In recent years new therapies have emerged for the management of metastatic renal cell carcinoma, from agents directed to the inhibition of the vascular endothelial growth factor (VEGF) pathway and mammalian target of rapamycin (mTOR) to novel immunotherapy agents targeting immune checkpoint inhibitors. Such is the case with nivolumab, a fully human monoclonal IgG4 antibody directed to block the interaction between PD-1 and PD 1 and 2 ligands, thus avoiding T-cell inhibition by tumor cells. We report two patients with metastatic renal cell carcinoma (mRCC) treated at the University Cancer Center at a tertiary level teaching hospital in Northeastern Mexico with nivolumab and ipilimumab, an anti-cytotoxic T-lymphocyte-associated antigen (CTLA-4) antibody directed to block the interaction between the B-7 family of molecules (CD80–D86) on antigen presenting cells and CTLA-4 expressed by T cells, which inhibits T-cell proliferation and function when triggered.
Renal cell carcinoma (RCC) is the second most common urogenital malignancy worldwide, accounting for 2.4% of all cancers. Incidence is greater in high income countries such as in the USA (3.6%) and Europe (3.3%), while in Mexico kidney cancer represents 2.6% of all cancer diagnoses.1 According to Globocan, in the year 2012 there were 3851 new cases, and 2115 deaths due to kidney cancer in Mexico.1 Median age at diagnosis varies according to geographical regions; data from the Surveillance, Epidemiology and End Results Program (SEER) of the National Cancer Institute reports a median age of diagnosis in the United States of 64 years, and a men to women ratio of two to one (2:1),2 meanwhile data from our University Cancer Center from 2004 to 2014, report a total of 234 patients with RCC, 63% male and 37% female, and a median age at diagnosis of 55 years, 9 years younger in comparison with the statistic from the SEER program.
The WHO classifies RCCs into clear cell RCC (ccRCC), type 1 and 2 papillary RCC, cromophobe RCC, medullary RCC, and others. There are 24 known subtypes of renal cell carcinomas, but the most frequent of all is the clear cell subtype (ccRCC) which accounts for 70 to 75% of all kidney malignancies.3 Finally, up to 30% of all patients with RCC present metastases at diagnosis. In these individuals, prognosis will depend upon the histologic subtype, as well on clinical characteristics such as performance status, time from the diagnosis to systemic treatment, hemoglobin, DHL, neutrophil count, platelet count and corrected calcium levels. One of the most commonly-used prognostic systems is the one published by the Memorial Sloan Kettering Cancer Center (MSKCC) which organizes groups into good risk, intermediate risk and poor risk, with a median time of survival of 29.6 months, 13.8 months and 4.9 months, respectively.4 In this paper, we present two case reports of patients with metastatic renal cell carcinoma (mRCC) treated at the University Cancer Center at a tertiary level teaching hospital in Northeastern Mexico with nivolumab and ipilimumab.
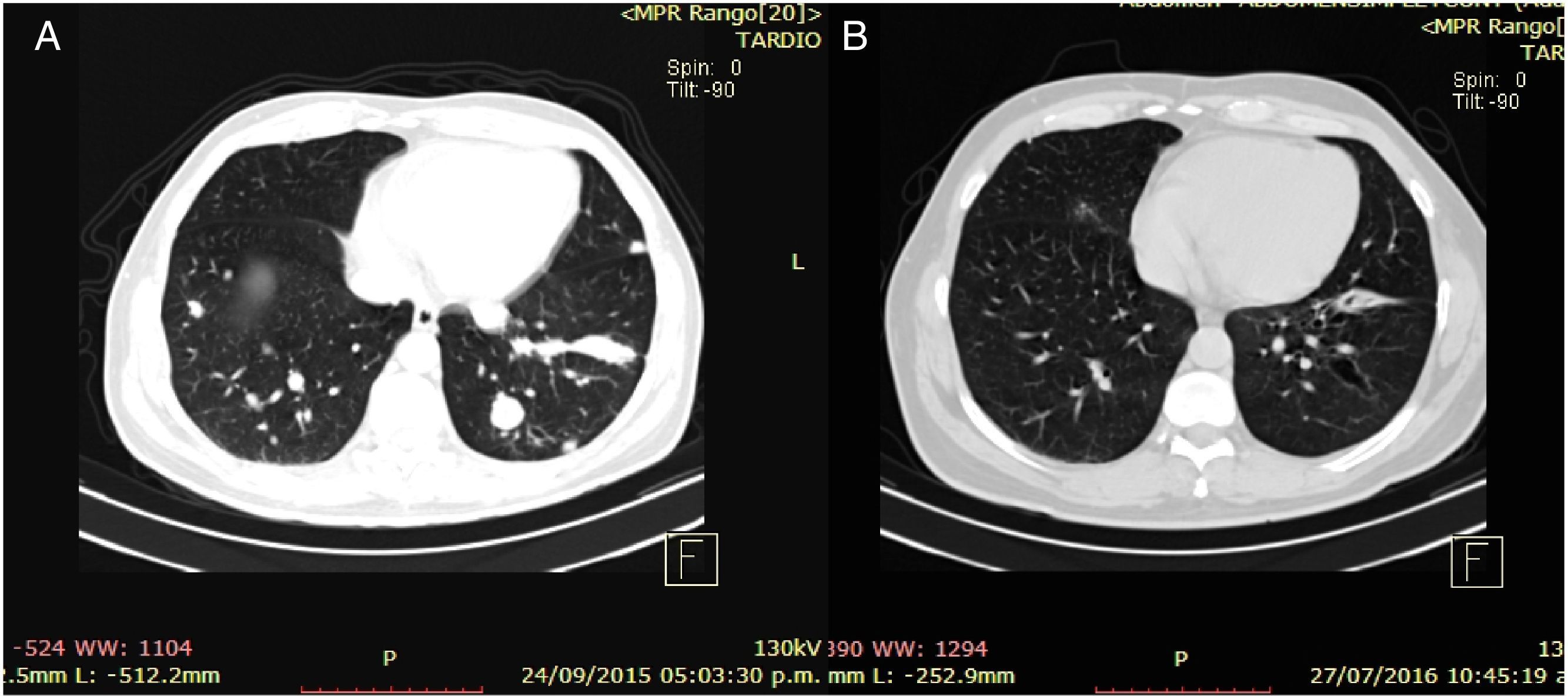
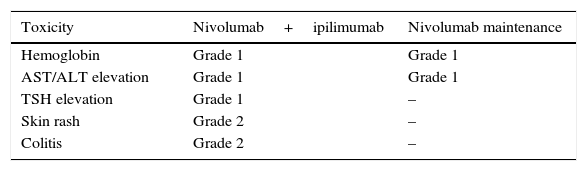
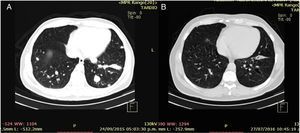
Clinical case 1A 45-year-old man presented to the Emergency Department (ED) with a two-week history of intermittent macroscopic hematuria. His past medical history, social history and family history were all unremarkable and he had no other significant complaints. Baseline laboratory tests were also unremarkable. A CT Scan showed multiple bilateral pulmonary metastases, the biggest of them measuring 21×18mm, and a 15cm right kidney tumor with a malignant appearance. The patient underwent a radical nephrectomy and pathology results revealed a renal cell carcinoma, Furhman 4, with 15% sarcomatoid differentiation, invasion of the gerota fascia, extension to peri-renal soft tissues, and lymph node metastases in two out of three nodes resected (pT4,N1,M1). The patient disease was classified as clinical stage IV for distant metastases with good risk by the MSKCC criteria. Following the pathology results, the patient was referred to the Oncology Department. At the first visit, the patient was found to have a good performance status (ECOG 0), and was initiated on systemic treatment with immunotherapy based on the combination of nivolumab at 3mg/kg and ipilimumab at 1mg/kg every two weeks for four cycles, following maintenance therapy with single agent nivolumab at 3mg/kg every two weeks. The most notable toxicity occurred during the combination regimen, the patient presented grade three (G3) elevation of liver enzymes which required a delay on the third cycle and a short course of prednisone, having a complete resolution of toxicity (Table 1). After the initial four cycles of immunotherapy (nivoluman and ipilimumab), the patient underwent a CT scan showing a partial response of target lesion 1 (21mm to 13mm), and a complete response in target lesion 2 (Table 2). Subsequently maintenance therapy was initiated, and after another four cycles of nivolumab monotherapy the CT scan was repeated, showing a complete response of both target lesions (Fig. 1). Currently, the patient remains on maintenance therapy (+16 cycles), remains asymptomatic and persists with a complete response of both target lesions (Table 2).
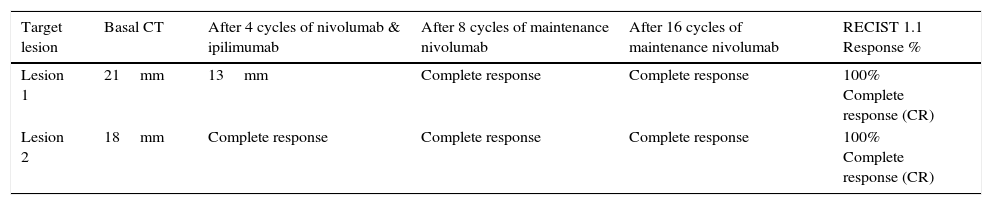
Response evaluation of patient 1. RECISIT 1.1.
| Target lesion | Basal CT | After 4 cycles of nivolumab & ipilimumab | After 8 cycles of maintenance nivolumab | After 16 cycles of maintenance nivolumab | RECIST 1.1 Response % |
|---|---|---|---|---|---|
| Lesion 1 | 21mm | 13mm | Complete response | Complete response | 100% Complete response (CR) |
| Lesion 2 | 18mm | Complete response | Complete response | Complete response | 100% Complete response (CR) |
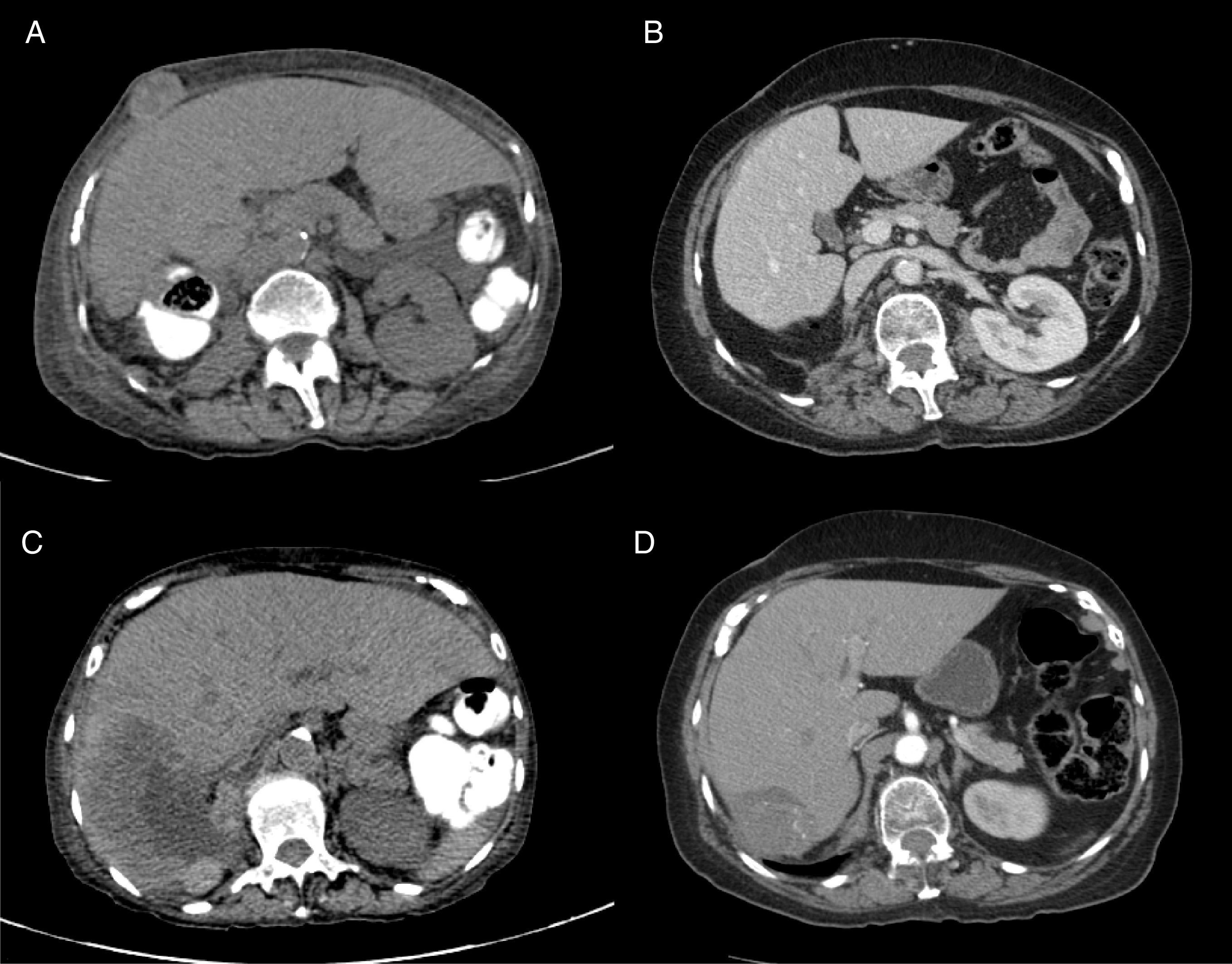
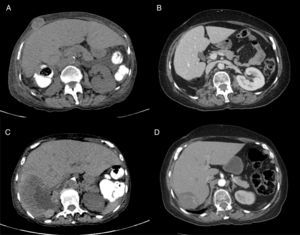
A 71-year-old woman was presented to the ED with a two-month history of macroscopic hematuria as flank pain, and weight loss of approximately 10 kilograms. Her past medical history was significant for hypertension, long term NSAID consumption for chronic pain and tobacco smoking for 20 years (20 packs/years). Laboratory: Hgb 9.8g/dl, Hto 26%, Leucocytes 6.8×109/L, Neut. 2.9×109/L, Platelet count 444×109/L, Corrected Calcium 9mg/dl, DHL 200UI/L. The initial CT scan revealed a 14cm renal mass, with destruction of 90% of the renal parenchyma, a 12cm hepatic lesion and another 2.7cm lesion in the abdominal wall compatible with metastatic disease. She underwent a radical nephrectomy with a pathologic result of renal cell carcinoma, Furhman 3 and borders without malignant neoplasm (pT4,N0,M1). After surgery, she was referred to the Oncology Department. At first visit, she was found with the diagnosis of MRCC, poor risk by MSKCC Criteria, and with a poor performance status (ECOG 3). She was started on immunotherapy treatment with four cycles of biweekly Nivolumab and Ipilimub, following nivolumab maintenance therapy. After the initial four cycles of treatment, performance status improved greatly (ECOG 1) and only experienced grade 1 and 2 toxicity during the combination therapy phase and grade 1 toxicity during the maintenance phase (Table 3). The first CT scan to assess radiologic response was performed after the initial four cycles of nivolumab and ipilimumab showing a partial response of the hepatic metastatic lesion (120mm to 7.5cm), as well as a partial response for the metastatic lesion located in the abdominal wall (39mm to 22mm) (Fig. 2). The patient continued with nivolumab as a single agent, and after four more cycles of maintenance therapy a CT scan was repeated showing a greater response of the hepatic lesion (75mm to 67mm) and a complete response of the abdominal wall metastatic lesion (Table 4). Currently the patient is asymptomatic and with a stable disease after 24 cycles of nivolumab monotherapy.
Response evaluation of patient 2. RECISIT 1.1.
| Target lesion | Basal CT | After 4 cycles of nivolumab & ipilimumab | After 4 cycles of maintenance nivolumab | After 24 cycles of maintenance nivolumab | RECIST 1.1 Response % |
|---|---|---|---|---|---|
| Lesion 1 | 120mm | 75mm | 67mm | 67mm | 44% reduction Partial response (PR) |
| Lesion 2 | 39mm | 22mm | Complete response | Complete response | 100% reduction Complete response (CR) |
Renal cell carcinoma is known to be a chemo-resistant malignancy, thus conventional chemotherapy is currently not recommended for the management of the metastastatic or recurrent disease. Until 2005, immunotherapies such as high-dose interleukin (IL-2) and interferon alpha (INF-a) were the only treatments available. Fyfe et al. published the results of 225 patients with mRCC treated with high-dose IL-2 and found an overall objective response rate of 14%, with 5% showing a complete response and 9% showing partial responses; nevertheless the treatment was associated with severe acute toxicity and 4% of patients die in relation to its adverse events. Nowadays, VEGF and mTOR inhibitors (sunitinib, pazopanib, everolimus, temsirolimus) have become the standard of care in the first and second line settings of metastatic RCC, and are currently recommended by NCCN (National Comprehensive Cancer Network) guidelines.9 Two of the most-used VEGF inhibitors, sunitinib and pazopanib, were compared in a first line setting of mRCC in a phase III study (COMPARZ). In this trial, 1100 patients were randomly assigned to receive 800mg of pazopanib once daily or 50mg of sunitinib once daily. Objective response rate was found to be 31% and 25% respectively, higher than previously reported with (HD IL-2), while median progression-free survival and overall survival were similar between both groups (8.4 vs 9.5 months and 28.4 vs 29.3 months, respectively).5 However, in recent years, new therapies have emerged for the management of metastatic renal cell carcinoma, from agents directed toward the inhibition of the vascular endothelial growth factor (VEGF) pathway (sunitinib, pazopanib, sorafenib) and mammalian target of rapamycin (mTOR) inhibitors (temsiloriumus, everolimus) to novel immunotherapies such as nivolumab, a fully humanized IgG4 antibody that selectively blocks the interaction between PD-1 (expressed on T cells) and PD-L1 and PD-L2 (expressed on immune cells and tumor cells), avoiding the inhibition of the cellular immune response against the tumor cells,6 and ipilimumab, a monoclonal antibody directed to block the interaction between CTLA4 (T Cells) and the B7 family of molecules (CD80 and CD86) on antigen-presenting cells, which if triggered inhibits T-Cell proliferation and function.7 Nivolumab was investigated in the management of metastatic RCC in comparison with everolimus (mTOR inhibitor) in a randomized phase III study with patients who had previously received one or two lines of metastatic treatment, median progression-free survival was similar between the two groups (4.6 vs 4.4 months), nevertheless median overall survival with nivolumab resulted in a 5.4 month improvement in comparison with everolimus (25 vs 19.6 moths HR 0.73 p=0.002). On the other hand, ipilimumab was explored in the management of mRCC in a phase II study where 5 out 40 patients experienced a partial response. In this study, a high association was found between autoimmune events (AEs) and tumor regression (response rate=30% with AEs, 0% without AEs). In this paper, we presented two cases treated at the University Cancer Center at a tertiary level teaching hospital in Northeastern Mexico with a combination of two novel immunotherapies (nivolumab and ipilimumab) working at two different levels of the cancer-immunity cycle.10 Patient 1 had a complete response of all target lesions and currently continues without progression after more than 10 months, which is higher than the standard median time of progression for the standard of care (pazopanib, sunitinib). Patient 2 experienced a complete response of one target lesion and a partial response of 44% of the other target lesion, and as of the last visit a survival time without progression of more than 14 months, exceeding the expected time with the anti-VEGF and mTOR inhibitors. In both patients the treatment was well tolerated. Patient 1 only experienced grade 1 (G1) toxicities during therapy with the combination regimen, and only a grade 3 elevation of liver enzymes (AST/ALT) during maintenance with nivolumab, which was rapidly corrected after a short course of prednisone. Patient 2 also tolerated the combination of immunotherapy well, only experiencing G2 colitis, nevertheless in this case the patient had a remarkable recovery of her performance status (PS). At first visit her she was on PS 3 (capable of only limited care and confined to a bed or chair for more than 50% of waking hours) and after the induction treatment of 4 cycles of nivolumab and ipilimumab, the patient recovered her performance status to a PS of 1 (restricted in physically strenuous activity, but ambulatory and able to carry out work of a light nature).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors have none conflicts of interest to declare