The relationship between impulse control disorder (ICD) and REM sleep behaviour disorder (RBD) has not yet been clarified, and the literature reports contradictory results. Our purpose is to analyse the association between these 2 disorders and their presence in patients under dopaminergic treatment.
MethodsA total of 73 patients diagnosed with Parkinson's disease and treated with a single dopamine agonist were included in the study after undergoing clinical assessment and completing the single-question screen for REM sleep behaviour disorder and the short version of the questionnaire for impulsive–compulsive behaviours in Parkinson's disease.
ResultsMean age was 68.88±7.758 years. Twenty-six patients (35.6%) were classified as probable-RBD. This group showed a significant association with ICD (P=.001) and had a higher prevalence of non-tremor akinetic rigid syndrome and longer duration of treatment with levodopa and dopamine agonists than the group without probable-RBD. We found a significant correlation between the use of oral dopamine agonists and ICD. Likewise, patients treated with oral dopamine agonists demonstrated a greater tendency towards presenting probable-RBD than patients taking dopamine agonists by other routes; the difference was non-significant.
ConclusionsThe present study confirms the association between RBD and a higher risk of developing symptoms of ICD in Parkinson's disease.
La relación entre el trastorno del control de impulsos (TCI) y el trastorno de conducta del sueño REM (TCSR) no se ha aclarado todavía y los resultados de la literatura son contradictorios. Nuestro objetivo es valorar la asociación entre estos 2 trastornos y, a su vez, su presencia en dependencia de la terapia dopaminérgica.
MétodosUn total de 73 pacientes diagnosticados de enfermedad de Parkinson, en tratamiento con un único agonista dopaminérgico, fueron incluidos en el estudio, tras valoración clínica y habiendo completado el cuestionario de pregunta única para el TCSR y el cuestionario abreviado para los trastornos impulsivo-compulsivos en la enfermedad de Parkinson.
ResultadosLa edad media ± desviación estándar de los pacientes fue de 68,88±7,758 años. De ellos, 26 pacientes (35,6%) se clasificaron dentro de un TCSR-probable, presentando mayor prevalencia de síndrome rígido acinético no tremórico, más años de tratamiento con levodopa y con agonistas dopaminérgicos, y una relación significativa con el TCI (p=0,001) en comparación con el grupo sin TCSR-probable.
En cuanto al tratamiento con agonistas dopaminérgicos, se demostró la asociación significativa de la administración por vía oral con una mayor prevalencia de TCI, mientras que esta vía también se relacionó con mayor tendencia a desarrollar TCSR, diferencias en este caso no significativas.
ConclusionesNuestros datos confirman que el TCSR se relaciona con el TCI en la enfermedad de Parkinson.
REM sleep behaviour disorder (RBD) is a sleep disorder characterised by vigorous motor behaviour, unpleasant dreams, and lack of normal voluntary muscle atonia during REM sleep.1 RBD most commonly presents in patients with neurodegenerative diseases, especially such synucleinopathies as Parkinson's diseases (PD), Lewy body dementia, and multiple system atrophy.2
All the conditions linked to RBD are characterised by marked brainstem degeneration, which probably alters the structures modulating REM sleep in normal circumstances. Furthermore, the emotional, unpleasant, distressing content of dreams suggests that in addition to the pontine tegmentum, the limbic system may be involved in the pathophysiology of RBD.1,3
In the case of PD, the frequency of RBD has been reported to range from 20% to 72%2 and may precede motor symptom onset by years or even decades.
According to several studies, patients with PD and RBD present more motor and non-motor disorders, which points to an extensive degenerative process.4 RBD has been associated with such factors as older age, longer disease duration, more severe motor impairment associated with akinetic-rigid syndrome (ARS), hallucinations, autonomic dysfunction, and high levodopa doses. A direct association has recently been described between this sleep disorder and increased risk of impulse control disorder (ICD).4,5
ICD-related behaviours (ICDRB) constitute a non-motor complication of PD. These psychological disorders are characterised by persistent failure to resist the urge to perform actions that may be damaging to the patient and/or the people surrounding him or her.6 ICDRB include such behaviours as punding (repetitive purposeless stereotyped behaviours), hobbysm (compulsively seeking a hobby, such as cleaning or tidying up), walkabout, and poor impulse control (sexual impulses, eating, shopping, gambling), and even symptoms of dopamine dysregulation syndrome. These are all reward-seeking behaviours whose aetiopathogenesis seems to be related to decreased dopamine transporter activity in the ventral striatum,7 mesocorticolimbic pathway dysfunction, and presence of polymorphisms in D3 and D4 dopamine receptors.8 The prevalence of these disorders in patients with PD is estimated at 8% to 28%, depending on the study methodology.9–12 ICD has been associated with younger ages, early symptom onset, a personal or family history of ICD, substance abuse, bipolar disorder, and impulsivity.4
Given that patients with PD and ICD display more severe motor and non-motor symptoms, it has been hypothesised that these patients have more severe mesocorticolimbic pathway alterations, one of the pathophysiological factors of ICD.
To confirm this hypothesis, we decided to analyse the association between probable RBD and ICDRB, and to determine whether ICDRB is a non-motor complication of PD closely linked to dopamine replacement therapy.8 As a secondary objective, we analysed the association between these 2 disorders and treatment for PD.
Patients and methodsOur study included 73 patients with a clinical diagnosis of PD13 attending the PD section of the movement disorders unit at Hospital Clínico Universitario Lozano Blesa in Zaragoza between September 2014 and July 2015. All patients signed informed consent forms prior to inclusion in the study. They all met the inclusion criteria and had been receiving a dopaminergic agent (DA; pramipexole, ropinirole, or rotigotine) for at least 6 months previously. We excluded patients with cognitive impairment or psychosis and those previously treated with other DAs, deep brain stimulation, or continuous infusion of levodopa/apomorphine.
We gathered the following clinical and demographic data: sex, age, onset of PD, degree of motor impairment according to the Hoehn and Yahr (H&Y) scale, presence of motor fluctuations and dyskinesia, and concomitant treatment. The levodopa equivalent daily dose (LEDD) was calculated using the conversion formula proposed by Tomlinson et al.14
To evaluate probable RBD (pRBD), participants completed the REM sleep behaviour disorder-single-question screen (RBD-1Q), a validated tool for detecting RBD consisting of a single question.15 An affirmative response to the RBD-1Q indicated pRBD.
Participants also completed the short version of the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease (QUIP-S).16 This self-administered questionnaire was specifically designed and validated to detect symptoms of ICD (compulsive gambling, shopping, sexual behaviour, or eating) and ICDRB (punding, hobbysm, walkabout, and dopamine dysregulation syndrome) in the previous 4 weeks. An affirmative answer to any of the questions of the QUIP-S was considered to indicate presence of ICDRB (ICDRB+). Patients with no affirmative responses were assigned to the ICDRB− group.
Statistical analysis was performed using SPSS statistical software version 22.0. An alpha risk of 5% was considered statistically significant (P<.05). Clinical and demographic characteristics are expressed as means±SD for quantitative variables and as percentages for qualitative variables. The bivariate analysis used the chi-square test for qualitative variables and the t test for quantitative variables.
ResultsOur study included a total of 73 patients with clinically diagnosed PD who completed the RBD-1Q and the QUIP-S. Mean age was 68.88±7.758 years; 50.7% of participants were men. A total of 26 patients (35.6%; 14 men) were included in the pRBD group. The clinical and demographic characteristics of patients with and without pRBD are shown in Table 1.
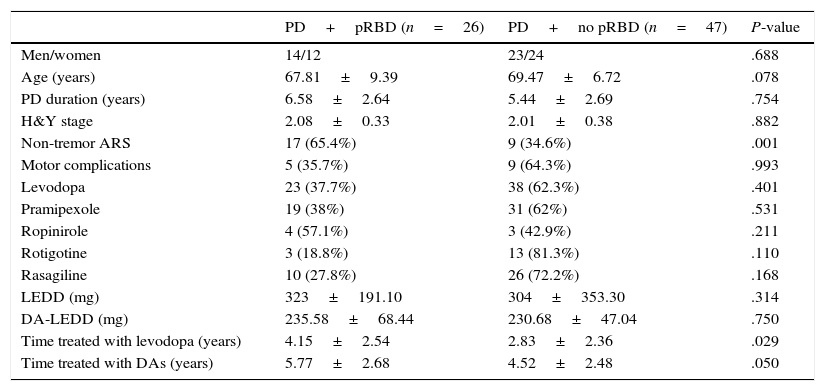
Clinical and demographic characteristics of PD patients with and without pRBD.
| PD+pRBD (n=26) | PD+no pRBD (n=47) | P-value | |
|---|---|---|---|
| Men/women | 14/12 | 23/24 | .688 |
| Age (years) | 67.81±9.39 | 69.47±6.72 | .078 |
| PD duration (years) | 6.58±2.64 | 5.44±2.69 | .754 |
| H&Y stage | 2.08±0.33 | 2.01±0.38 | .882 |
| Non-tremor ARS | 17 (65.4%) | 9 (34.6%) | .001 |
| Motor complications | 5 (35.7%) | 9 (64.3%) | .993 |
| Levodopa | 23 (37.7%) | 38 (62.3%) | .401 |
| Pramipexole | 19 (38%) | 31 (62%) | .531 |
| Ropinirole | 4 (57.1%) | 3 (42.9%) | .211 |
| Rotigotine | 3 (18.8%) | 13 (81.3%) | .110 |
| Rasagiline | 10 (27.8%) | 26 (72.2%) | .168 |
| LEDD (mg) | 323±191.10 | 304±353.30 | .314 |
| DA-LEDD (mg) | 235.58±68.44 | 230.68±47.04 | .750 |
| Time treated with levodopa (years) | 4.15±2.54 | 2.83±2.36 | .029 |
| Time treated with DAs (years) | 5.77±2.68 | 4.52±2.48 | .050 |
Data are expressed as percentages or means±SD.
Non-tremor ARS was more frequent and treatment with levodopa and other DAs was longer in patients with pRBD than in those without. However, no significant differences were found between groups in sex, age, disease duration, use of different DAs, or motor complications. We should highlight that 81.3% of the patients receiving rotigotine did not have pRBD, compared to 62% and 42.9% of those receiving pramipexole and ropinirole, respectively. However, these differences were not significant.
A significant association was observed between pRBD and ICDRB (P=.001). Around 84.6% of the patients with pRBD (n=22) answered affirmatively to at least one question on the QUIP-S; only 15.4% (n=4) of patients showed no ICDRB. Only 31.9% of the patients without pRBD (n=15) were found to have ICDRB according to the QUIP-S.
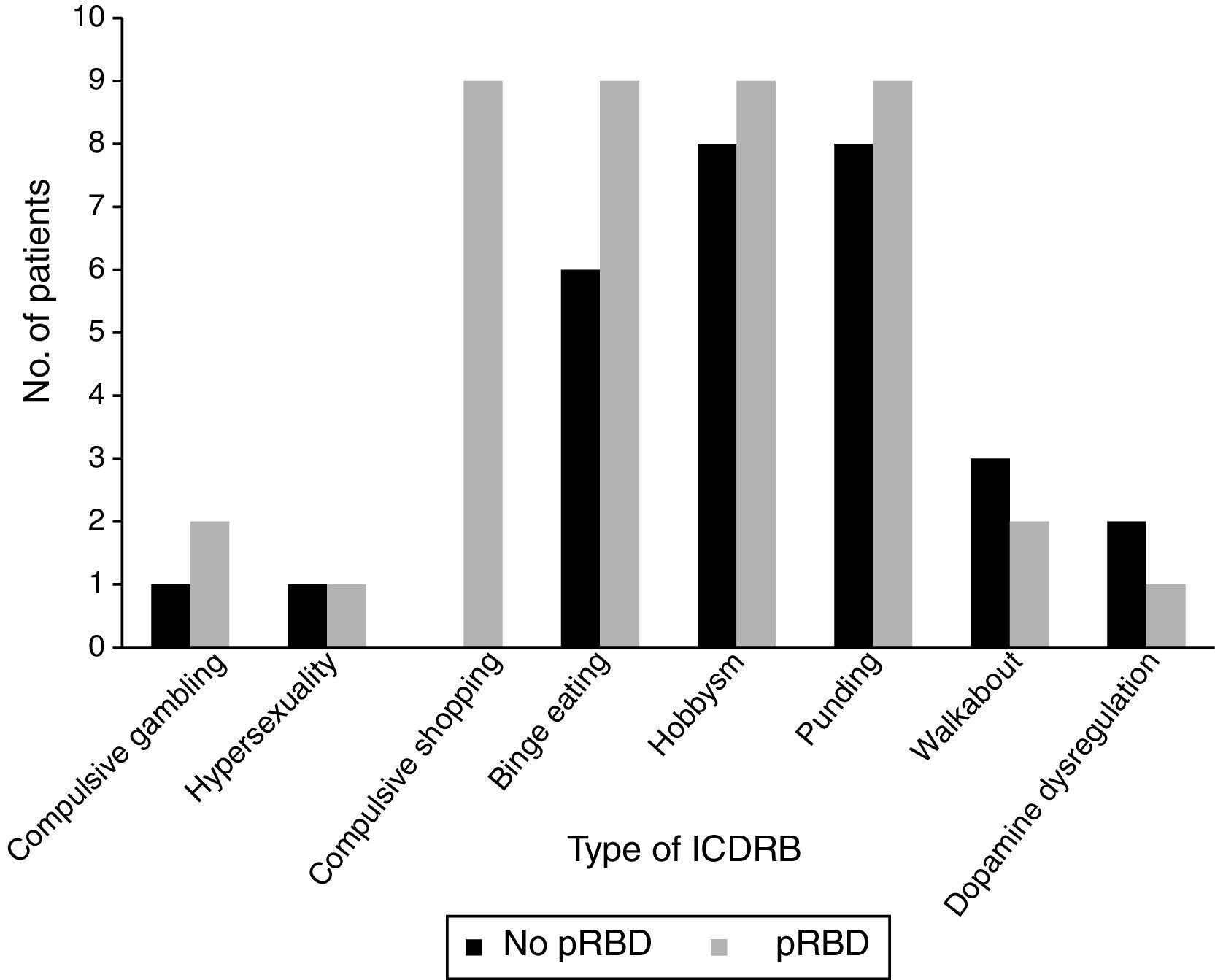
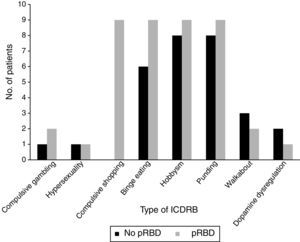
Frequencies of the different ICDRB in patients with and without pRBD are shown in Fig. 1. Compulsive shopping and binge eating were found to be significantly associated with pRBD (P=.001 and .028, respectively).
Lastly, we classified our sample into patients receiving DAs by oral route (pramipexole or ropinirole) and those receiving DAs by transdermal route (rotigotine). Table 2 compares different variables between administration routes. In our sample, 57% of the patients receiving oral DAs and 25% of those treated with rotigotine displayed ICDRB (P=.021). The frequency of pRBD was also higher in the group of patients receiving oral treatment (40.4% vs 18.8%), although differences were not significant in this case.
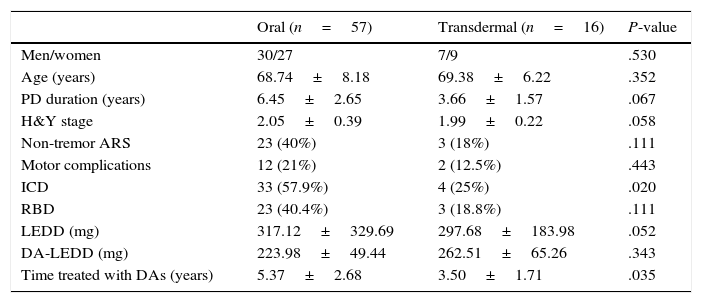
Clinical and demographic characteristics by route of administration.
| Oral (n=57) | Transdermal (n=16) | P-value | |
|---|---|---|---|
| Men/women | 30/27 | 7/9 | .530 |
| Age (years) | 68.74±8.18 | 69.38±6.22 | .352 |
| PD duration (years) | 6.45±2.65 | 3.66±1.57 | .067 |
| H&Y stage | 2.05±0.39 | 1.99±0.22 | .058 |
| Non-tremor ARS | 23 (40%) | 3 (18%) | .111 |
| Motor complications | 12 (21%) | 2 (12.5%) | .443 |
| ICD | 33 (57.9%) | 4 (25%) | .020 |
| RBD | 23 (40.4%) | 3 (18.8%) | .111 |
| LEDD (mg) | 317.12±329.69 | 297.68±183.98 | .052 |
| DA-LEDD (mg) | 223.98±49.44 | 262.51±65.26 | .343 |
| Time treated with DAs (years) | 5.37±2.68 | 3.50±1.71 | .035 |
Data are expressed as percentages or means±SD.
Our results seem to demonstrate that presence of symptoms suggestive of ICD may be associated with an increased risk of developing ICDRB during the course of PD.
This association has already been described in previous studies. Kim et al.5 interviewed 944 patients with PD to assess presence of RBD and its association with ICD, which was found to be statistically significant. In a subsequent study, Bayard et al.17 concluded that such an association does not exist, based on the results of gold standard tests (polysomnography for RBD and semi-structured interview for ICD). In February 2015, Fantini et al.4 conducted another study including 260 patients with PD who were classified by presence or absence of pRBD. According to their results, ICDRB were significantly more frequent in patients with pRBD than in those without (53% vs 28%; P=.0002).
Our study aimed to further analyse this association based on the hypothesis that patients with PD and RBD have more severe mesocorticolimbic pathway alterations and are therefore more likely to develop ICD. The mesocorticolimbic dopaminergic pathway plays a crucial role in the reward system, reinforcement learning, and regulation of impulse control. This pathway involves the ventral striatum, amygdala, hippocampus, and the ventromedial and orbitofrontal regions of the prefrontal cortex.4
There is a well-documented association between the limbic areas involved in impulse control and those involved in REM sleep, including the pedunculopontine nucleus. The pedunculopontine nucleus, located at the level of the brainstem, is responsible for modulating REM sleep. It is connected to the basal ganglia and limbic system; as a result, this structure forms part of the reward system and plays a major role in motivation, reinforcement learning, and response control.18 The limbic system is known to regulate emotions during wakening and to be active during REM sleep (especially the amygdala), probably as a result of the emotional content of dreams.19
In light of the above, and based on the hypothesis that damage to the limbic system may be responsible for both vivid dreaming and ICD, we feel that the association observed in this study should be further analysed in prospective studies with greater sample sizes.
We should point out that the percentage of patients with ICDRB was higher in our series (50.7%) than in other studies,9,16 which may be due to the use of the QUIP-S to detect these disorders. The QUIP-S constitutes a good screening tool due to its high sensitivity (94%)16; this helps detect a great number of cases, which would require confirmation with close follow-up or more specific tests. According to a multicentre study conducted in June 2012, RBD-1Q and polysomnography, the gold standard test for this condition, have similar sensitivity and specificity.15 Based on the above, RBD-1Q seems to be a good screening tool for RBD.
The association between DA consumption and ICDRB is well documented, although very few studies have analysed the association between this disorder and each specific type of DAs. A study conducted in Spain in 201420 concluded that ICD was significantly more frequent in patients receiving DAs orally than in those treated with DAs by transdermal route. The same was found to be true in our sample (P=.021).
Before analysing the association between ICD and RBD in patients with PD, we reviewed the available literature on DAs in patients with sleep disorders and found that the role of this type of treatment was heterogeneous. We now know that DAs may improve nocturnal sleep, reducing motor and non-motor symptoms associated with treatment interruption at night. Continuous dopamine release, as in the case of rotigotine, results in better scores on the sleep-related domains of the Non-Motor Symptoms Scale.21 In the RECOVER study, for instance, transdermal rotigotine patches achieved significant improvements in nocturnal motor symptoms and overall sleep quality, and a subsequent study of the same treatment revealed significant improvements in sleep fragmentation.7
Based on the above, and considering the association between DAs and ICDRB, we sought to analyse the connection between RBD and DAs by route of administration. We did find differences, though these were not statistically significant.
Our results suggest that RBD should be considered a risk factor for ICDRB in PD. Given that RBD is on many occasions a pre-motor symptom of PD, PD patients with RBD should be followed up more closely as they may also develop ICDRB throughout the course of the disease.
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bellosta Diago E, Lopez del Val LJ, Santos Lasaosa S, López Garcia E, Viloria Alebesque A. Relación entre el trastorno de conducta del sueño REM y el trastorno de control de impulsos en pacientes con enfermedad de Parkinson. Neurología. 2017;32:494–499.