and Sex and cognitive profile may be related to the laterality of motor symptoms in idiopathic Parkinson's disease.
IntroductionParkinson's disease (PD) is well recognised as an inherently asymmetric disease with unilateral onset of motor symptoms. The laterality of motor symptoms may be linked to sex, clinical and demographic variables, and neuropsychological disorders. However, the available data are inconsistent. This study aimed to explore the potential association between the laterality of motor symptoms and clinical and demographic variables and deficits in specific cognitive domains.
Material and methodsWe retrospectively recruited 97 participants with idiopathic PD without dementia; 60 presented motor symptoms on the left side and 37 on the right side. Both groups were comparable in terms of age, age at disease onset, disease duration, and severity of the neurological deficits according to the Unified Parkinson's Disease Rating Scale and the Hoehn and Yahr scale.
ResultsParticipants with left-side motor symptoms scored lower on the Schwab and England Activities of Daily Living scale. Our sample included more men than women (67% vs. 33%). Both sexes were not equally represented in the 2 groups: there were significantly more men than women in the group of patients with left-side motor symptoms (77% vs. 23%), whereas the percentages of men and women in the group of patients with right-side motor symptoms were similar (51% vs. 49%). Both groups performed similarly in all neuropsychological tasks, but women, independently of laterality, performed better than men in the naming task.
ConclusionWe found a clear prevalence of men in the group of patients with left-side motor symptoms; this group also scored lower on the Schwab and England Scale. Female sex was predictive of better performance in the naming task. Sex should always be considered in disorders that cause asymmetric involvement of the brain, such as PD.
La enfermedad de Parkinson (EP) es una enfermedad asimétrica en la que los primeros síntomas se presentan solo en un lado del cuerpo. El lado de inicio de la sintomatología puede depender del sexo, de variables clínicas y demográficas y de la presencia de trastornos neuropsicológicos. Sin embargo, la evidencia disponible no es consistente. Nuestro estudio pretende determinar si el lado que presenta síntomas motores tiene alguna relación con variables clínicas y demográficas y con déficits en determinados dominios cognitivos.
Materiales y métodosIncluimos 97 individuos con EP y sin demencia; 60 de ellos tenían síntomas motores en el lado izquierdo y 37 en el lado derecho. Ambos grupos presentaban similitudes en cuanto a edad, edad de inicio de la enfermedad, duración de la enfermedad, y gravedad de los síntomas neurológicos, según la Unified Parkinson's Disease Rating Scale y la Hoehn and Yahr Scale.
ResultadosLos participantes con síntomas en el lado izquierdo obtuvieron puntuaciones más bajas en la Escala de Actividades de la Vida Diaria de Schwab y England. Nuestra muestra incluía más hombres que mujeres (67 vs. 33%). Además, la distribución de hombres y mujeres no era equitativa entre los dos grupos; había un número significativamente mayor de hombres en el grupo de pacientes con síntomas en el lado izquierdo (77 vs. 23%), mientras que la distribución por sexo era similar en el grupo de pacientes con síntomas en el lado derecho (51 vs. 49%). No encontramos diferencias en las puntuaciones de ninguna de las pruebas neuropsicológicas entre los grupos. Sin embargo, las mujeres, independientemente del lado afecto, obtuvieron mejores resultados que los hombres en la prueba de denominación.
ConclusionesLos hombres eran mucho más numerosos en el grupo de pacientes con afectación del lado izquierdo; este grupo mostró peores puntuaciones en la escala de Schwab y England. El sexo femenino fue predictor de un mejor desempeño en la prueba de denominación. El sexo podría desempeñar un papel fundamental en la lateralidad de los síntomas en enfermedades como la EP.
Parkinson's disease (PD) is an asymmetric syndrome either at the onset or, to some extent, in the later stages of its evolution.1 Asymmetry is a characterising feature that differentiates PD from other Parkinsonian syndromes.2,3 The loss of dopaminergic neurons in the nigrostriatal pathway is the neuropathological correlate which, although bilateral, shows prevalence in one side.4 No definitive explanation exists on the genesis of asymmetry, which is probably derived from the interaction of a series of factors – genetic and environmental,3,5 which characterise not only sporadic but also monogenic6,7 and Parkin mutation forms.8
Clinical asymmetry with lateralised motor symptoms can be traced back to more severe damage in the contralateral nigrostriatal pathway,9 as neuroimaging studies performed with different techniques seem to confirm this observation (see3 for review). The relationship between hemispheric dominance and the side of onset is an important matter of discussion. According to some studies, the side of onset and the dominant hand are independently related.10 According to others, motor symptoms would emerge more frequently in the dominant hand side,11,12 supporting the conclusion of the non-random susceptibility of the left nigrostriatal pathway,13,14 although hemispheric dominance alone does not explain the laterality of damage.14 The relationship between the side of onset and the severity of the syndrome is also discussed; some studies suggest that motor impairment is more severe on the side of handedness, either right or left,15 while Munhoz et al.16 demonstrated that left-sided onset in left-handed subjects corresponds to a more benign course of the disease.
A specific aspect of the relationship between disease severity and the side of the motor syndrome is the severity of cognitive impairment; for example, to a marked left hemisphere, motor symptoms would correspond to a more significant cognitive impairment,17 while a right-onset tremor would correspond to better cognitive preservation.18
Neural damage is not confined to the basal ganglia but may also extend to the cortical regions,19 with some evidence of an asymmetric distribution20 related to the side of motor symptoms.21 No full agreement exists as to which hemispheric side has more severe cortical thinning,22 although, in right-handed subjects with left-sided motor symptoms, the atrophy seems less severe in the left hemisphere, suggesting a neuroprotective role for the dominant left hemisphere.23 The asymmetric distribution of the hemispheric damage correlates with the pattern of non-motor symptoms of both cognitive and behavioural nature.6,24 It has also been proposed that cortical involvement in PD can vary with disease evolution stage, with the atrophy involving the left frontal regions at the earlier stages and then extending to the posterior regions, with prevalence on the right hemisphere.25
The side of the hemispheric damage may predict the general severity of cognitive disorders, with more severe attentional and executive deficits in patients with predominant right-sided motor impairment26 (but see also27). Other reports suggest instead that the side of motor symptoms would predict different types of cognitive decline,28 with prevalent deficits for language and verbal memory and visuospatial abilities in subjects with right and left motor symptoms, respectively.29,30
In conclusion, the presence and type of cognitive disorder in PD might be influenced by different variables, principally handedness, the side of the motor syndrome/hemispheric damage, and disease duration; however, probably for methodological reasons, the reports are mostly inconsistent.
This study aimed to explore whether the motor syndrome side might be related to clinical and demographic variables and the type of neuropsychological disorder.
Materials and methodsParticipants and selection criteriaNinety-seven participants who had received the diagnosis of idiopathic PD according to standard criteria (the United Kingdom Brain Bank criteria)31 formed the study group. Since the presence of diffuse cognitive decay could represent a confounding factor when investigating specific cognitive deficits, only PD patients without dementia were recruited.
Participants were retrospectively recruited in a tertiary Movement Disorders Centre according to the following inclusion criteria: asymmetric motor syndrome; diagnosis of PD made at least three years previously; stable dopaminergic therapy for at least three months; no major cognitive disorders at a screening neuropsychological examination (Mental Deterioration Battery)32; no history of major internal diseases (including vascular disease) or psychiatric disorders excluding mild signs of depression; alcohol or drug abuse; the absence of atypical signs. Handedness was clinically evaluated by asking the participant about her/his preferred hand (for writing or using a spoon and knife) and whether there was a history of left-handedness in her/his family. Only two left-handed patients were identified and excluded from the sample since they could not be analysed as a subgroup.
Laterality was defined as the difference between the score of the right versus the left upper and lower limbs’ motor disorder in Part III of the Unified Parkinson's Disease Rating Scale (UPDRS).33 The difference (positive or negative) between the right and left total scores at the UPDRS III was taken to attribute the participants to the right-sided or left-sided group. Each participant was attributed to the right-sided group if the difference was positive and to the left-sided group if the difference was negative. The participants were also asked what the first motor symptom they had noticed was and if it had appeared on the right or left side; the side of the prevalent motor syndrome identified by the clinician at the first neurological examination, was also considered. The correlation between the three criteria was very high (p<0.001). The presence of dementia was evaluated by the CDR score (dementia=CDR>1).34
The study was approved by the local ethics committee and was performed following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All the participants signed informed consent forms.
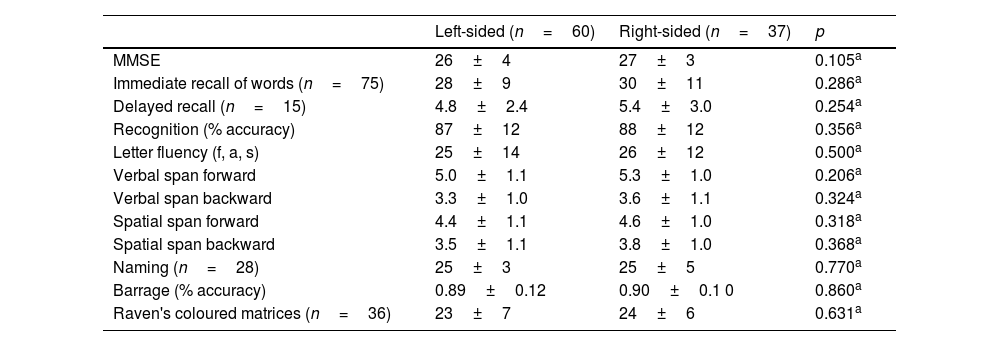
TasksAll the participants underwent the MMSE and an extensive neuropsychological examination at the admission, including language, long-term and short-term memory, visuospatial, constructional, executive, and intelligence tasks31 (see Table 2).
The functional status was evaluated by the Schwab and England Activities of Daily Living Scale (S&E),32 and the severity of the neurological syndrome by the UPDRS33 and the Hoehn and Yahr Staging Scale.34 Dopaminergic therapy was quantified using the Levodopa Equivalent Daily Dose (LEDD).35
Statistical analysisRaw neuropsychological test scores were used for statistical analyses via Statistical Package for Social Science (SPSS) version 15.0. Continuous variables were expressed as mean±SD, categorical variables were displayed as frequencies, and the parametric t-test or non-parametric Mann–Whitney U test and 2 tests were used to assess the significance of the differences between subgroups, as appropriate.
Univariate correlations were calculated with the Spearman correlation coefficient. Multiple linear regressions with the backwards-stepwise method were also performed to study the relationships among the clinical variables and S&E scores, the naming scores, and the LEDD values; the covariates introduced in the model were causal variables which were significantly different at the univariate analysis. A p-value <0.05 was considered statistically significant. Moreover, in addition to statistical significance, the effect size was calculated for each comparison to measure the relationship's strength and clinical relevance.
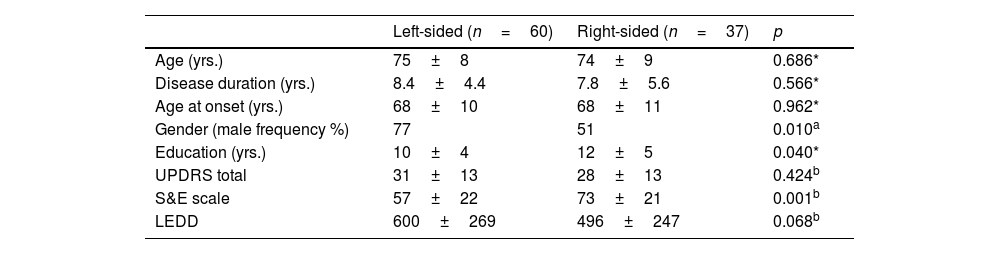
ResultsRight-sided and left-sided PD groupsNinety-seven right-handed PD participants were enrolled: sixty were left-sided, and thirty-seven were right-sided. The main clinical characteristics of the left- and right-sided participants are reported in Table 1.
No significant difference emerged in terms of age (p=0.686), age of onset (p=0.962), or disease duration (p=0.566), and only a marginal discrepancy in education (p=0.040) was found between the two groups. Also, the motor picture (total UPDRS) was of comparable severity (p=0.424).
Demographic and clinical characteristics of PD participants with left-sided and right-sided motor symptoms.
| Left-sided (n=60) | Right-sided (n=37) | p | |
|---|---|---|---|
| Age (yrs.) | 75±8 | 74±9 | 0.686* |
| Disease duration (yrs.) | 8.4±4.4 | 7.8±5.6 | 0.566* |
| Age at onset (yrs.) | 68±10 | 68±11 | 0.962* |
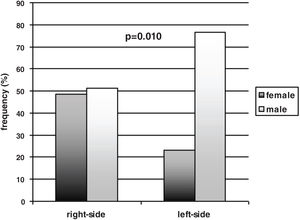
| Gender (male frequency %) | 77 | 51 | 0.010a |
| Education (yrs.) | 10±4 | 12±5 | 0.040* |
| UPDRS total | 31±13 | 28±13 | 0.424b |
| S&E scale | 57±22 | 73±21 | 0.001b |
| LEDD | 600±269 | 496±247 | 0.068b |
However, comparison between the right- and left-sided P.D. participants revealed a more severe functional limitation of the left-sided participants, with a significantly lower score (p=0.001) at the functional scale (S&E) and L.E.D.D. values that approached significance (p=0.068). The two groups did not differ in any neuropsychological task score (Table 2).
Neuropsychological performance of the left- and right-sided PD participants.
| Left-sided (n=60) | Right-sided (n=37) | p | |
|---|---|---|---|
| MMSE | 26±4 | 27±3 | 0.105a |
| Immediate recall of words (n=75) | 28±9 | 30±11 | 0.286a |
| Delayed recall (n=15) | 4.8±2.4 | 5.4±3.0 | 0.254a |
| Recognition (% accuracy) | 87±12 | 88±12 | 0.356a |
| Letter fluency (f, a, s) | 25±14 | 26±12 | 0.500a |
| Verbal span forward | 5.0±1.1 | 5.3±1.0 | 0.206a |
| Verbal span backward | 3.3±1.0 | 3.6±1.1 | 0.324a |
| Spatial span forward | 4.4±1.1 | 4.6±1.0 | 0.318a |
| Spatial span backward | 3.5±1.1 | 3.8±1.0 | 0.368a |
| Naming (n=28) | 25±3 | 25±5 | 0.770a |
| Barrage (% accuracy) | 0.89±0.12 | 0.90±0.1 0 | 0.860a |
| Raven's coloured matrices (n=36) | 23±7 | 24±6 | 0.631a |
Note: The mean scores and SD are reported.
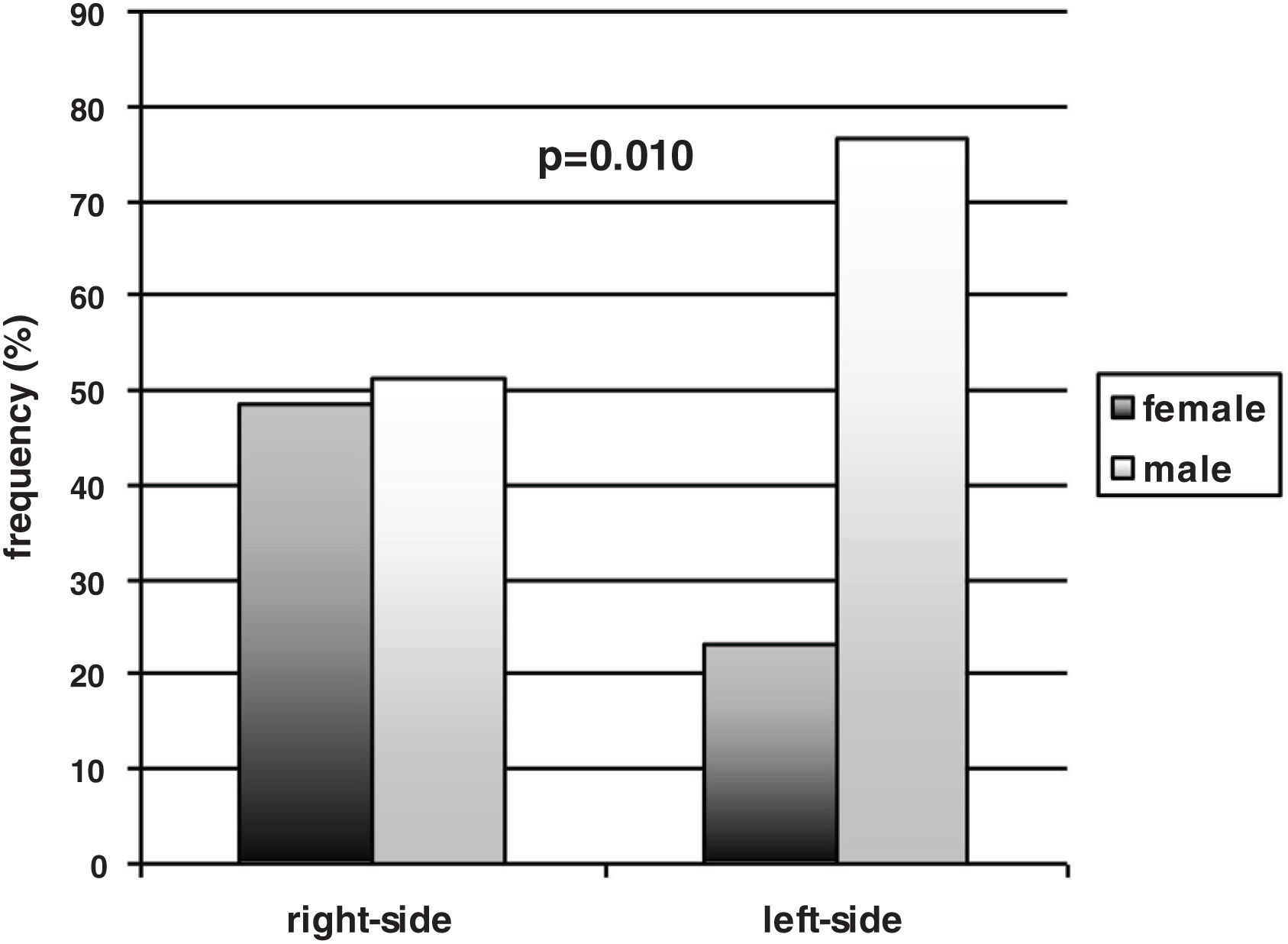
In the total sample of participants, the males prevailed over the females [65/97 (67%) vs. 32/97 (33%)]. Gender, however, was not equally represented between the left- and right-sided participants. A significant prevalence of males compared to females was found within the left-sided participants [males vs. females 46/60 (77%) vs. 14/60 (23%) (p=0.010, effect size h=0.56 – medium), while the males and the females were equally distributed in the right-sided group [males vs. females 19/37 (51%) vs. 18/37 (49%)] (Fig. 1). Thus, the females were equally represented in the left- and right-sided groups (14 vs. 18), and the males prevailed in the left-sided group (46 vs. 19), with a significant difference between the two subgroups’ distribution (p=0.010, effect size h=0.56 – medium). The side of motor symptoms was significantly related to gender (Spearman correlation coefficient 0.26 – effect size small; p=0.010) and to the S&E scale (Spearman correlation coefficient=0.33 – effect size medium; p=0.001); gender was also significantly related to naming performance (Spearman correlation coefficient=−0.37 – effect size medium; p<0.001).
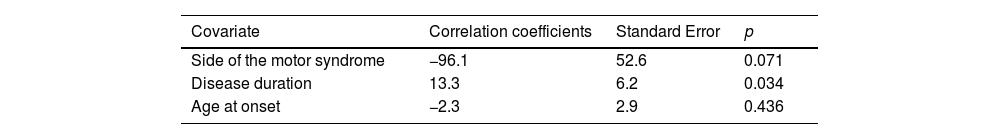
Multiple linear regression models showed that: the side of the motor syndrome significantly predicted the S&E score and disease duration (p=0.001 and p=0.001, respectively) (Table 3a) and the LEDD value by the side of the motor syndrome and disease duration (p=0.074 and p=0.003, respectively) (Table 3b); age at onset and gender significantly predicted the naming score (p=0.001 and p=0.002, respectively) (Table 3c).
To summarise, right-sided motor symptoms predicted a higher level of functionality (S&E) and a lower assumption of dopaminergic drugs. Higher age at onset and the female gender predicted better performance in the naming task.
Multiple linear regression. Dependent variable: LEDD. r2=0.13.
| Covariate | Correlation coefficients | Standard Error | p |
|---|---|---|---|
| Side of the motor syndrome | −96.1 | 52.6 | 0.071 |
| Disease duration | 13.3 | 6.2 | 0.034 |
| Age at onset | −2.3 | 2.9 | 0.436 |
| Reduced model of the regression obtained with a backward-stepwise method. r2=0.12 | |||
|---|---|---|---|
| Covariate | Correlation coefficients | Standard error | p |
| Side of the motor syndrome | −94.8 | 52.4 | 0.074 |
| Disease duration | 15.9 | 5.2 | 0.003 |
Multiple linear regression. Dependent variable: naming score. r2=0.21.
| Covariate | Correlation coefficients | Standard error | p |
|---|---|---|---|
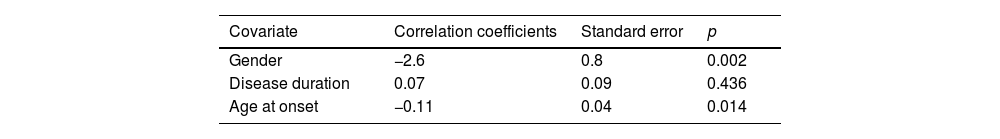
| Gender | −2.6 | 0.8 | 0.002 |
| Disease duration | 0.07 | 0.09 | 0.436 |
| Age at onset | −0.11 | 0.04 | 0.014 |
| Reduced model of the regression obtained with a backward-stepwise method. r2=0.21 | |||
|---|---|---|---|
| Covariate | Correlation coefficients | Standard Error | p |
| Gender | −2.5 | 0.8 | 0.002 |
| Age at onset | −0.13 | 0.04 | 0.001 |
The right-sided and left-sided subgroups did not differ in most demographic variables; the age of onset, the disease duration, and the severity of the motor syndrome was also comparable. The neuropsychological task scores did not differ between the two groups, ranging from normal to mild impairment in agreement with the selection criteria, which excluded dementia patients. The left- and right-sided groups showed similar neuropsychological profiles and were relatively normal. The functionality level was different, presenting the right-sided subjects with higher scores on the S&E scale associated with a lower (although not significant) need for dopaminergic drugs. Lower functionality was, as expected, also associated with longer disease duration.
In agreement with the literature,36 the number of males in the whole sample was larger than females. However, the males’ prevalence (67%) was higher than generally reported.37 The exclusion of subjects with dementia could have generated a bias towards male hyperinflation since the prevalence of dementia in females with PD would start to increase steadily after the age of sixty-five.38 Thus, in an ageing population such as ours, more women than men might have been excluded from the sample.
Moreover, gender was not homogeneously distributed between the two subgroups, with the number of males significantly higher in the left-sided group, while the males and females were equally represented in the right-sided group. In other words, more than 70% of the left-sided patients were males, while the females were equally represented in the two groups. Right-sided motor symptoms predicted higher functionality and lower dopaminergic drug use. The female gender also predicted better performance in naming.
Different hemispheric lateralisation between males and females might influence the side of motor symptoms. Studies on the laterality of functional connectivity density indicate that males’ hemispheric lateralisation is greater than that of females; in addition, males show greater rightward connectivity than females, who, instead, show greater leftward connectivity.39 General greater lateralisation and prevalent rightward lateralisation in males might suggest the lower possibility of compensation and thus greater sensitivity of the right hemisphere to the effect of neurodegeneration compared to females, consistent with both the higher prevalence of PD in males and a higher probability of right-hemisphere damage. This interpretation is consistent, to some extent, with previous observations of a more marked cognitive decline27 and more rapid disease progression in left-sided onset compared to right-sided onset, which is attributed to the greater neural reserve of the left hemisphere40 (see also7).
As for cognition, the only significant result was the predictive value of the female gender for a better naming performance, independently of the side of the motor syndrome. This result is of particular interest since it suggests that the weaker hemispheric lateralisation in females (see41) could make them capable of counteracting the prevalent left hemisphere damage (about half of the female participants were in the right-sided group) by compensatory mechanisms.
The more preserved language competence in females might contribute to the higher level of functionality of the right-sided group (where females represent about half of the subjects) despite comparable severity in the motor syndrome in the left- and right-sided groups.
Study limitationsOur study has some limitations. The population is relatively small, and the study is cross-sectional, and we cannot make inferences about causality. Future research are required to confirm our findings.
We used the S&E scale to assess functional capacity. This scale is crude in that it asks the subjects to rate their level of functionality using epochs of 10 from 0 to 100. Additional measures of functional capacity are necessary to confirm in futures studies, our results.
Since our work was retrospective, we used the MDB31 that is a screening battery administered to all patients at the admission. We are aware that other batteries might be more specific to assess the cognitive status of parkinsonian patients; nevertheless, we believe that the battery we used was able to detect major cognitive impairment that was an exclusion criterion for selection of patients and to assess executive and visuospatial functions that are typically affected in Parkinson's disease.
A further limitation is that excluding dementia patients could be a possible biasing factor favouring those who are more resilient to the dementia process. This limits the clinical implications.
We were able to recruit only right-handed patients. However, studies suggest that only approximately 10% of the world population is left-handed42 and the percentage is even lower in Italian population44; left-handed subjects in our sample were few (only two patients that were excluded from the sample) for the handedness factor to be explored.
ConclusionResults of interest in our study are the evident prevalence of males in the left-sided subgroup that also shows a lower level of functionality on the S&E scale compared to right-sided subgroup; from the neuropsychological point of view, the parameter ‘female gender’ as predictive of better performance in the naming task.
In conclusion, the relationship between motor syndrome, cognitive disorders, and clinical parameters is still an open question as the wide variability of clinical observations indicates.3,7 We suggest that the gender variable should always be taken into account in pathologies with asymmetric involvement of the cerebral hemispheres, such as PD.
NoteThe manuscript was proofread by a professional service and was revised and approved by all the authors for final submission.
Conflict of interestsThe authors declare that they have no conflict of interest.