The antiglobulin, or Coombs test is part of the compatibility tests that any patient who will receive a red blood cell transfusion must undergo. This test is also essential in the diagnostic work of patients with anemia whose origin is not easily determined and when the etiology must be identified precisely.
In 1945, Robin Coombs, Arthur Mourant and Rob Race described a test to detect anti-Rho (anti-D) non-agglutinant antibodies.1 Originally, the test was devised by Robin Coombs as part of his postgraduate studies at Race and Mourant's laboratory in Cambridge, England in 1945. His goal was to study the characteristics of the antibodies involved in the context of what was known as fetal erythroblastosis, which is now known as hemolytic disease of the newborn (HDN), caused most frequently by the incompatibility between an Rh-negative mother sensitized during a previous pregnancy, who produces IgG anti-D antibodies able to pass the placenta barrier due to their small size that then cover the fetal red blood cells. These are later phagocytosed in the spleen and liver, organs which, in addition to their other functions, maintain extramedullary hematopoiesis in the fetus to compensate for anemia resulting from hemolysis. Later, the Coombs test was used to demonstrate the presence of incomplete antibodies which covered the erythrocytes in vivo, such as is seen in cases of autoimmune hemolytic anemia (AHA). The description of the method, and its application in various hematological diseases, was published in The Lancet and The British Journal of Experimental Pathology in 1945 and 1946, respectively.2
The principles of the antiglobulin test are as follows: when immunoglobulins of the IgG class (gamma globulin) and the complement (beta globulin) of human origin is injected into different rabbits, they produce IgG antibodies against these globulins, which are later mixed in the laboratory to produce the broad spectrum Coombs reagent, which is used in daily blood banking practice. The rabbit's IgG antiglobulin molecules act as a bridge, which unites the incomplete IgG antibodies covering adjacent red blood cells, causing agglutination and visualization of the reaction to the naked eye, which can be seen in a test tube or gel card, interpreted as a positive Coombs test.
There are two variants of this test. When it is employed to detect antibodies bound to erythrocytes in vivo it is known as a direct antiglobulin test or direct Coombs test. On the other hand, when the antiglobulin is used to detect the presence in vitro of free antibodies in serum after incubation in the Coombs phase of the cross match, it is known as an indirect antiglobulin test, or an indirect Coombs test. This variant of the test is the one that is used in the detection of anti-D antibodies in the maternal serum of women who are D antigen negative, as well as the search for and identification of antibodies in serum when a panel of commercial erythrocytes, whose phenotype is known, is employed.3,4
ApplicationsThe direct Coombs test- 1.
In the investigation of antibodies, such as those in patients with autoimmune hemolytic anemia.
- 2.
In the investigation of alloantibodies, such as in newborns suffering hemolytic disease of the newborn.
- 3.
To document a hemolytic transfusion reaction.
- 1.
In the detection of irregular antibodies in the patient's serum.
- 2.
In the determination of red blood cell phenotypes.
- 3.
As part of the crossmatch test.
These can be considered, in general: (1) test tube techniques, which were traditional and are now practically unused, (2) microplate techniques, and (3) agglutination techniques in gel columns. The solid phase methods (gel, glass beads and microplates) appeared in the 90s and have seen generalized use in Mexico since the year 2000. Currently, the most-used technique is that of cards with gel columns.
The Gel testIn 1990, Lapierre developed a system of gel tests.5 These were based on the use of dextran acrylamide gel particles, a gel matrix which acted as a filter, stopping the agglutinated red blood cells from passing through and thus trapping red blood cells on the surface of the gel according to their size, which constitutes a positive test. A negative reaction forms a button of non-agglutinated red blood cells at the bottom of the microtube. The gel card contains 6 microtubes, which allows for the determination of 6 different antigens and antibodies, usually ABO blood type (direct and inverse) and Rh (D-antigen), compatibility tests, and Coombs tests. These tests can be carried out manually or automatically.5
Among the advantages of automation are the following: a larger number of samples can be analyzed, washed erythrocytes are not required, precision and reproducibility are improved, subjectivity in the interpretation of the results is eliminated, the results can be preserved for hours, and manual labor is reduced, minimizing susceptibility to error in the different phases of the test.
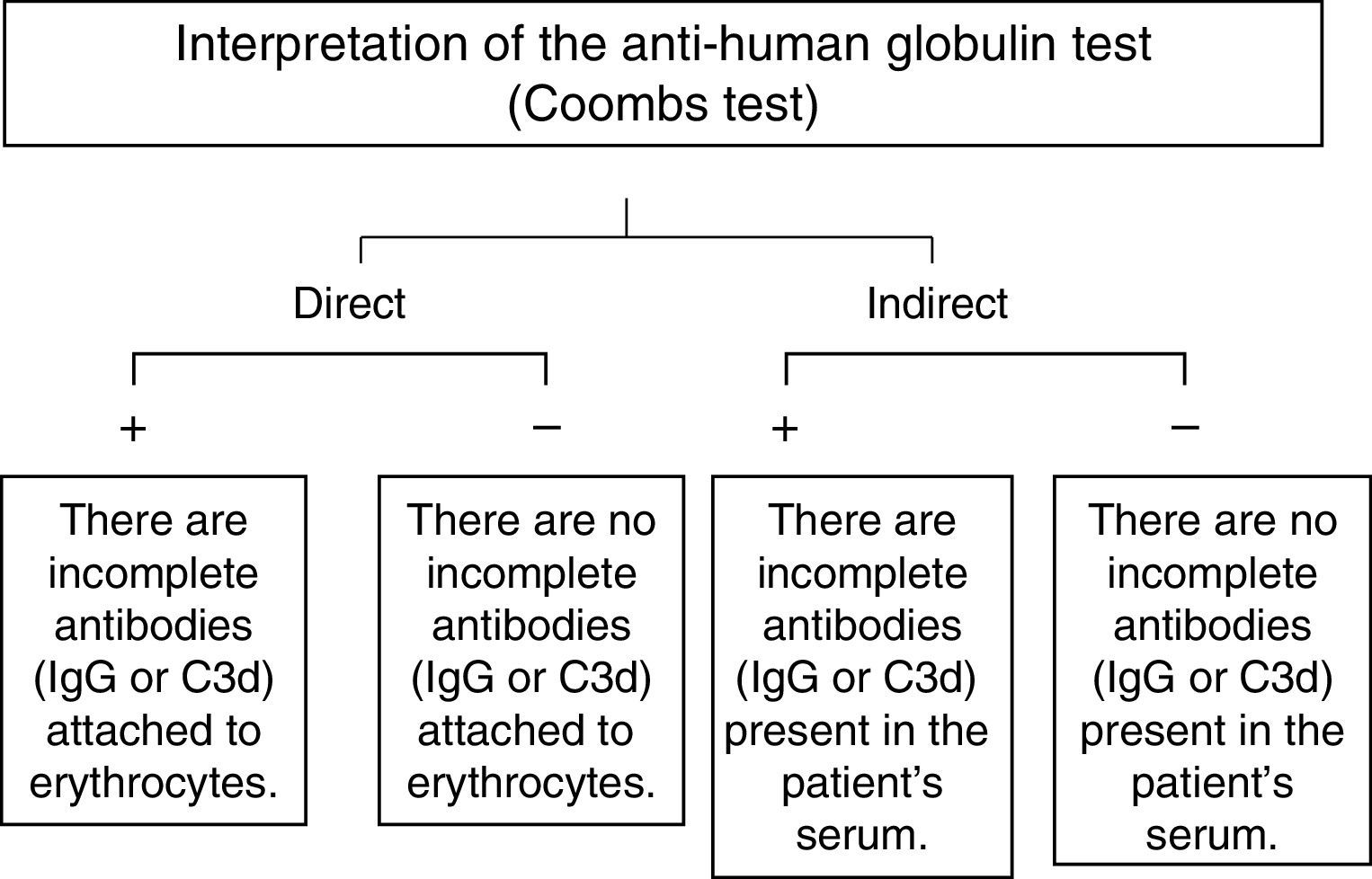
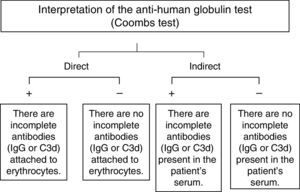
The direct Coombs testWhen the direct antiglobulin test is positive, this means that incomplete IgG antibodies or fragments of the complement (specifically C3d), are present, on the erythrocytes. On the other hand, when there is no agglutination (a negative test), these antibodies are not present on the erythrocytes’ surface (Fig. 1).6
A negative direct Coombs test does not necessarily mean that there are no incomplete antibodies on the red blood cells, since the usual reagents for human polyspecific antiglobulins, which are used daily in blood banks, only react when there are at least 200 IgG molecules or more per erythrocyte. In the same manner, if the antibody involved is an IgA, as in some cases of autoimmune hemolytic anemia, the test will be negative, since the standard Coombs reagent does not contain anti-IgA antibodies.7,8
The indirect Coombs testThe indirect human antiglobulin test is positive when there are free IgG antibodies in the serum, as is the case with hemolytic disease of the newborn, the test is also routinely used for identification of irregular (unexpected) antibodies employing a commercial panel of red blood cells (Fig. 1).6
In conclusion, since its introduction to the clinical laboratory 70 years ago, the Coombs test has evolved technically, and its principles continue to be applicable today, which makes this simple and economic test one of the most versatile and important used in the diagnosis of hematological diseases, and in the safe practice of transfusion medicine.
FundingNo financial support was provided.
Conflict of interestThe authors have no conflicts of interest to declare.