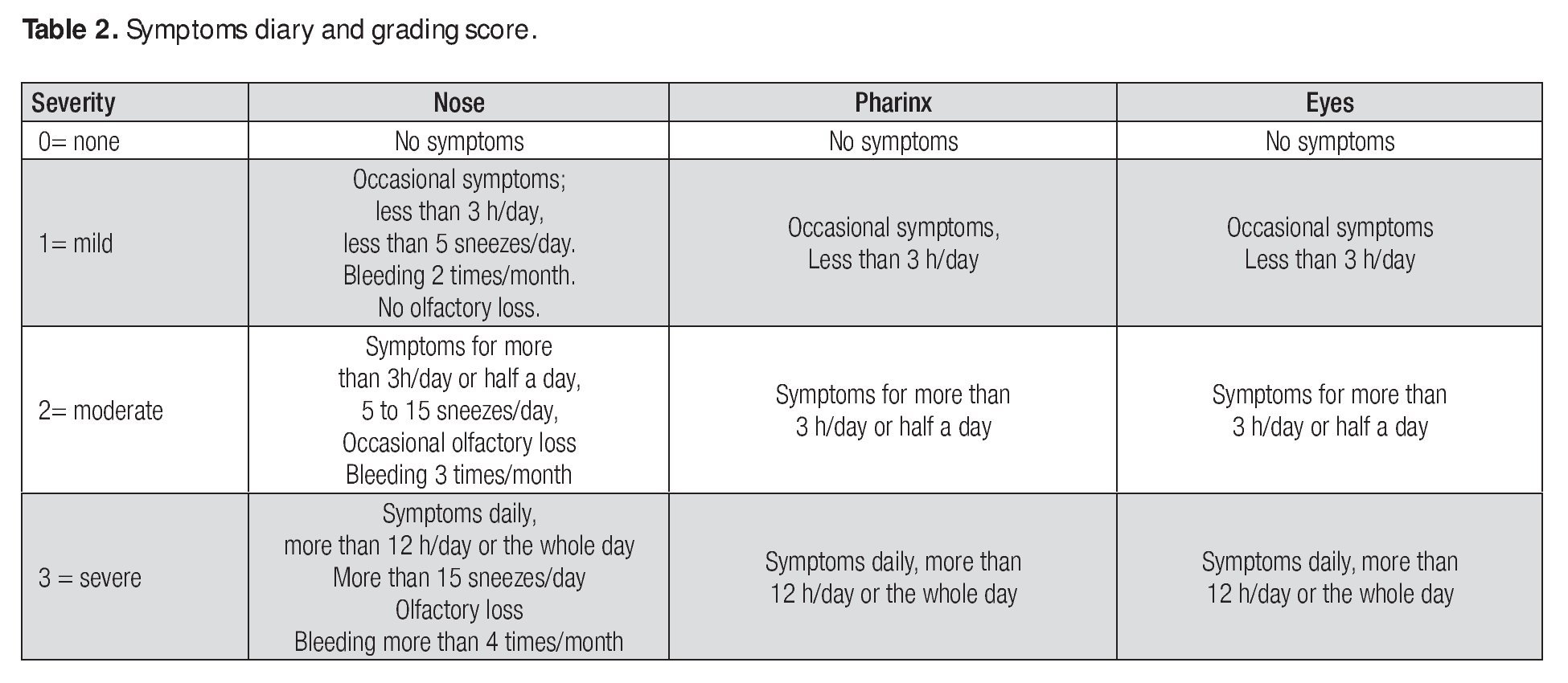
The diary was given to the patients for accurate record keeping at each of the four evaluation visits throughout the 24 week study and they recorded symptoms based on their presence and severity. Symptoms were graded as: negative (0), mild (1), moderate (2) and severe (3) (Table 2) Patients were required to record their drug intake and any symptoms or discomfort, local or systemic, possibly related to treatment, in the diary.
Skin tests
A percutaneous test for 24 aeroallergens was performed, and included Dermatophagoides pteronyssinus. The reaction was read after 20 minutes and was then compared against the positive controls histamine and the negative control diluent solution. The reaction size was graded from 0 to +++. Skin tests were performed again at Stage 4 for an evaluation of change in skin reactivity.
Immunoglobulin E levels
Total serum IgE was measured by a commercial ELISA- sandwich test (Sanofi-Pasteur, France). Specific IgE levels to house dust mite Dermatophagoides pteronyssinus (made only for the purpose of meeting diagnostic inclusion criteria) was produced in an independent laboratory.
Eosinophil count in blood and nasal mucosa
Nasal cytology was performed using the technique described elsewhere.14 Eosinophilia in the nasal mucosa membrane was graded by the number of cells in each microscopic field and the scores ranged from 0 to +++. Eosinophilia in blood was determined by automated means.
Allergen preparation
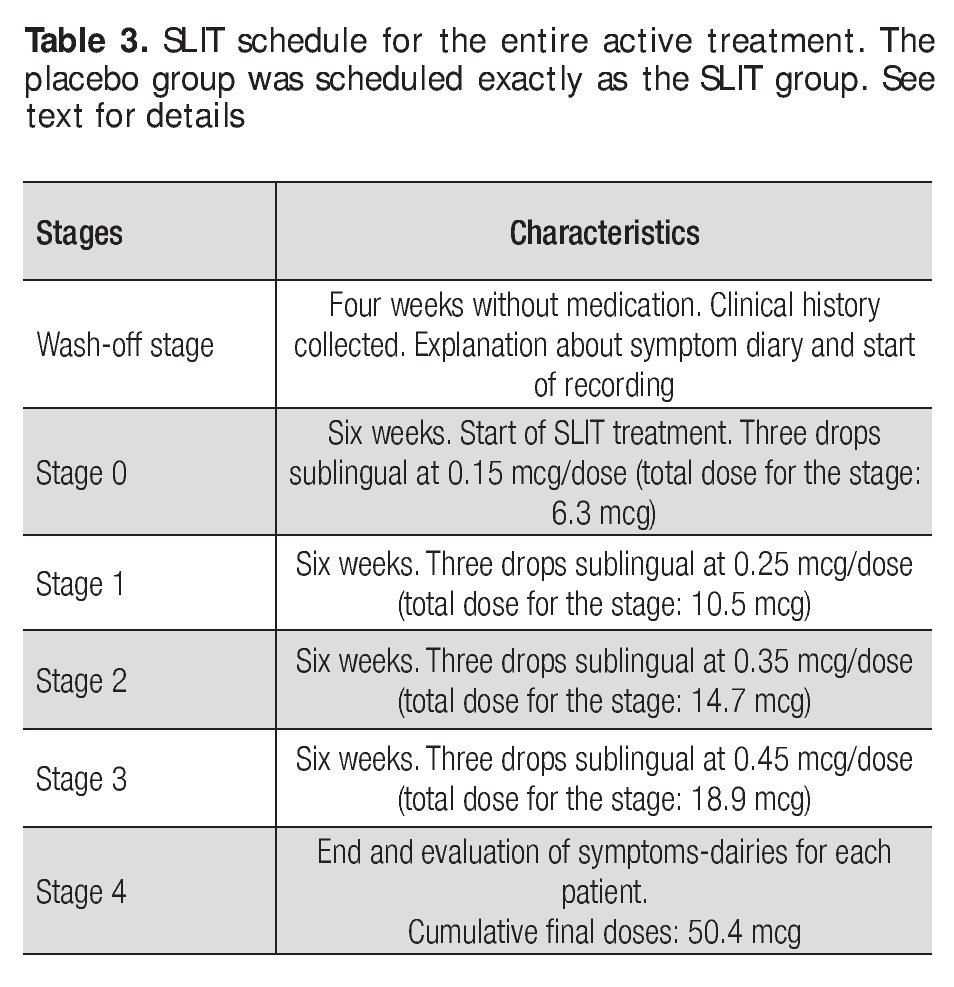
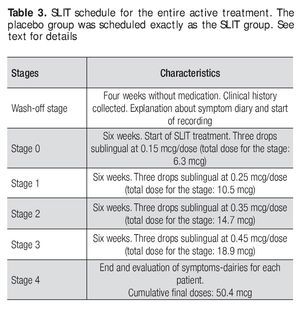
The allergen extract of D. pteronyssinus prepared for treatment was made by the Allergen Laboratory of the Immunology Service University Hospital of UANL The base solution was prepared a concentration allergen protein of 1mg/ml, from which all of the diluted concentrations for treatment were derived. The active SLIT was scheduled and administered as described below, in the form of three drops applied sublingually. This routine was performed daily (in the morning before breakfast) and the patients were told to hold the drops sublingually for one minute, prior to swallowing Table 3.
Treatment
The treatment was given for 24 weeks. After an initial period of 30 days (wash-out period), patients with a diagnosis of AR, were randomly divided in two groups, one with 16 patients who received the active SLIT treatment and a second with 16 patients who received the placebo treatment. All patients were allowed to use antihistaminic and nasal steroid medication for symptoms control and were advised to accurately record its use in the symptoms diary. Record keeping was used to assess the need for medication at the end of the treatment. The treatment of the SLIT group was administered in 4 stages with increasing doses given as follows: a) recruiting period (no treatment) b) wash-out stage (no treatment) c) Stage 0: 0.15 mcg/day dose for 6 weeks, d) Stage 1: 0.25 mcg/day dose for 6 weeks e) Stage 2: 0.35 mcg/day dose for 6 weeks, f) Stage 3: 0.45 mcg/day dose for 6 weeks and g) Stage 4 (end of study). At the end of each stage (including the wash-out period) a new symptom dairy was given to the patients. Eosinophil counts and scratch tests were done at the end of the wash-out period and at Stage 4. The placebo group followed the same schedule, but the flasks given to these patients were prepared only with diluent solution (Table 3).
Compliance
In the symptoms diary, patients had to fill a daily form to assess compliance. We evaluated patients' compliance reviewing the dairies every six weeks, to detect any failure or interruption of treatment.
Statistical methods
To determine the number of patients to be included in the study for a significant difference between groups, we calculated the number of patients for a = 0.05 and b = 0.80 to be 14 per group. For possible dropouts during the study, we choose a samples size of 16. The variables with parametric distribution were expressed as mean and standard deviation; those with non-parametric distribution are presented as median and rank. Comparison of mean were made by ´Students T test. Comparison of median was made by Mann-Withney test and for proportion, x2 test was used. For comparisons a significance level of 0.05 was used The statistical management of data was performed by a licensed program STAT-IC-2008
Results
Demographic characteristics
We screened 166 patients with symptoms of AR and 32 were selected who met the criteria to be included in the study. The features of the groups are shown in Table 1. No differences were observed between the two groups in terms of patients features (age, sex and duration of disease) as confirmed by statistic analysis. There were no dropouts from the two groups, so the final number of each group was 16. Analysis of symptom diary showed neither systemic nor local side effects recorded during SLIT. Based on the clinical records and the inclusion criteria the AR was considered to be produced by D pteronyssinus, a household allergen affecting the indoor population.
Furthermore, there were no exacerbation of symptoms that could be attributed to seasonal allergens, confirming our inicial observation that no other major seasonal allergens were identified as leading symptom cause in the selected patients.
Symptom scores
We compared the symptom data recorded at the wash-out stage with that of Stage 4 after 24 weeks of treatment. Analysis of the symptoms diaries showed changes in the intensity and frequency of symptoms, as shown in Table 4; differences between the active and placebo groups were recorded with regard to nasal, ocular and pharyngeal symptoms. Clear, statistically significant differences were observed between SLIT and placebo group. Table 4 show differences between the two groups with regard to nasal symptoms, in which a statistically significant difference was recorded. Furthermore, a comparison of symptoms between groups showed a significant improvement in the active SLIT group versus the placebo group regarding nasal pharyngeal and ocular symptoms.
Medication scores
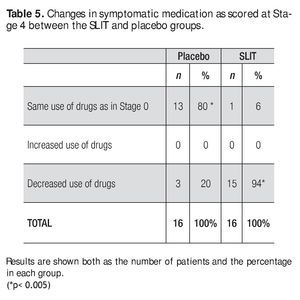
Regarding the use of medication for symptoms control, there was a significant difference between groups: 94% of patients in the active group reported a reduction or withdrawal of symptomatic drug use during the treatment period of 24 weeks, whereas it happened in the 20% of patients in the placebo group (Table 5).
Skin reactivity
Skin reactivity was measured from 0 to +++ and tested at the start and end of treatment schedule: the active group showed a reduction in reactivity or a negative test result in 50% of the SLIT treated patients, whereas in the placebo group no patients had reduced reactivity or negative test results. In this latter group all patients remained with either enhanced or identical skin reactivity as when compared to the start of the study (Table 6). In eight (50% of patients in the active Group, there was a reduction or a negative skin test, whereas eight remained unchanged. In the placebo Group four patients had an augmented reaction whereas 12 remained unchanged.
Eosinophil count in blood and nasal mucosa
There was no significant difference between the two groups regarding eosinophils in the nasal cytology mucous mucosa membrane, for in the active SLIT group mucosal eosinophils were reduced in 37%, whereas in placebo group it was reduced in 25% of patients. However, the blood eosinophil count was significantly reduced in the active SLIT group in 69% of patients and in placebo group it was reduced only in 13% of patients. (Table 7).
Side effects
After the assessment of the symptoms diary of each patient, we found that SLIT was well tolerated by all 16 patients studied in the active group. No adverse effects were recorded in the placebo group either. In any of the clinical evaluations made every six weeks, adverse reactions were recorded by the patients.
Compliance
We evaluated patients' compliance reviewing the dairies every six weeks, and as a whole at the end of the scheduled treatment with SLIT /placebo. No patients referred failure in the administration of the therapy, or any failure or interruption of treatment.
Discussion
The present work is a double-blind, placebo-controlled trial (DBPC), which compared the clinical efficacy of SLIT in a group of Mexican adults with moderate to severe chronic AR. Our results showed a efficacy of SLIT over a 24 week period and with standardized doses increasing every 6 weeks. Our results are in agreement with the mainstream of DBPC studies on SLIT that have been analyzed in several meta-analyses13,15,16,17 and confirm the efficacy of this route for desensitization therapy. Thus, we have added a study carried out in a Latin American population to the body of evidence in favor of SLIT. However, some other aspects of SLIT therapy, such as dose and time of treatment remain key issues for analysis and discussion.18 In the present study we used a doses 3-fold higher than the subcutaneous dose starting at 0.15 mcg/ day and increasing every 6 weeks to the end with a dose of 0.45 mcg/day (3 times higher than the starting dose). In the available studies made from 1986 -2003 of SLIT Clinical studies with SLIT from 1986 to 2003 showed variably efficacy from 20% to 50% using different allergens, the magnitude of clinical efficacy ranged between 20% and 50%, and no severe systemic adverse events were reported in the literature over 15 years17 A remarkable finding was that no patients in this study had any adverse effects related to SLIT. This could be attributed to a lower dose,19 and may have resulted in the absence of dropouts and very good compliance shown by patients. It has been recorded that higher doses could be related to a higher efficacy of SLIT, but at the same time they may have a higher frequency of adverse effects.20,21 According to some authors that have reviewed immunotherapy doses, SLIT involves doses calculated between 2 and 500 times those used for subcutaneous immunotherapy.7,17
Our results with the use of 3 times the corresponding doses of SCIT and cumulative doses of 50.4 mcg belong to the low dose group and therefore cannot appear to be in agreement with the current consensus that the use of high doses of allergen is the better way to deliver SLIT with good results.22,23
Passalacqua et al,24 using an 8-month scheme with a cumulative Parietaria dose of 16 mcg for AR and asthma, showed a significantly improved symptom score and a reduction in the drug use and nasal neutrophil and eosinophil counts. No adverse reactions were recorded. Similarly, Purello-D'Ambrosio et al. had positive results using a cumulative dose of 12.7 mcg of Parietaria in a 7-month scheme, with respect to AR and asthma, both in the clinical responses and drug scores and without any patients reporting adverse effects.24,25 These two studies were similar to ours, as we also had positive results using a low cumulative doses over a 6 to 8 month period of treatment. Our study shows further evidence that low doses of SLIT with D pteronyssinus, may provide an effective alternative to SCIT without any adverse effects. The standarization of the doses for SLIT is an important issue in need of further study and clear recommendations. One factor affecting the standardization of allergen solutions for SLIT is the use of different units for the grading of potency/activity of allergens. In the extended use of SLIT, references to international units (IU), biological units, (BU), the index of reactivity (IR), standard therapeutic units (STU), allergy units (AU) and the simple w/v allergen concentration are made,26 all of them indicating standardized allergens and making the field confusing and unclear. This hampers a direct comparison of schemes and schedules for SLIT. Choosing to use micrograms /ml concentrations of allergen protein, we deliberately used protein doses expressed as weight units, which we believe are beyond subjective interpretations or changes in biological activity that cannot be easily determined. Regardless of the consensus on units to be used in the near future in SLIT, it remains to be further determined whether the clinical efficacy of SLIT is unequivocally and directly proportional to the administered dose.7,16,27,28
Compliance for treatment is another issue that is under debate for SLIT.29 As for any form of immunotherapy, compliance is determinant for a successful SLIT scheme.29 This was again observed in our study were a high compliance by the patients was recorded. Passalacqua et al. point out that adherence to SLIT could be expected to be lower than in SCIT, for SLIT self-managed by the patient. Although a direct comparison of SCIT vs SLIT regarding compliance was not done and was not their goal, by studying compliance on SLIT at 3 and 6 months of treatment they found compliance to be satisfactory, with once-daily administration and a simple updosing schedule playing a relevant role in improving compliance.30 We had no dropouts or irregularities in treatment compliance and all patients took all scheduled doses. Interestingly, many of our patients were reactive to multiple allergens; nevertheless they had a good response to Dermatophagoides monotherapy. This is an important issue that should be addressed in further research, SLIT monotherapy could be administered in patients with skin positivity to different allergens, but with symptoms to a specific allergen that are referred to and related by the patient.
In conclusion, our study shows that this SLIT-scheme is effective reducing symptoms, reduction of symptomatic drug use and has no adverse reaction.
Acknowledgements: This study was by Grants PAYCITSA09698, UANL and financial support form the Faculty of Medicine, UANL (Drs Jesus Ancer-Rodríguez and Santos Guzmán-López). We acknowledge the valuable support from Drs. Donato Saldívar-Rodríguez, Sandra González-Diaz, José E. Gonzalez-Morales, Marisela García-Hernández, MSc. Graciela Ramírez-Guerrero and Carmen Canizales Tejada.
Corresponding author:
Dr. Carlos E. Medina De la Garza.
Departamento de Inmunología, Facultad de Medicina y Hospital Universitario Dr. José E. González. Universidad Autónoma de Nuevo León. Avenida Gonzalitos No. 235 Norte. Colonia Mitras Centro. CP64460. Monterrey, Nuevo León, México.
Telephone: (+52 81) 8329 4211.
E mail:carlos.medina@uanl.mx
Received: November, 2009.
Accepted: January, 2010.