Gait impairment, a frequent sign in multiple sclerosis (MS), places a major burden on patients since it results in progressive loss of personal and social autonomy, along with work productivity. This guide aims to provide recommendations on how to evaluate gait impairment and use prolonged-release fampridine (PR-fampridine) as treatment for MS patients with gait impairment in Spain.
DevelopmentPR-fampridine dosed at 10mg every 12hours is currently the only drug approved to treat gait impairment in adults with MS. Additionally, PR-fampridine has been shown in clinical practice to significantly improve quality of life (QoL) in patients who respond to treatment. Treatment response can be assessed with the Timed 25-Foot Walk (T25FW) or the 12-item MS Walking Scale (MSWS-12); tests should be completed before and after starting treatment. The minimum time recommended for evaluating treatment response is 2 weeks after treatment onset. Patients are considered responders and permitted to continue the treatment when they demonstrate a decrease in their T25FW time or an increase in MSWS-12 scores. A re-evaluation is recommended at least every 6 months. The SF-36 (Short Form-36) and the MSIS-29 (MS Impact Scale-29) tests are recommended for clinicians interested in performing a detailed QoL assessment. This drug is generally well-tolerated and has a good safety profile. It should be taken on an empty stomach and renal function must be monitored regularly.
ConclusionsThese recommendations will help ensure safer and more efficient prescription practices and easier management of PR-fampridine as treatment for gait impairment in Spanish adults with MS.
La alteración de la marcha es frecuente en la esclerosis múltiple (EM) y tiene un gran impacto negativo en los pacientes pues conlleva a la pérdida progresiva de autonomía personal y social, y de productividad laboral. Esta guía pretende establecer recomendaciones para la evaluación del deterioro de la marcha y el uso de fampridina de liberación prolongada (fampridina-LP) como tratamiento de pacientes con EM y deterioro de la marcha en España.
DesarrolloFampridina-LP a dosis de 10 mg cada 12 h es actualmente el único fármaco autorizado para mejorar el trastorno de la marcha en adultos con EM. En la práctica clínica, el fármaco ha demostrado además que mejora de forma significativa la calidad de vida de los pacientes que responden al tratamiento. La respuesta se puede evaluar mediante la prueba cronometrada de la marcha de 25 pies (T25FW) o el cuestionario MSWS-12 que deben realizarse antes y después del inicio del tratamiento. El tiempo mínimo recomendado para evaluar la respuesta inicial es de 2 semanas. Para considerar a un paciente como respondedor y continuar el tratamiento debe presentar, según indica la ficha técnica, una disminución en el tiempo T25FW o mejoría en el MSWS-12. Se recomienda realizar las revaluaciones al menos cada 6 meses. En los casos en que se considere la valoración de la calidad de vida, se recomienda la utilización del cuestionario de salud Short Form-36 (SF-36) o la escala MS Impact Scale-29 (MSIS-29). Es un fármaco en general bien tolerado y con buen perfil de seguridad. Se recomienda su administración en ayunas y control periódico de la función renal.
ConclusionesEstas recomendaciones permiten garantizar una prescripción eficiente y más segura, y ayudan al manejo de fampridina-LP como tratamiento del deterioro de la marcha en pacientes adultos con EM en España.
Multiple sclerosis (MS) is a chronic inflammatory autoimmune disease of the central nervous system whose aetiology is yet to be determined. In MS, the immune system attacks the myelin sheaths, oligodendrocytes, and axons, causing neurological deficits and physical and cognitive impairment.1 MS has traditionally been classified by clinical course into relapsing-remitting (RR), secondary progressive (SP), primary progressive (PP), or progressive relapsing (PR).2 A new classification of clinical phenotypes of MS (by disease activity and progression: clinically isolated syndrome, RRMS, and progressive MS) has recently been proposed, with phenotypes established according to clinical and MRI data.3 MS is 2 to 3 times more common in women than in men and is usually diagnosed between ages 20 to 40. There are estimated to be 46000 patients with MS in Spain (prevalence of 100 cases per 100000 population). The incidence of MS in Spain is estimated at ≥4 cases per 100000 person-years.4
Reduced mobility, and more specifically difficulty walking, is frequent in MS and has a major impact on patients’ lives.5 Walking is one of the most valued functions for patients. MS-related gait alterations involve considerable economic and social burden, leading to progressive loss of productivity and independence.5,6
This study provides a series of recommendations for the evaluation of gait impairment and treatment of this manifestation with prolonged-release fampridine (PR-fampridine) in patients with MS in Spain.
MethodsSpanish experts in MS established the most relevant topics to be addressed in these treatment guidelines with a view to establishing clinical recommendations for the evaluation and treatment of walking disability in patients with MS. Treatment recommendations focus on PR-fampridine, the only drug currently approved by the European Medicines Agency and the United States Food and Drug Administration for improving walking ability in patients with MS.7,8 We also conducted a literature review to analyse the use of PR-fampridine in clinical trials and observational studies based on normal clinical practice.
DevelopmentImpact of gait impairment on patients with MSGait impairment results from the combination of multiple frequent symptoms of MS, including fatigue, weakness, impaired sensitivity, spasticity, ataxia, and imbalance.9,10 According to the literature, mobility is reduced to some extent in nearly 90% of patients with MS.5,11 Walking disability, however, affects over two-thirds of these patients.5 We now know that nearly 50% of patients will require some degree of walking assistance after 15 years of disease progression.3,9 Although gait impairment has been observed to increase in line with disability and disease progression, patients with Expanded Disability Status Scale (EDSS) scores<3.5 may also experience considerable gait alterations.10,12
Multiple studies have shown the negative impact of MS on health-related quality of life (HRQoL),13,14 and suggest that preserving adequate physical function and walking ability significantly reduces this impact.9,10,15
In comparison to pain or other cognitive or visual alterations,3,16–20 gait impairment results in greater losses of HRQoL in patients with MS since it has a negative impact on patients’ independence in the activities of daily living (91%), self-esteem (84%), working life (83%), and mobility (87%).15 Furthermore, loss of mobility is independently correlated with high Instrumental Activities of Daily Living (IADL) scale scores and is associated with a decrease in socioeconomic status.9,21 The need for a carer is twice as frequent in patients with walking disability as in the general population with MS (37% vs 18%). The condition affects not only patients’ HRQoL but also carers’ QoL.9,22
Pharmacological treatment of gait impairmentMost traditional treatments for gait impairment in MS are non-pharmacological and include physical therapy and orthopaedic devices, which may increase the stigmatisation of patients.
From a pharmacological viewpoint, disease-modifying treatments, which focus on preventing relapses and delaying the progression of disability, may partially stabilise the progression of gait impairment but do not normally improve gait.
Symptomatic treatment, on the other hand, focuses on improving symptoms, providing functional improvements, and increasing patients’ HRQoL.23
Some symptomatic medications used to improve symptoms associated with gait impairment include baclofen, tizanidine, botulinum toxin, and nabiximols (a cannabis derivative containing delta 9-tetrahydrocannabinol and cannabidiol) for spasticity24,25; gabapentin and pregabalin for pain26; and beta-blockers and botulinum toxin for tremor.27 None of these drugs has been proven in clinical trials to significantly improve gait in patients with MS, and none of them is specifically indicated for gait impairment.
PR-fampridine is currently the only authorised treatment for improving walking ability in adults with MS.8,28
Pharmacokinetic and pharmacodynamic properties of PR-fampridineAxonal demyelination exposes voltage-dependent potassium (K+) channels in the axon membrane, leading to increased K+ current from the axon, which results in action potential conduction delay or block.19,29
PR-fampridine, whose active ingredient is prolonged-release 4-aminopyridine (4-AP), is a broad-spectrum voltage-dependent K+ channel blocker which can increase action potential propagation in demyelinated axons.8,28 The compound, which is lipid soluble, crosses the axon membrane, blocking the exposed K+ channels; this decreases K+ loss, increases action potentials and conduction, and promotes synaptic and neuromuscular transmission of nerve signals.28,30
PR-fampridine is characterised by linear pharmacokinetics. It is completely absorbed from the gastrointestinal tract; PR-fampridine is absorbed more slowly than immediate-release 4-AP (IR-4-AP).8 This decreases peak plasma concentration by 50%, which consequently reduces dose-related adverse effects.16
A recent study by Jensen et al.31 analysed the safety of PR-fampridine and IR-4-AP in all human clinical trials. Studies of IR-4-AP and PR-fampridine vs placebo caused adverse effects in 9.60% and 1.64% of the cases, respectively. Although most adverse effects of these 2 oral-route drugs are of mild to moderate severity, IR-4-AP tends to cause severe adverse effects more frequently.
PR-fampridine should be administered under fasting conditions: administration with food increases peak plasma concentration, leading to increased risk of adverse effects.8,32
Over 97% of PR-fampridine does not bind to plasma proteins,8 and approximately 10% is metabolised by cytochrome P450 2E1. Its 2 main metabolites have no pharmacological activity against K+ channels.8,28,32 The major route of elimination for PR-fampridine is renal excretion. Nearly 90% of the drug is excreted intact within 24hours; this drug should therefore be used with caution in patients with renal insufficiency.8,16,28,32
Pharmacological recommendations for PR-fampridine: candidate patient and assessment of responseAccording to the summary of product characteristics, PR-fampridine is indicated for improving walking in adult MS patients scoring 4.0 to 7.0 on the EDSS.8 Evidence supports the idea that the effectiveness of PR-fampridine is independent of the type of MS, the disease-modifying drug administered, disease duration, and the main demographic variables.20,23,33
The recommended dose of PR-fampridine is 10mg (1 tablet) every 12hours. The drug should not be administered with food, or more frequently or at higher doses than recommended.8
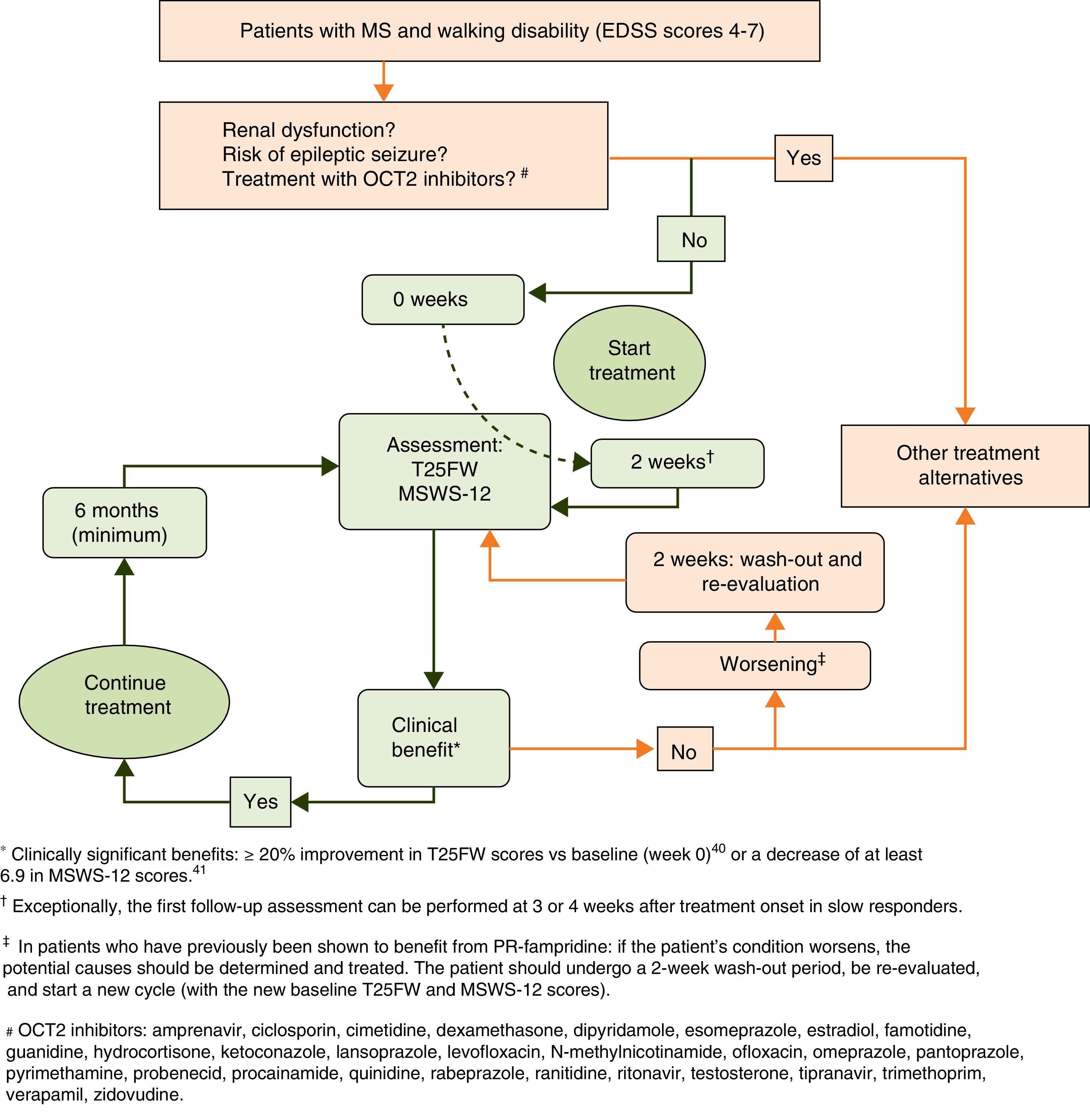
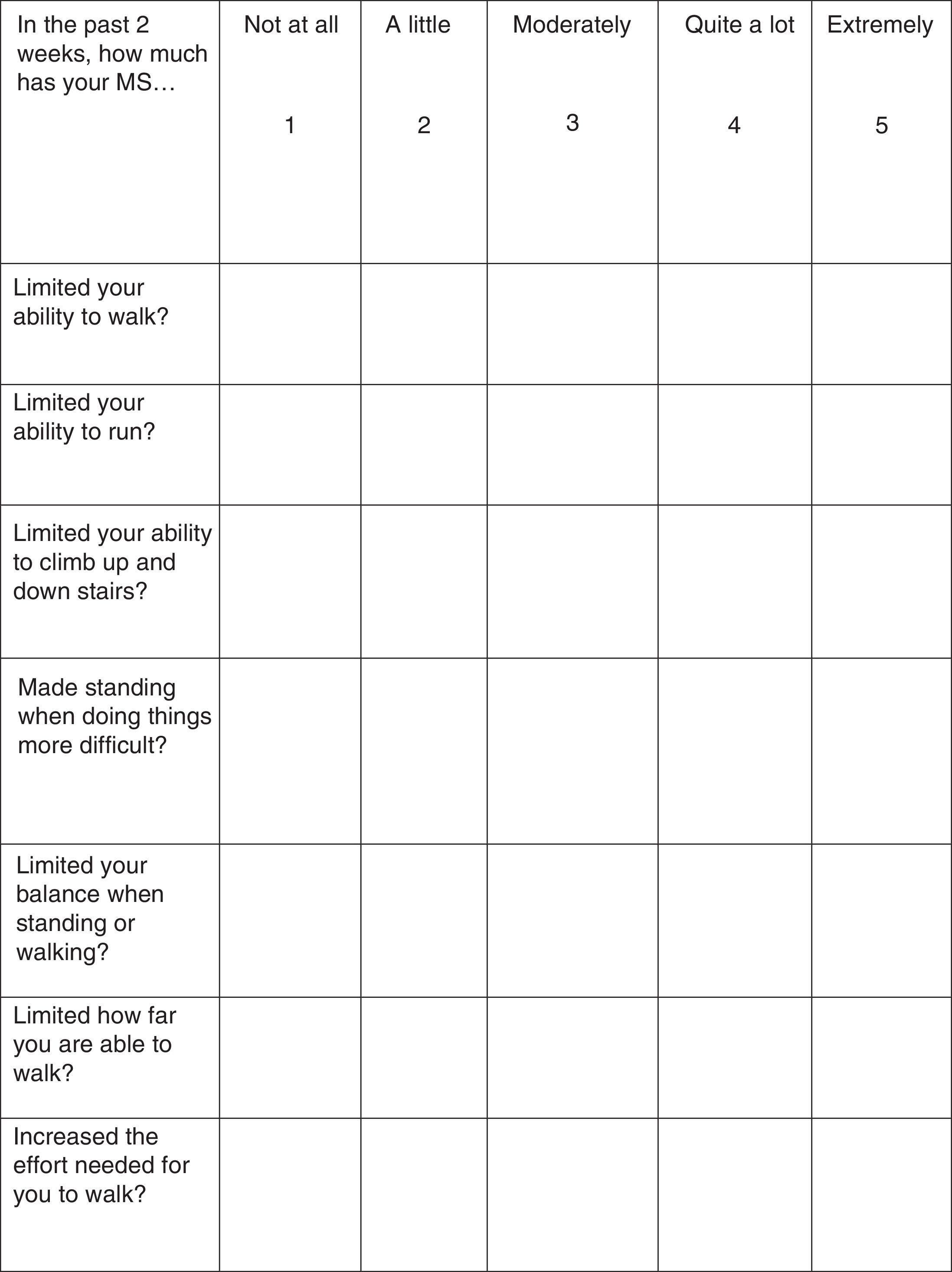
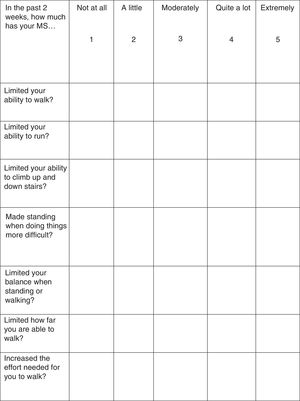
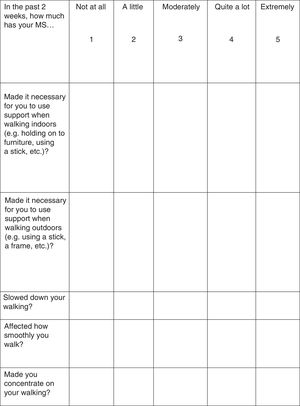
Treatment response should be assessed with the Timed 25-Foot Walk (T25FW) test and the 12-item MS Walking Scale (MSWS-12) (Fig. 1). These tests should be conducted at baseline (before starting treatment) and some time after treatment initiation in order to be able to make an objective comparison and assessment of treatment response. When deemed appropriate, the impact of walking disability on QoL may also be assessed; the 36-item Short Form Health Survey (SF-36) and the MS Impact Scale-29 (MSIS-29) may be used for this purpose.34–37
According to the summary of product characteristics, patients are considered responders and eligible for continuing treatment when they display a decrease in T25FW completion time38,39 (a decrease of 20% or over is considered clinically significant40) or in MSWS-12 scores37 (6.9 points or over41) after the evaluation period.
Initial response to PR-fampridine should be assessed after 2 weeks, the time which is normally necessary to observe the effects of the drug on gait.7,32,42 Clinical practice has shown that some patients display slow response to PR-fampridine. Exceptionally, initial assessment may be delayed until the 4-week mark in these cases. Treatment should be continued in responders and discontinued in non-responders.
Response to treatment in responder patients should be re-evaluated at least every 6 months, comparing follow-up results with baseline status. Administration of PR-fampridine should be discontinued if a patient who was initially responsive ceases to show improvements in walking in a 6-month follow-up assessment. In these cases, physicians should aim to detect the presence of any intercurrent processes associated with MS (relapses, progression) or of other origin (e.g. infections) that may have contributed to gait impairment. Patients with intercurrent processes should receive appropriate treatment; evaluation based on the new baseline status may be resumed following a 2-week wash-out period (Fig. 2).
Efficacy of PR-fampridineClinical trialsThe efficacy of PR-fampridine has been demonstrated in multiple clinical trials. The first trial, published by Schwid et al.,43 included 10 patients with MS; patients received either placebo or 17.5mg PR-fampridine twice daily for one week. According to their results, PR-fampridine had a significant, positive effect (P=0.02) on gait speed and improved muscle strength according to the Lower Extremity Manual Muscle Test (LEMMT).43
Goodman et al. subsequently published a trial of the efficacy of PR-fampridine44 after their 2007 dose-ranging trial of the safety of the drug.45 The former trial, a multicentre, randomised, double-blind, placebo-controlled phase II study, examined the efficacy and safety of 3 different doses of PR-fampridine (10, 15, and 20mg) administered twice daily for 15 weeks in 206 patients with MS.44 In this study, walking speed increased by over 20% in the T25FW in 23.5%, 26.0%, and 15.8% of the patients receiving 10, 15, and 20mg of PR-fampridine, compared to only 12.8% of patients in the placebo group. Responders in the treatment groups displayed an increase in walking speed of between 25% and 29%, compared to 4% in controls. The authors concluded that the most appropriate dose of PR-fampridine for the treatment of these patients was 10mg every 12hours.
The same research group subsequently published 2 multicentre, double-blind, placebo-controlled phase III trials evaluating the efficacy and safety of PR-fampridine in patients with MS and gait alterations.7,42 In the 2009 trial, 301 patients were randomised at a 3:1 ratio, and administered either 10mg PR-fampridine or placebo twice daily for 14 weeks. Around 34.8% of the patients receiving PR-fampridine were responders (mean increase in walking speed of 25.2% and a mean change in MSWS-12 scores of −6.8). In the placebo group, in contrast, 8.3% of patients were responders (mean increase in walking speed of 4.7% and mean change in MSWS-12 scores of +1.4).42
In the 2010 trial, 239 patients were randomised at a 1:1 ratio, and administered either 10mg PR-fampridine or placebo twice daily for 9 weeks. A total of 42.9% of the patients treated with PR-fampridine responded to treatment (mean increase in walking speed of 24.7% and a mean change in MSWS-12 scores of −6.04), compared to 15.3% of responders in the placebo group (mean increase in walking speed of 9.3% and a mean change in MSWS-12 scores of +1.3).7
Experience in clinical practicePR-fampridine was authorised in 2010 in the US and in 2011 in Europe for improving walking ability in patients with any type of MS.8 Observational studies began to be published in 2013; these studies were based on clinical practice and followed the recommendations of the summary of product characteristics of PR-fampridine.
An observational study conducted in Germany assessed 52 MS patients over 9 to 12 months; 58% of these were still receiving the treatment by the end of the study period due to demonstrable clinical improvement, treatment adherence, and lack of adverse effects. More specifically, patients showed improvements in T25FW and the Fatigue Scale for Motor and Cognitive Functions (FSMC) at 2 weeks from treatment onset and at the end of the follow-up period, increased walking speed (maximum walked distance/time) at 2 weeks, and improvements in Paced Auditory Symbols Audition Test (PASAT) scores at the end of follow-up.46
In the US, Cameron et al.47 conducted an observational study including 39 patients who were treated for 12 months. Sixty-two percent of the patients continued treatment after 3-4 weeks, showing significant improvements in the T25FW test at 6-8 weeks, and in the MSWS-12 and the 2-Minute Timed Walk (2MTW) test at 6-8 weeks and at one year.47
In an observational study conducted in Austria, which included 67 patients, 71.6% of the sample were still receiving the treatment at 6 months since they perceived clinical benefits and had experienced no adverse effects.48 Four weeks after starting treatment with PR-fampridine, 32.8% of the patients walked ≥20% faster (T25FW test) and 65.7% showed improvements in MSWS-12 scores. After 6 months, 16.4% of the patients walked ≥20% faster, 59.7% showed improvements in MSWS-12 scores, and patients reporting clinical benefits had a mean improvement of one point on the Fatigue Severity Scale (FSS) and 10 points on the MSWS-12.48
Quality of life with PR-fampridineGait impairment has a significant impact on HRQoL; improving walking ability may therefore have a beneficial effect on responders.3,49
HRQoL can be reliably estimated in patients with MS using the SF-3613,14 and the EuroQol-5 dimension (EQ-5D)50 questionnaires, 2 instruments designed for the general population, and with the MSIS-2951 and the Patient-Reported Outcome Indices for Multiple Sclerosis (PRIMUS)52 scales, which are specifically designed for patients with the disease. In a study evaluating patients with the abbreviated version of the SF-36 survey, patients with gait impairment were observed to present a significantly lower HRQoL in the physical component than those without (P<.001).53 In another study, changes in T25FW scores in patients with progressive forms of MS were found to be associated with positive changes in MSIS-29.54
In the study by Goodman et al.,42 301 patients completed the MSWS-12, a questionnaire evaluating patients’ subjective perception of their walking disability over the 2 previous weeks. Limone et al.55 mapped MSWS-12 scores to the EQ-5D, demonstrating that HRQoL improved significantly in PR-fampridine responders compared to controls; these improvements continued during treatment and were lost after the drug was discontinued. No differences were observed between non-responders and controls throughout the study period.
ENABLE,56 a recent open-label multicentre phase IV study, evaluated the efficacy of PR-fampridine for improving HRQoL in responder MS patients after a 48-week period. Of a total of 901 patients, 707 (78.5%) responded to treatment. Compared to baseline values, responders showed statistically significant improvements in SF-36 physical component summary scores, the 8 SF-36 subscales, SF-36 mental component summary scores, MSIS-29, PRIMUS (activity limitations scale), and EQ-5D in all follow-up visits (weeks 12, 24, 36, and 48). Non-responders were followed up despite treatment discontinuation and displayed no significant improvements in any questionnaire and at any of the follow-up assessments.
The studies by Cameron et al.47 and Prugger and Berger48 also report positive results on the scales used to measure HRQoL.
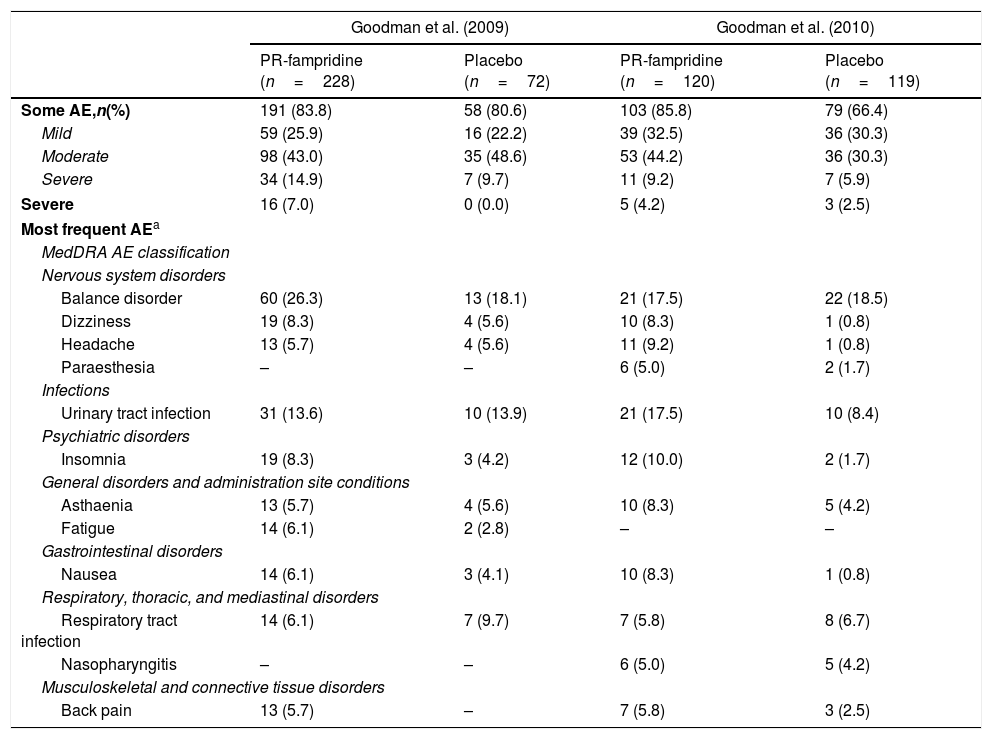
Safety of PR-fampridineClinical trialsOf the total of 539 patients with MS included in all phase III studies, 348 received 10mg PR-fampridine every 12hours and 191 received a placebo (Table 1).8 In the most recent study, 103 patients (85.8%) receiving PR-fampridine and 79 patients (66.4%) receiving placebo experienced some adverse effect (P<.001). The most common adverse effects were urinary tract infection and balance disorder, followed by insomnia, dizziness, asthaenia, headache, nausea, and paraesthesia. In most cases, adverse effects were mild (32.5% vs 30.3% in the placebo group) or moderate (44.2% vs 30.3%). Some 4.2% of the patients in the treatment group and 2.5% of controls experienced severe adverse effects. Falls were reported in both groups, although they were less frequent in the treatment group (11.7% vs 16.8%; P=.2724).
Summary of adverse effects of PR-fampridine dosed at 10mg every 12hours according to the results of phase III clinical trials.
| Goodman et al. (2009) | Goodman et al. (2010) | |||
|---|---|---|---|---|
| PR-fampridine (n=228) | Placebo (n=72) | PR-fampridine (n=120) | Placebo (n=119) | |
| Some AE,n(%) | 191 (83.8) | 58 (80.6) | 103 (85.8) | 79 (66.4) |
| Mild | 59 (25.9) | 16 (22.2) | 39 (32.5) | 36 (30.3) |
| Moderate | 98 (43.0) | 35 (48.6) | 53 (44.2) | 36 (30.3) |
| Severe | 34 (14.9) | 7 (9.7) | 11 (9.2) | 7 (5.9) |
| Severe | 16 (7.0) | 0 (0.0) | 5 (4.2) | 3 (2.5) |
| Most frequent AEa | ||||
| MedDRA AE classification | ||||
| Nervous system disorders | ||||
| Balance disorder | 60 (26.3) | 13 (18.1) | 21 (17.5) | 22 (18.5) |
| Dizziness | 19 (8.3) | 4 (5.6) | 10 (8.3) | 1 (0.8) |
| Headache | 13 (5.7) | 4 (5.6) | 11 (9.2) | 1 (0.8) |
| Paraesthesia | – | – | 6 (5.0) | 2 (1.7) |
| Infections | ||||
| Urinary tract infection | 31 (13.6) | 10 (13.9) | 21 (17.5) | 10 (8.4) |
| Psychiatric disorders | ||||
| Insomnia | 19 (8.3) | 3 (4.2) | 12 (10.0) | 2 (1.7) |
| General disorders and administration site conditions | ||||
| Asthaenia | 13 (5.7) | 4 (5.6) | 10 (8.3) | 5 (4.2) |
| Fatigue | 14 (6.1) | 2 (2.8) | – | – |
| Gastrointestinal disorders | ||||
| Nausea | 14 (6.1) | 3 (4.1) | 10 (8.3) | 1 (0.8) |
| Respiratory, thoracic, and mediastinal disorders | ||||
| Respiratory tract infection | 14 (6.1) | 7 (9.7) | 7 (5.8) | 8 (6.7) |
| Nasopharyngitis | – | – | 6 (5.0) | 5 (4.2) |
| Musculoskeletal and connective tissue disorders | ||||
| Back pain | 13 (5.7) | – | 7 (5.8) | 3 (2.5) |
AE: adverse effect; n: number of patients; MedDRA: Medical Dictionary for Regulatory Activities.
In all the clinical trials together, epileptic seizures were observed in one patient receiving PR-fampridine and in one control.31,57
In the clinical trials conducted by Goodman et al.7,42,44 patients were exposed to PR-fampridine for up to 5 years; during the study period, the rate of epileptic seizures was 4.1 cases per 1000 patient-years, a risk similar to that in the general population with MS.58 However current data suggest that epileptic seizures may be caused by other factors, including renal insufficiency or concomitant treatment, which may increase the risk of seizures in these patients.2
Regarding the potential cardiotoxicity of PR-fampridine, a study by March and Cardi59 found no clinically significant changes in heart rate or in QT, PR, and QRS intervals with either therapeutic or supratherapeutic doses of PR-fampridine.
Experience in clinical practiceJara et al.58 analysed postmarketing safety of PR-fampridine in the US in 46000 patients with MS after one year of treatment. The study identified a total of 11549 instances of adverse effects, largely coinciding with those described in clinical trials: dizziness (5.7%), insomnia (4.5%), falls and balance disorder (3.9%), headache (3.2%), nausea (2.8%), and urinary tract infection (2.4%). A total of 573 instances of adverse effects (5.0%) were caused by inappropriate dosing. The study reported 85 cases of epileptic seizure, occurring within a week of treatment onset in 31% of cases. The incidence rate of seizures after the first week of treatment was 3.9 cases per 1000 patient-years of use.
Although safety data drawn from clinical practice are coherent with the safety profile observed during the clinical development of PR-fampridine, long-term postmarketing surveillance is necessary to correctly characterise the clinical safety profile of the drug.
ConclusionsClinical trials and observations from clinical practice show that PR-fampridine is a well-tolerated, easy-to-administer drug that is useful for the treatment of walking disability. It is indicated for adults with any form of MS and who score 4.0 to 7.0 on the EDSS.
Dosed at 10mg (1 tablet) every 12hours, PR-fampridine causes mild-to-moderate transient adverse effects. Although randomised clinical trials report clinical benefits in 35% of patients, the ENABLE study and other observational studies of clinical practice have observed benefits in over 50% of patients. Responders are easily identified after an initial treatment period of 2 weeks (or in exceptional cases, 4 weeks); this ensures that only patients with objective clinical benefits continue treatment.
Although data on 5-year exposure is now available,60 only patient follow-up and future observational studies will be able to provide an accurate description of the benefits of PR-fampridine on walking disability and other MS-related symptoms and to establish the drug's safety profile.
FundingThe authors have received no funding for this study.
Conflict of interestLluís Ramió-Torrentà has received research grants, funds for attending congresses, or fees as a consultant to committees from Almirall, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
José Carlos Álvarez-Cermeño has received research grants, funds for attending congresses, or fees as a consultant to committees from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
Rafael Arroyo has received lecture honoraria and fees as a scientific consultant from Almirall, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, and TEVA.
Bonaventura Casanova-Estruch has received research grants, funds for attending congresses, or fees as a consultant to committees from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
Óscar Fernández has received lecture honoraria, fees as a scientific consultant, and research funding from Actelion, Almirall, Allergan, Bayer, Biogen, Merck-Serono, Novartis, Roche, and TEVA.
Juan Antonio García-Merino has received research grants, funds for attending congresses, or fees as a consultant to committees from Bayer, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
Miguel Ángel Hernández has received research grants, funds for attending congresses, or fees as a consultant to committees from Almirall, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
Guillermo Izquierdo has received research grants, funds for attending congresses, or fees as a consultant from Almirall, Roche, Bayer, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
Sergio Martínez-Yélamos has no conflicts of interest to declare.
José Meca has received funds for attending congresses or fees as a consultant from Almirall, Biogen, Genzyme, Merck-Serono, Novartis, and TEVA.
Ester Moral has no conflicts of interest to declare.
Javier Olascoaga has received lecture honoraria, funds for attending congresses, fees as a consultant, or research grants from Biogen, Bayer, Genzyme, Merck-Serono, Novartis, and TEVA.
José María Prieto has received lecture honoraria, funds for attending congresses, fees as a consultant, or research grants from Bayer, Biogen, Genzyme, Novartis, Merck-Serono, Sanofi-Aventis, TEVA, Roche, and Almirall.
Albert Saiz has received research grants, funds for attending congresses, or fees as a consultant to committees from Bayer, Biogen, Genzyme, Merck, Novartis, and TEVA.
Please cite this article as: Ramió-Torrentà L, Álvarez-Cermeño JC, Arroyo R, Casanova-Estruch B, Fernández O, García-Merino JA, et al. Guía de tratamiento del deterioro de la marcha con fampridina de liberación prolongada (Fampyra®) en pacientes con esclerosis múltiple. Neurología. 2018;33:327–337.