Parkinson's disease (PD) is recognized as the second most common neurodegenerative disorder after Alzheimer's; in PD, a patient's nutritional status may be compromised. This study involved 15 adults, aged 47–80 years of age, with idiopathic Parkinson's disease. Twenty percent of the population had low weight, of which 66% were over 60 years old and, according to the AMA, 26.7% presented malnutrition. In conclusion, PD can contribute to reduced nutritional status, so a nutritional assessment is essential for the early detection of PD patients at risk of malnutrition and for the development and implementation of nutritional intervention. The aim of our study was to describe an outpatient nutritional status with PD in a third level hospital.
Parkinson's disease (PD) is a result of the progressive degeneration of dopaminergenic neurons in the brain, and a group of monoaminergic cells in the brain tissue. This disease is recognized as the second most common neurodegenerative disorder after Alzheimer's, affecting 1% of the population over 65 worldwide, with an incidence of 14–100 cases per 100,000 a year.1
PD causes motor dysfunctions, such as resting tremors, bradykinesia, rigidity and postural instability, as well as non-motor symptoms such as depression, changes in taste, gastrointestinal dysfunction and sleep disorders.2 The objective of PD treatment is to increase dopamine levels, in addition to alleviating symptoms, for which medications like levodopa are utilized.3
Nutritional status is an important contributor to a good quality of life and the general state of everyday life in the elderly.4 This nutritional status may become compromised in patients with PD, usually by the loss of weight. Among the possible weight loss factors in these patients are: low dietary intake due to dysphagia and/or anorexia, a lowering of absorption caused by slow gastric emptying, and the increase of energy consumption due to high muscular activity.5 Moreover, the disease itself and the side effects of the medication may be involved as well.6 Hence, correct nutrition plays a major role in the prevention of malnutrition and complications in patients with PD.
The objective of this work is to describe the nutritional status of ambulatory patients with PD in a third level hospital.
Material and methodsA descriptive study was conducted, including adults between 30 and 80 years old with idiopathic Parkinson's who voluntarily attended the Outpatient Clinic of Neurology and the Outpatient Clinic of Nutrition of the hospital in Northeastern Mexico, during the months of September, 2013 to December, 2014.
Each patient was assessed through a clinical history, where different data were obtained, such as gender, age, initial symptoms, data on dietary habits (using a 24-hour reminder), as well as anthropometrics (weight, size, body mass index [BMI], tricipital skin fold thickness [TSF], mid-upper arm circumference [(M)UAC], and mid-upper arm muscle area [(M)UAMA]).
Intake determination of macro and micro nutrients was conducted through the use of NUTRIS® (FaSPyN, UANL) software; subsequently, there was an intake adaptation of all analyzed nutrients, applying a percentage formula by Inano et al.,7 which categorizes a deficient intake as below 67% of the Dietary Reference Intake (DRI), a recommended intake as from 67% to 89%, an adequate intake as from 90% to 110% and an excess intake as a consumption greater than 110%. The mathematical proportionality value of the operation was applied based on the dietary intake recommendation for the Mexican population according to their age and gender.8,9
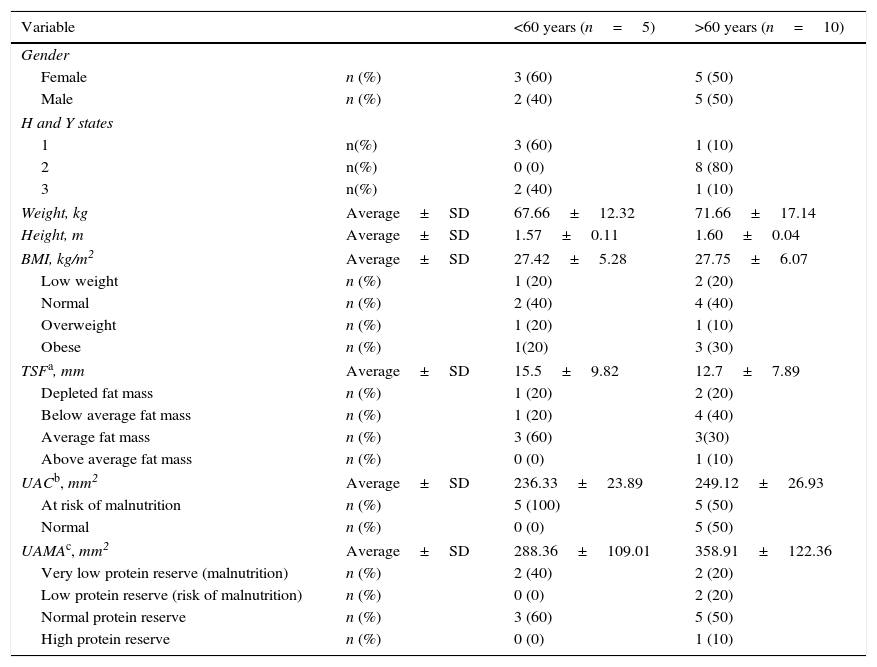
ResultsFifteen patients (47% male) were studied, and an average duration of disease of 5.64 years was identified; according to the Höen and Yahr (HyY) scale, 27% were in stage 1, 53% were in stage 2 and 20% were in stage 3. In addition, 80% of the patients reported tremors as an initial symptom, while 13.3% reported stiffness and 6.7% reported bradykinesia. The subjects presented a mean age of 63.33±9.4 years, a mean weight of 70.33±15.86kg, a mean height of 1.59±0.07m, and a mean BMI of 27.64±5.63kg/m2. Twenty percent of the population was identified as having low weight, and 40% were identified as overweight or obese. Body fat, according to the triceps skinfold thickness, which was 13.63±8.34mm, showed that 20% of the subjects studied had a depleted fat mass, 33.3% had below average fat mass, 40% had average fat mass and 6.7% with above average fat mass. According to the somatic protein reserve based on CMB (244.86±25.85mm2), 66.7% of the individuals presented a risk of malnutrition. However, on the basis of AMB, only 13.3% of the population presented a risk of malnutrition (low protein reserve, 315.69mm2), while 26.7% presented malnutrition (very low protein reserve, 195.55±47.71mm2). In addition to PD, 26.7% and 40% of the population presented diabetes and hypertension, respectively. Table 1 shows the clinical and anthropometric characteristics of the studied subjects classified by age (under 60 years and over 60 years).
Clinical and anthropometric characteristics of patients with Parkinson's disease in a third level hospital in Northeastern Mexico.
| Variable | <60 years (n=5) | >60 years (n=10) | |
|---|---|---|---|
| Gender | |||
| Female | n (%) | 3 (60) | 5 (50) |
| Male | n (%) | 2 (40) | 5 (50) |
| H and Y states | |||
| 1 | n(%) | 3 (60) | 1 (10) |
| 2 | n(%) | 0 (0) | 8 (80) |
| 3 | n(%) | 2 (40) | 1 (10) |
| Weight, kg | Average±SD | 67.66±12.32 | 71.66±17.14 |
| Height, m | Average±SD | 1.57±0.11 | 1.60±0.04 |
| BMI, kg/m2 | Average±SD | 27.42±5.28 | 27.75±6.07 |
| Low weight | n (%) | 1 (20) | 2 (20) |
| Normal | n (%) | 2 (40) | 4 (40) |
| Overweight | n (%) | 1 (20) | 1 (10) |
| Obese | n (%) | 1(20) | 3 (30) |
| TSFa, mm | Average±SD | 15.5±9.82 | 12.7±7.89 |
| Depleted fat mass | n (%) | 1 (20) | 2 (20) |
| Below average fat mass | n (%) | 1 (20) | 4 (40) |
| Average fat mass | n (%) | 3 (60) | 3(30) |
| Above average fat mass | n (%) | 0 (0) | 1 (10) |
| UACb, mm2 | Average±SD | 236.33±23.89 | 249.12±26.93 |
| At risk of malnutrition | n (%) | 5 (100) | 5 (50) |
| Normal | n (%) | 0 (0) | 5 (50) |
| UAMAc, mm2 | Average±SD | 288.36±109.01 | 358.91±122.36 |
| Very low protein reserve (malnutrition) | n (%) | 2 (40) | 2 (20) |
| Low protein reserve (risk of malnutrition) | n (%) | 0 (0) | 2 (20) |
| Normal protein reserve | n (%) | 3 (60) | 5 (50) |
| High protein reserve | n (%) | 0 (0) | 1 (10) |
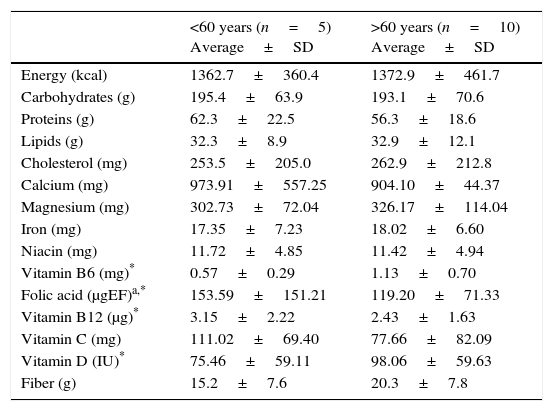
Regarding the characterization of diet, the group over 60 years old presented a higher consumption of kcal, lipids, cholesterol and fiber, and a lower intake of carbohydrates and protein, compared to the group younger than 60 years of age. However, in micronutrient intake, a greater consumption of magnesium, iron, vitamin B6 and vitamin D was observed in individuals over 60 years of age, as well as a lower intake of calcium, niacin, folic acid, vitamin B12 and vitamin C compared to their counterparts (Table 2).
Dietary intake of macronutrients, micronutrients and fiber of patients with Parkinson's disease at a third level hospital in Northeastern Mexico.
| <60 years (n=5) Average±SD | >60 years (n=10) Average±SD | |
|---|---|---|
| Energy (kcal) | 1362.7±360.4 | 1372.9±461.7 |
| Carbohydrates (g) | 195.4±63.9 | 193.1±70.6 |
| Proteins (g) | 62.3±22.5 | 56.3±18.6 |
| Lipids (g) | 32.3±8.9 | 32.9±12.1 |
| Cholesterol (mg) | 253.5±205.0 | 262.9±212.8 |
| Calcium (mg) | 973.91±557.25 | 904.10±44.37 |
| Magnesium (mg) | 302.73±72.04 | 326.17±114.04 |
| Iron (mg) | 17.35±7.23 | 18.02±6.60 |
| Niacin (mg) | 11.72±4.85 | 11.42±4.94 |
| Vitamin B6 (mg)* | 0.57±0.29 | 1.13±0.70 |
| Folic acid (μgEF)a,* | 153.59±151.21 | 119.20±71.33 |
| Vitamin B12 (μg)* | 3.15±2.22 | 2.43±1.63 |
| Vitamin C (mg) | 111.02±69.40 | 77.66±82.09 |
| Vitamin D (IU)* | 75.46±59.11 | 98.06±59.63 |
| Fiber (g) | 15.2±7.6 | 20.3±7.8 |
SD=Standard Deviation.
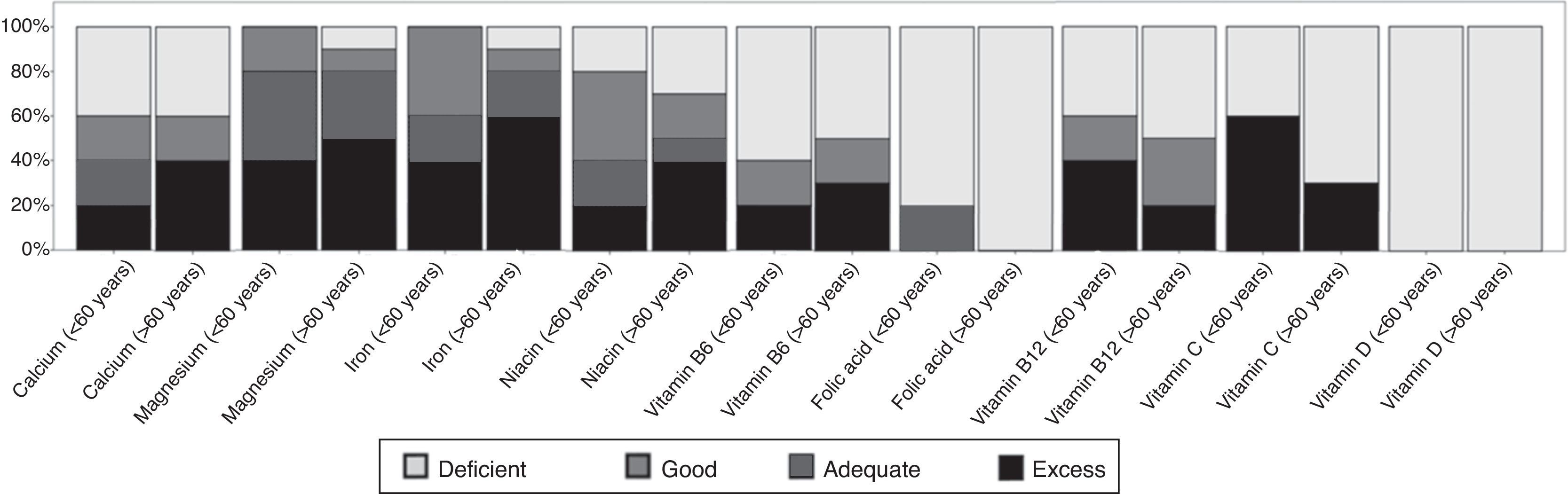
Based on the adequacy of the intake of the micronutrients studied, it was determined that 100% and 70% of subjects over 60 years of age had a deficient intake of folic acid and vitamin C, respectively. In addition, the entire population studied had poor vitamin D intakes (Fig. 1).
Adequacy of the intake of the micronutrients analyzed in both groups. Deficient: <67% of IDR; good: from 67 to 89% of IDR; adequate: from 90 to 110% of IDR. Excess: >110% of IDR.7–9
Nutritional status is defined as the body condition of the balance between food intake and its use by the body.10 In this study, it was observed that 20% of the population were underweight, of which 66% were older than 60 years. Different studies have reported that the prevalence of malnutrition due to deficiency in patients with PD ranges from 3% to 60%, and that the prevalence of malnutrition due to deficiency ranges from 0% to 24%.11–13 In our study, according to the WBA, 40% of the population presented a low or very low protein reserve, being considered at risk of malnutrition (13.3%) and presenting malnutrition (26.7%), respectively. These differences could be the result of the different methods used, or the criteria of classification of malnutrition or low weight used, as well as the characteristics of the patients.
In most cases, the progression of PD is accompanied by a consequent loss of weight.14–16 In this study, 73.3% of the patients reported having lost weight in the last three months, and 66.7%, mostly subjects older than 60 years of age (70%), reported a decrease in food intake in the last week. We do not know if both the weight loss and the decrease in the dietary intake of the patients are caused by the disease itself, or are due to the side effects of the drugs, since all subjects were being treated with levodopa, which has been associated with an increased likelihood of malnutrition risk,15,17,18 or for some other reason.
Nutritional deficiencies in the elderly are common; in spite of fortification, these deficiencies remain a problem.19,20 The elderly are particularly at risk for vitamin D deficiency due to low sunlight exposure, in addition to age-related decreases in cutaneous synthesis and low vitamin D diets contributing to the high prevalence of insufficiency of vitamin D.21 On the other hand, recent studies indicate that low levels of vitamin D are associated with an increased risk of PD,22 besides being an important factor in the pathogenesis of this disease23. In our study, the entire population studied presented a percentage of adequacy of deficient vitamin D intake (Table 2).
In this study, all subjects over 60 years of age had a poor intake of folic acid, while 50% of the patients at this age reported deficient intakes of vitamin B6 and B12. For subjects younger than 60 years, 80%, 60% and 40% had deficient intakes of folic acid and vitamins B6 and B12, respectively. The use of folic acid and vitamins B6 and B12 is that they reduce homocysteine levels.24,25 According to other authors, levodopa increases homocysteine levels, which in addition to favoring other diseases is a possible cause of Parkinson's progression (Table 2).26,27
In conclusion, this study identified that 26.7% of the patients in the study presented malnutrition. Together with the findings of other authors, this indicates that PD can contribute to a decrease in nutritional status. Therefore, nutritional assessment is essential for the early detection of patients with PD at a risk of malnutrition, as is the implementation of both individualized nutritional intervention and the development of strategies that favor food intake to improve nutritional status and the general conditions of a patient with idiopathic PE.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.