Neurexins are a small family of cell adhesion proteins which participate in synapsis by binding to the post-synaptic ligand neuroligin and forming calcium-dependent trans-synaptic complexes in the central nervous system.1–3 Three neurexin genes have been identified in the human genome: NRXN1, NRXN2, and NRXN3. The NRXN1 gene, located at 2p16.3, is 1.1 Mb long and includes 24 exons.4
We present the case of a paediatric patient from south-western Colombia. He was the first child of non-consanguineous parents, and was born after a full-term pregnancy with medical care during delivery. The first examination of the neonate revealed right cryptorchidism and heart murmur; an echocardiography revealed peripheral pulmonary stenosis, tricuspid insufficiency, and left ventricular hypertrophy.
At 4 years of age, the patient was referred to a paediatric genetics clinic; physical examination revealed dolichocephaly, prominent forehead, coarse hair and dry skin, bitemporal hair thinning, bulbous nose, fingers with bilateral clinodactyly, autistic behaviour (including self-harm), and cognitive deficits.
The patient underwent several studies including G-banding (46, XY with no numerical or structural alterations), computed tomography, and magnetic resonance imaging, revealing posterior ventricular asymmetry with right ventricular dilatation, anatomical changes to the ventricular floor, and mega cisterna magna.
Metabolic screening, array comparative genomic hybridisation, and a molecular panel for RASopathies yielded negative results. Neuropsychological evaluation revealed severe autism with a score of 94 on an autism spectrum scale (minimum 24/maximum 96). We requested a 101-gene molecular panel for autism, including NRXN1, which showed 2 mutations in heterozygosis. In the first mutation, cytosine was replaced by thymine at position 1405 (c.1405C>T), presumably causing the replacement of proline by serine at position 469 (p.Pro469Ser) [rs7850316]; in the second mutation, adenine was replaced by guanine at position 4053 (c.4053A>G), resulting in the replacement of alanine by alanine at the 1351 position (p.Ala1351Ala) [rs7997075]. The parents were assessed for both mutations; the father was found to carry the c.1405C>T variant (p.Pro469Ser) [rs7850316] in heterozygosis and the mother was a carrier of the c.4053A>G variant (p.Ala1351Ala) [rs7997075] in heterozygosis.
The literature includes reports of 3 cases of patients with biallelic mutations of the NRXN1 gene with a similar phenotype, who were finally diagnosed with an entity called Pitt-Hopkins-like syndrome 2.5,6 We believe that our patient may be the fourth case of Pitt-Hopkins-like syndrome 2, but the first to present a heterozygous mutation of the NRXN1 gene not associated with deletions in the 2p16.3 region.
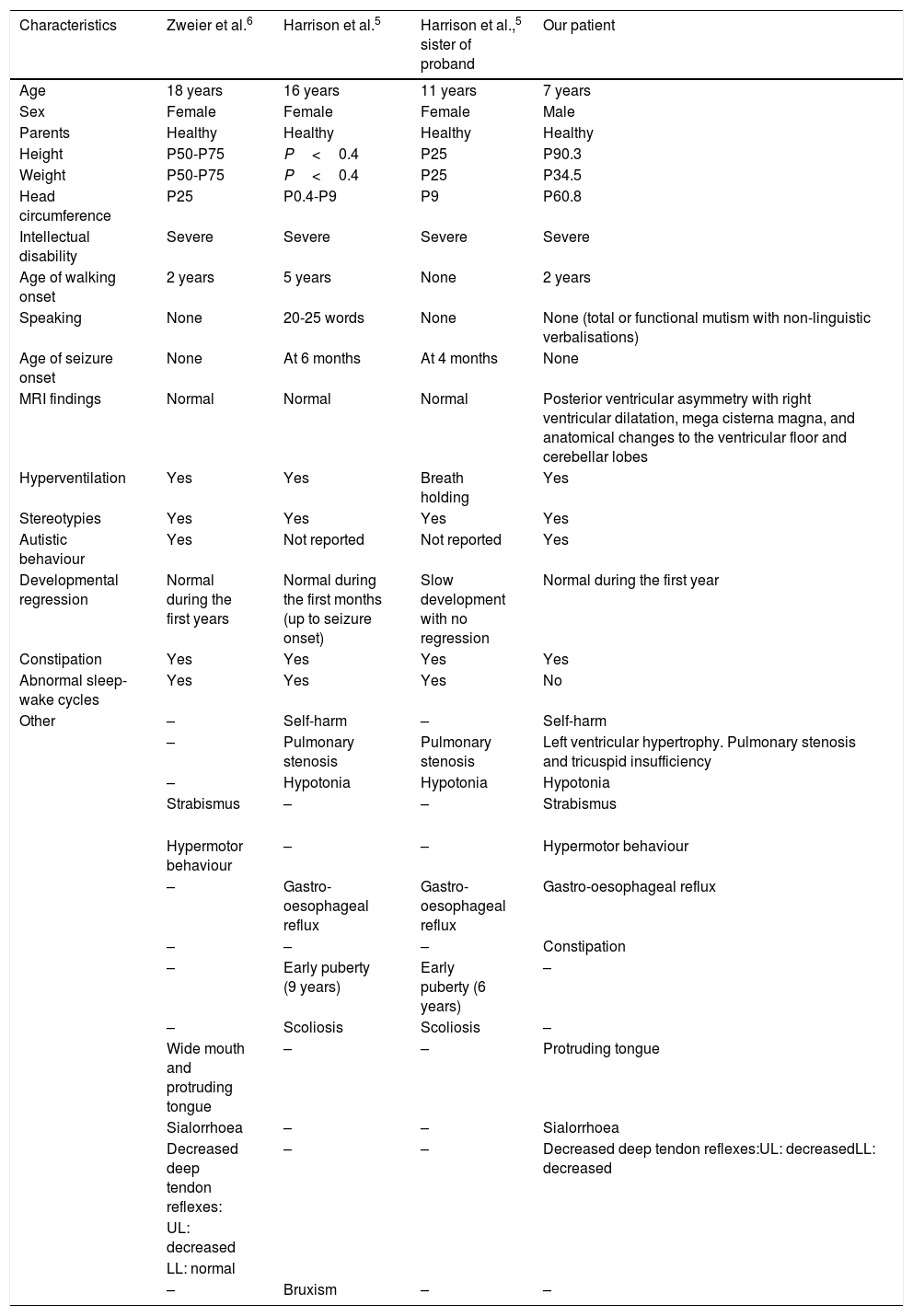
The c.1405C>T variant (rs78540316) is classified in the Clinical Variant database as a change of uncertain clinical significance. An in silico analysis provided contradictory results, with the SIFT software predicting that this is probably a tolerated change, whereas the PolyPhen-2 software predicted that it was probably pathological. However, a comparative analysis of the phenotypes of our case and those reported by Zweier et al.6 and Harrison et al.5 (Table 1) shows a significant overlap of signs and symptoms, the exceptions being the posterior ventricular asymmetry with right ventricular dilatation, the anatomical changes to the ventricular floor, and the mega cisterna magna.5–7 These findings may be a new addition to the phenotypic spectrum of Pitt-Hopkins-like syndrome 2, and are probably due to the specific mutations present in our patient.
Comparison of the phenotypes of patients previously reported with Pitt-Hopkins-like syndrome 2 and the present case.
| Characteristics | Zweier et al.6 | Harrison et al.5 | Harrison et al.,5 sister of proband | Our patient |
|---|---|---|---|---|
| Age | 18 years | 16 years | 11 years | 7 years |
| Sex | Female | Female | Female | Male |
| Parents | Healthy | Healthy | Healthy | Healthy |
| Height | P50-P75 | P<0.4 | P25 | P90.3 |
| Weight | P50-P75 | P<0.4 | P25 | P34.5 |
| Head circumference | P25 | P0.4-P9 | P9 | P60.8 |
| Intellectual disability | Severe | Severe | Severe | Severe |
| Age of walking onset | 2 years | 5 years | None | 2 years |
| Speaking | None | 20-25 words | None | None (total or functional mutism with non-linguistic verbalisations) |
| Age of seizure onset | None | At 6 months | At 4 months | None |
| MRI findings | Normal | Normal | Normal | Posterior ventricular asymmetry with right ventricular dilatation, mega cisterna magna, and anatomical changes to the ventricular floor and cerebellar lobes |
| Hyperventilation | Yes | Yes | Breath holding | Yes |
| Stereotypies | Yes | Yes | Yes | Yes |
| Autistic behaviour | Yes | Not reported | Not reported | Yes |
| Developmental regression | Normal during the first years | Normal during the first months (up to seizure onset) | Slow development with no regression | Normal during the first year |
| Constipation | Yes | Yes | Yes | Yes |
| Abnormal sleep-wake cycles | Yes | Yes | Yes | No |
| Other | – | Self-harm | – | Self-harm |
| – | Pulmonary stenosis | Pulmonary stenosis | Left ventricular hypertrophy. Pulmonary stenosis and tricuspid insufficiency | |
| – | Hypotonia | Hypotonia | Hypotonia | |
| Strabismus | – | – | Strabismus | |
| Hypermotor behaviour | – | – | Hypermotor behaviour | |
| – | Gastro-oesophageal reflux | Gastro-oesophageal reflux | Gastro-oesophageal reflux | |
| – | – | – | Constipation | |
| – | Early puberty (9 years) | Early puberty (6 years) | – | |
| – | Scoliosis | Scoliosis | – | |
| Wide mouth and protruding tongue | – | – | Protruding tongue | |
| Sialorrhoea | – | – | Sialorrhoea | |
| Decreased deep tendon reflexes: | – | – | Decreased deep tendon reflexes:UL: decreasedLL: decreased | |
| UL: decreased | ||||
| LL: normal | ||||
| – | Bruxism | – | – |
We should mention that the previously reported cases of Pitt-Hopkins-like syndrome 2 present a deletion-type mutation in homozygosis affecting NRXN1,5–7 suggesting an autosomal recessive inheritance pattern; however, our case presents a nucleotide substitution in heterozygosis, also present in the father. Therefore, in this case, the mutation (c.1405C>T) is considered to follow an autosomal dominant inheritance pattern with incomplete penetrance, or to be caused by the co-occurrence of mutations c.1405C>T and c.4053A>G, in which case our patient would be a compound heterozygote for NRXN, which would justify the healthy phenotypes of the parents.
The significant phenotypic similarities with the previously reported patients, in association with the presence of a mutation in the NRXN1 gene, lead us to believe that our patient has the same condition. This supports the idea that mutations in the NRXN1 gene cause a phenotype consisting in autism and cognitive deficit similar to that observed in Pitt-Hopkins syndrome.
Please cite this article as: Ruiz-Botero F, Gómez-Pineda E, Pachajoa H. ¿Un nuevo caso de síndrome de Pitt-Hopkins like 2? Neurología. 2019;34:607–609.