We aimed to analyse the effects of age and sex on performance and cost for patients with chronic peripheral neuropathic pain (PNP) who have started treatment with brand name gabapentin versus generic gabapentin (EFG).
MethodsWe conducted a retrospective multicentre study using electronic medical records (EMR) for patients of both sexes, older than 18, who began treatment with brand name or generic gabapentin. Adherence (medication possession ratio, MPR), persistence, use of healthcare resources, cost, and pain reduction were measured for one year.
ResultsWe analysed 1369 EMRs [61.1% women; mean age 64.6 (15.9), 52.4%≥65 years]. 400 used brand name drugs while 969 used generic gabapentin. Persistence and adherence were higher in patients using brand name gabapentin (7.3 vs 6.3 months, P<.001; 86.5% vs 81.3% MPR, P<.001). Lower healthcare costs were observed in patients using brand-name gabapentin in both age groups (<65 and ≥65). Mean difference in cost per patient amounted to €220 (95% CI: 59-382) and €216 (95% CI: 51-382) in the <65 and ≥65 age groups, respectively (P=.004). Mean difference in cost among men amounted to €197 (63-328), while mean difference in cost among women amounted to €239 (96-397) (P=.005 and P=.004, respectively). Compared with EFG, brand treatment showed greater pain relief: 13.5% (10.9-16.2) and 10.8% (8.2-13.5) in <65 and ≥65 year patients, respectively (P<.001), and 10.7% (8.2-13.2) and 13.8% (11.0-16.5) in women and men respectively (P<.001).
ConclusionsRegardless of sex and age, patients who started PNP treatment with brand name medication showed greater persistence and adherence to treatment than those taking generic drugs. Brand name treatment also involved lower healthcare costs, and greater pain relief.
Analizar el efecto de la edad y el género sobre el dolor y costes en pacientes con dolor Neuropático periférico (DNp) crónico que inician tratamiento con gabapentina (marca) frente a gabapentina genérica (EFG).
MétodosEstudio multicéntrico-retrospectivo, realizado con registros médicos electrónicos (RME) de pacientes de ambos géneros, >18 años, que iniciaron nuevo tratamiento con gabapentina de marca o genérico. Durante un año se midió adherencia (ratio posesión medicación, RPM) y persistencia, utilización de recursos sanitarios, costes y reducción del dolor.
ResultadosSe analizaron 1.369 RMEs [61,1% mujeres; edad 64,6 (15,9) años, 52,4% ≥65 años]: marca: 400, EFG: 969. Persistencia y adherencia fueron mayores con marca; 7,3 vs. 6,3 meses (p<0,001), y 86,5% vs. 81,3% de RPM (p<0,001). Con marca, se observaron costes sanitarios menores, tanto en <65 como ≥65 años; diferencias medias por paciente de 220€ (IC 95%; 59-382) y 216€ (51-382), respectivamente (p=0,004), como en hombres; diferencias medias de 197€ (63-328) o mujeres; diferencias de 239€ (96-397), p=0,005 y p=0,004, respectivamente. Comparado con EFG, el tratamiento con marca mostró una reducción mayor del dolor; 13,5% (10,9-16,2) y 10,8% (8,2-13,5), en <65 y ≥65 años, respectivamente (p<0,001), y 10,7% (8,2-13,2) y 13,8% (11,0-16,5), y en mujeres y hombres, respectivamente (p<0,001).
ConclusionesCon independencia del género o la edad, los pacientes que iniciaron tratamiento del DNp con gabapentina de marca vs. genérico, mostraron un mayor grado de adherencia y persistencia al tratamiento, repercutiendo en unos menores costes sanitarios, a la vez que se observaron mayores reducciones del dolor.
The International Association for the Study of Pain (IASP) defines peripheral neuropathic pain (PNP) as a pain caused by a lesion or disease of the somatosensory nervous system; PNP is a common symptom in a group or variety of diseases.1 Estimated prevalence ranges from 1% to 8% of the adult population; the condition represents approximately 40% of cases of chronic pain.2
The disease has a tendency to become chronic and is frequently incapacitating, and therefore involves high direct and indirect costs for society as a whole. PNP is considered one of the most important public health issues.3,4 This translates into reduced quality of life, affecting patients’ family, social, and professional environment.2,5 In many cases, patients do not receive a correct diagnosis, or drug therapy is inappropriate or prescribed at insufficient doses.1,5,6
Drug therapy is a fundamental part of treatment.7 In this context, the anticonvulsant gabapentin is a therapeutic option for managing PNP, with both brand-name formulations and generic substitutions (GS) available.8,9 GS have the same efficacy, safety, and quality as the original formulation, and are bioequivalent to the brand-name drug.10 A positive attitude, physicians’ familiarity with the drug, and healthcare policy on GS are factors influencing GS use.11 In Spain, the current policy regulating the prices of brand-name drugs vs GS is no longer a robust argument to demand the use of brand-name drugs assuming price equality (reference prices).12
After reviewing various articles, we observed that the pharmacological arguments for and against the prescription of these drugs are uncertain.9,13 Some studies manifest discrepancies (brand-name vs GS) due to several circumstances, such as treatment adherence, which generally cause a decrease in clinical effectiveness (patient confusion, poor therapeutic control, poor health outcomes) and a possible increase in healthcare costs.14,15 We have recently identified significant differences between original branded drugs and GS of the same active ingredient in the treatment of chronic PNP9; however, the effect of age and sex on these results was not analysed. Furthermore, limited evidence is available on the relationship between these variables, both in the international literature and in the context of Spanish healthcare; we therefore consider this study to be of relevance. The aim of this study is to analyse the effect of age and sex on the clinical and financial consequences associated with patients with chronic PNP who start treatment with brand-name vs generic gabapentin in the context of routine clinical practice.
Patients and methodsStudy design and populationWe analysed the secondary endpoint of a longitudinal, observational, multicentre study (of retrospective design) based on the review of electronic medical records (EMR) from electronic databases. Data were from patients attending outpatient and hospital follow-up, and were anonymised. The study population included patients from 6 primary care centres managed by the company Badalona Serveis Assistencials S.A. Most patients assigned to these centres came from predominantly industrial urban areas and were of medium-low socioeconomic status.
Inclusion and exclusion criteriaWe included patients seeking medical care and starting new treatment with gabapentin (Neurontin® or GS) between 2008 and 2012 (recruitment period, index date). Inclusion criteria were: a) age >18 years; b) patients registered in the database for at least 12 months before the start of the study; c) inclusion in the chronic medication programme for obtaining prescriptions (with a verified record of the daily dose, time interval, and the duration of each administered treatment); ≥2 prescriptions during the follow-up period; d) ensured regular follow-up of patients during the study period (≥2 healthcare records in the electronic system); and e) diagnosis of PNP (previous or at treatment onset). We excluded: a) patients who were transferred to other centres, displaced, or outside their healthcare district; b) patients who were permanently hospitalised; and c) patients who changed treatment (from brand-name to GS or vice versa). We divided patients into 4 groups, according to age and sex: a) <65 years; b) ≥65 years; c) men; and d) women. Follow-up period for these patients was one year beginning on the index date.
Diagnosis and scales usedRecords of patients with PNP were obtained using the International Classification of Primary Care (ICPC),16 codes N92-N99, and/or the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM, codes 350.1, 352.9, 353.1, 353.3, 353.8, 354.0, 355.1, 355.5, 357.2, 357.4, 357.8, 357.9, 053.13). Criteria were always applied according to the clinician's judgement. We considered PNP as a pain triggered or caused by a lesion or disease of the peripheral nervous system (i.e. nerve roots, plexuses, and nerves). As a measurement of clinical effectiveness, we obtained the information reported in the clinical histories using the numeric pain rating scale (NPRS),17 numerically graded to assess PNP intensity in a scale ranging from 0 (no pain) to 10 (worst pain imaginable).
Demographic variables and comorbiditiesWe recorded time since diagnosis and data on personal history based on the ICPC16: arterial hypertension (K86, K87), diabetes mellitus (T89, T90), dyslipidaemia (T93), obesity (T82), active smoking (P17), alcohol use (P15, P16), any type of organ dysfunction (heart, liver, kidney), ischaemic heart disease (K74, K76, K75), cerebrovascular accident (K90, K91, K93), dementia or memory disorders (P70, P20), neurological diseases (Parkinson's disease [N87], epilepsy [N88], multiple sclerosis [N86], and other neurological diseases [N99]), and malignant neoplasms (all types: A79, B72-75, D74-78, F75, H75, K72, L71, L97, N74-76, T71-73, U75-79, W72-73, X75-81, and Y77-79). To summarise general comorbidity, we recorded scores for each patient on: a) the Charlson comorbidity index18 as a measurement of patient severity, and b) individual case-mix index obtained using the Adjusted Clinical Group (ACG) system, which classifies patients by resource iso-consumption.19 The ACG system groups patients into one of 5 mutually exclusive resource utilisation bands (RUB) according to their general morbidity (1: healthy users/very low morbidity; 2: low morbidity; 3: moderate morbidity; 4: high morbidity; and 5: very high morbidity).
Medication used and treatment adherence/compliance and persistenceThe drug gabapentin (active ingredient), indicated for treating PNP, was identified according to the Anatomical Therapeutic Chemical Classification system (ATC)20: N03AX12. Data were obtained from the pharmaceutical prescription database using the CatSalut RCMPS application. The decision to choose a brand-name or GS drug for a patient was made by the clinician (clinical practice). Adherence/compliance rate was defined according to the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) criteria and calculated according to the medication possession ratio (MPR).21 MPR was assessed from the first to the last prescription; the value represents the number of days during the treatment period (from the index date) on which the medication was supplied.22Persistence was defined as the time in months during which the initial treatment is not abandoned or changed to another medication at least 30 days after the initial prescription. Data on persistence was obtained at 3, 6, 9, and 12 months of follow-up.
Use of resources and cost analysisHealthcare costs were considered to be those related to healthcare activity (medical consultations, days of hospitalisation, emergency services, and diagnostic or therapeutic requests) performed by professionals. Costs were expressed as mean cost per patient (average cost/unit). Unit costs for 2013 were obtained from centres’ analytic accounting, excluding medication costs, which were obtained from the Spanish General Council of Official Pharmacy Associations’ Bot Plus database. Prescriptions were quantified according to the recommended retail price per package at the time of prescription. We also included the following concomitant medication in the healthcare cost calculation: non-steroidal anti-inflammatory drugs (NSAID, M01), opiates (N02A), analgesics (N02B), sedative agents/hypnotics (anxiolytics: N05C), and antidepressants (N06A). Non-pharmacological unit costs included in the analysis correspond to primary care consultations (€23.7 per consultation), visits to the emergency department (€119.9), hospitalisation (€327.3 per day), consultation with specialists (€68.9), day hospitals (€184.8 per session), laboratory tests (€22.7 per conventional blood test), conventional radiology (€18.9), and diagnostic tests (€37.9).
Ethical considerationsThis study complies with the ethical principles for medical research on human subjects as set forth by the Helsinki Declaration. The study protocol was classified by the Spanish Agency for Medicines and Medical Devices as a post-authorisation study – other designs (PAS-OD) and approved by the Clinical Research Ethics Committee of Hospital Germans Trias i Pujol, Badalona (Barcelona). This non-interventional study was performed according to the regulation in force for observational studies based on retrospective databases. Data were anonymised to maintain patient confidentiality, as established by the Spanish Organic Law 15/1999, of 13 December, for the Protection of Personal Data.
Statistical analysisAs a previous step to analysis, we used an exploratory analysis to carefully review the data. We observed the frequency distribution and identified possible record or coding errors. We determined whether data were normally distributed using the Kolmogorov–Smirnov test. We performed a univariate descriptive statistical analysis, calculating the mean, median, standard deviation (SD), and 95% confidence intervals (95% CI) for parametric variables, and median and interquartile ranges (percentiles 25 and 75 of the distribution) for non-parametric variables. Persistence was calculated using the Kaplan–Meier estimator. We completed a bivariate analysis using ANOVA and the chi-square test, depending on data distribution. Healthcare costs were compared according to the recommendations of Thompson and Barber23 using a general linear model (analysis of covariance [ANCOVA]) adjusted for the covariables of sex, age, general comorbidity (RUB, Charlson index score), MPR, and treatment persistence. Pairwise comparisons were adjusted to the estimated marginal means by applying the Bonferroni correction to calculate statistical significance (P-value). Data were presented as differences of adjusted means between treatments with their corresponding 95% confidence intervals calculated with bias-corrected bootstrapping techniques, given the non-normally distributed variables ‘resource use’ and ‘costs’.
The clinical variable ‘pain intensity’, measured with the NPRS, was analysed after performing a single imputation of missing values using a general linear model (ANCOVA) for the absolute and relative variation of pain scores between treatment onset and discontinuation. A logistic regression model was used to analyse the proportion of responsive patients (baseline pain decreased by ≥50%), and patients with no pain/mild pain (scoring <4 on the NPRS). The covariables age, sex, and NPRS score at treatment onset were used in ANCOVA and in the logistic regression model. The NPRS was not completed by 9.7% of patients; no significant difference was observed between the brand-name drug (8.5%) and the GS (10.2%, P=.382). Imputation used the worst observation carried forward, which in this case was the score obtained in the consultation when treatment was started. We compared the scores obtained during the initial and final consultations, the absolute (points) and relative (%) variation in pain intensity between the 2 consultations, the proportion of patients who were responsive, and the proportion of patients with no pain/mild pain.
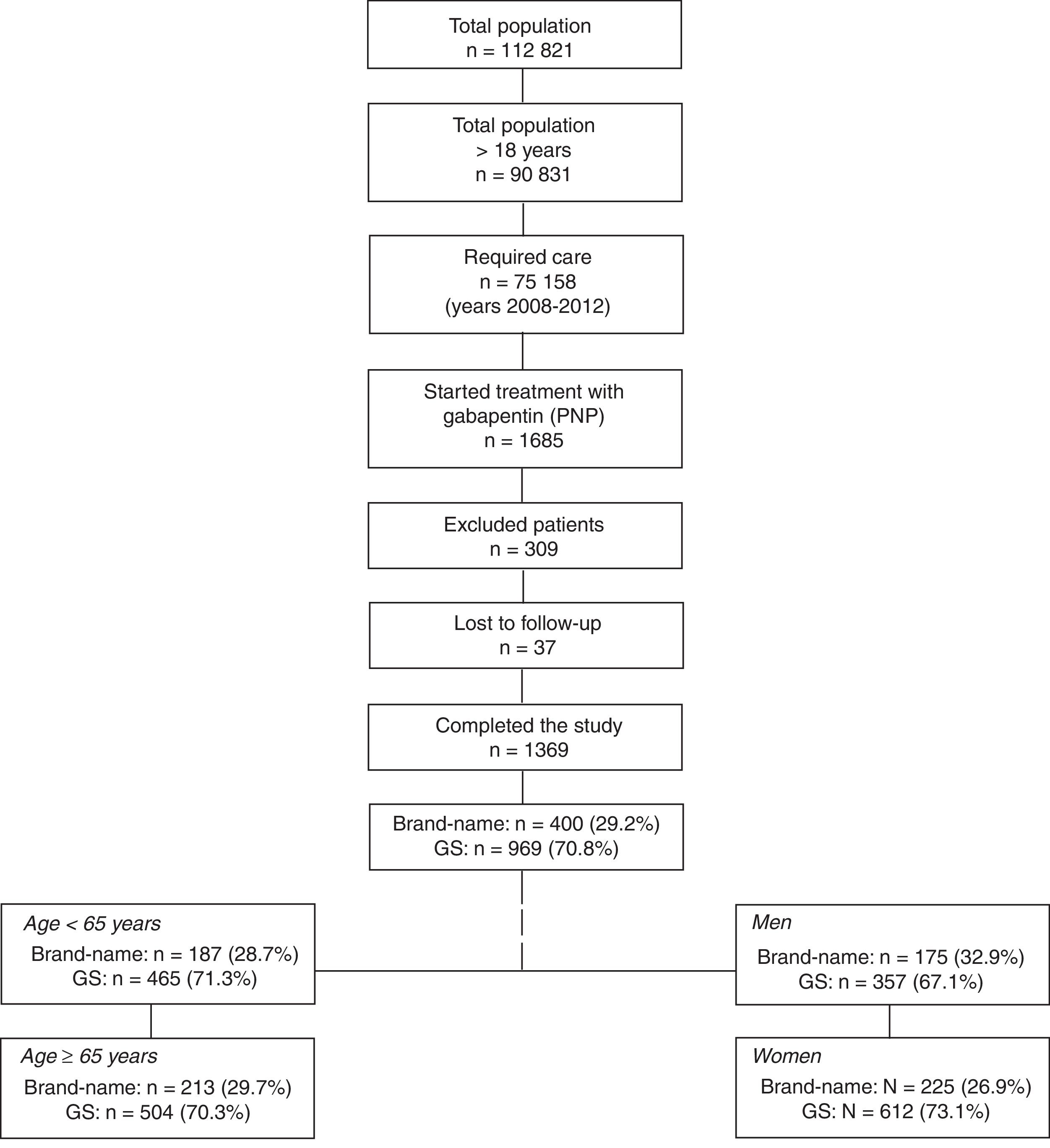
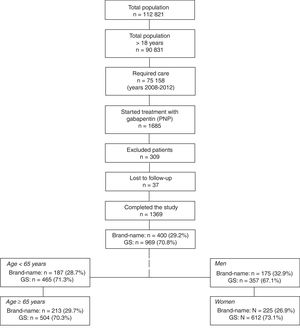
ResultsFrom an initial selection of 90 831 individuals who were older than 18 and assigned to the centres, we recruited 1369 patients meeting the study selection criteria (Fig. 1). Of the patients receiving treatment with gabapentin, 400 (29.2%) were treated with a brand-name drug and 969 (70.8%) with a GS; mean age was 64.6 (SD: 15.9) and 61.1% of patients were women. Forty-three percent of all patients had arterial hypertension and 41.2% dyslipidaemia. We compared the 4 groups of patients treated with a brand-name drug or GS: a) age <65 years: branded (n=187; 28.7%) vs GS (n=465; 71.3%); b) age ≥65 years: branded (n=213; 29.7%) vs GS (n=504; 70.3%); c) men: branded (n=175; 32.9%) vs GS (n=357; 67.1%), and d) women: branded (n=225; 26.9%) vs GS (n=612; 73.1%).
General structure of the study. We used an observational, retrospective design based on a review of existing medical records (in electronic databases; data were anonymised) of patients treated with gabapentin (brand-name vs GS) and followed up in outpatient consultations and hospitals.
GS: generic substitutions.
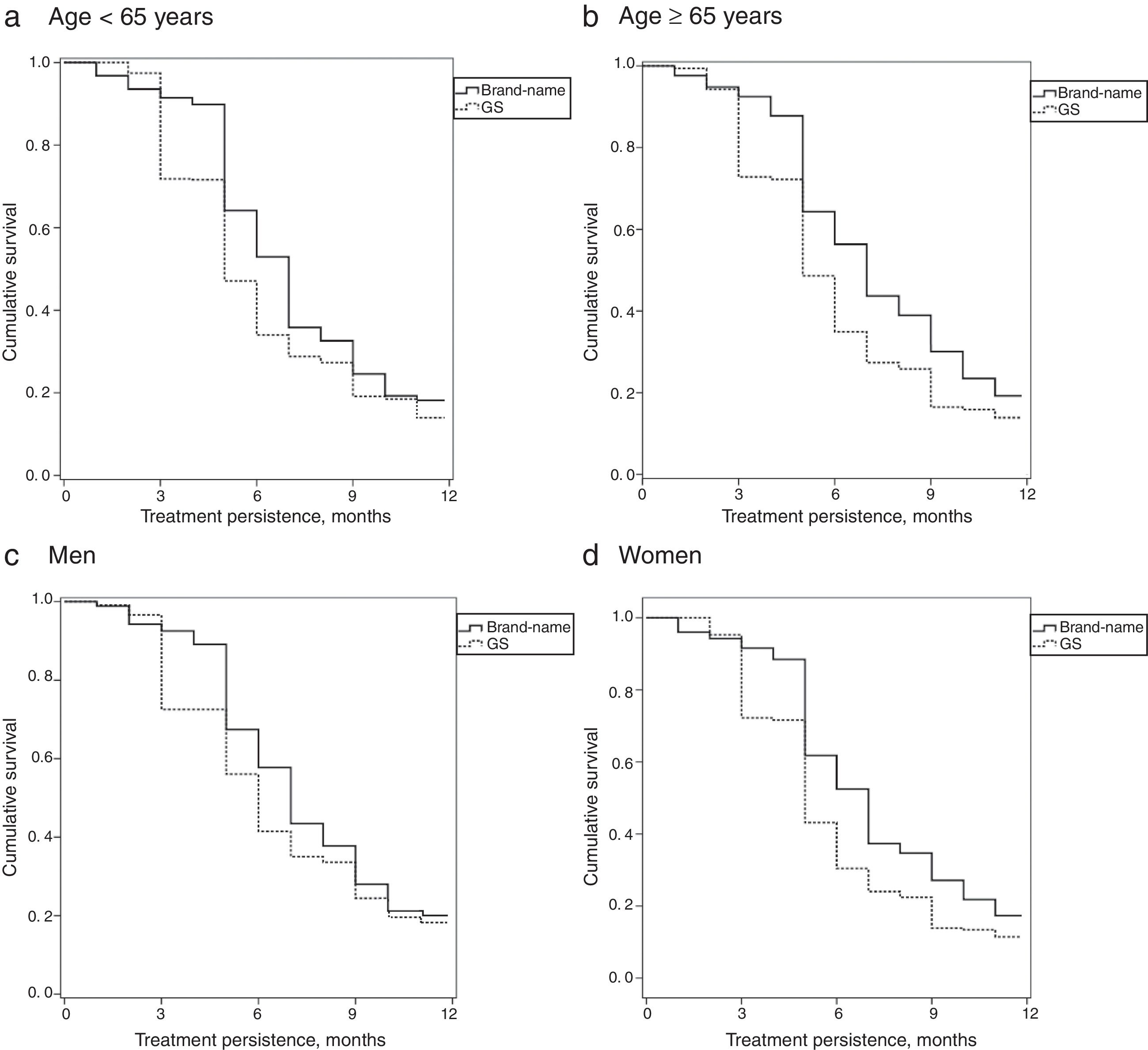
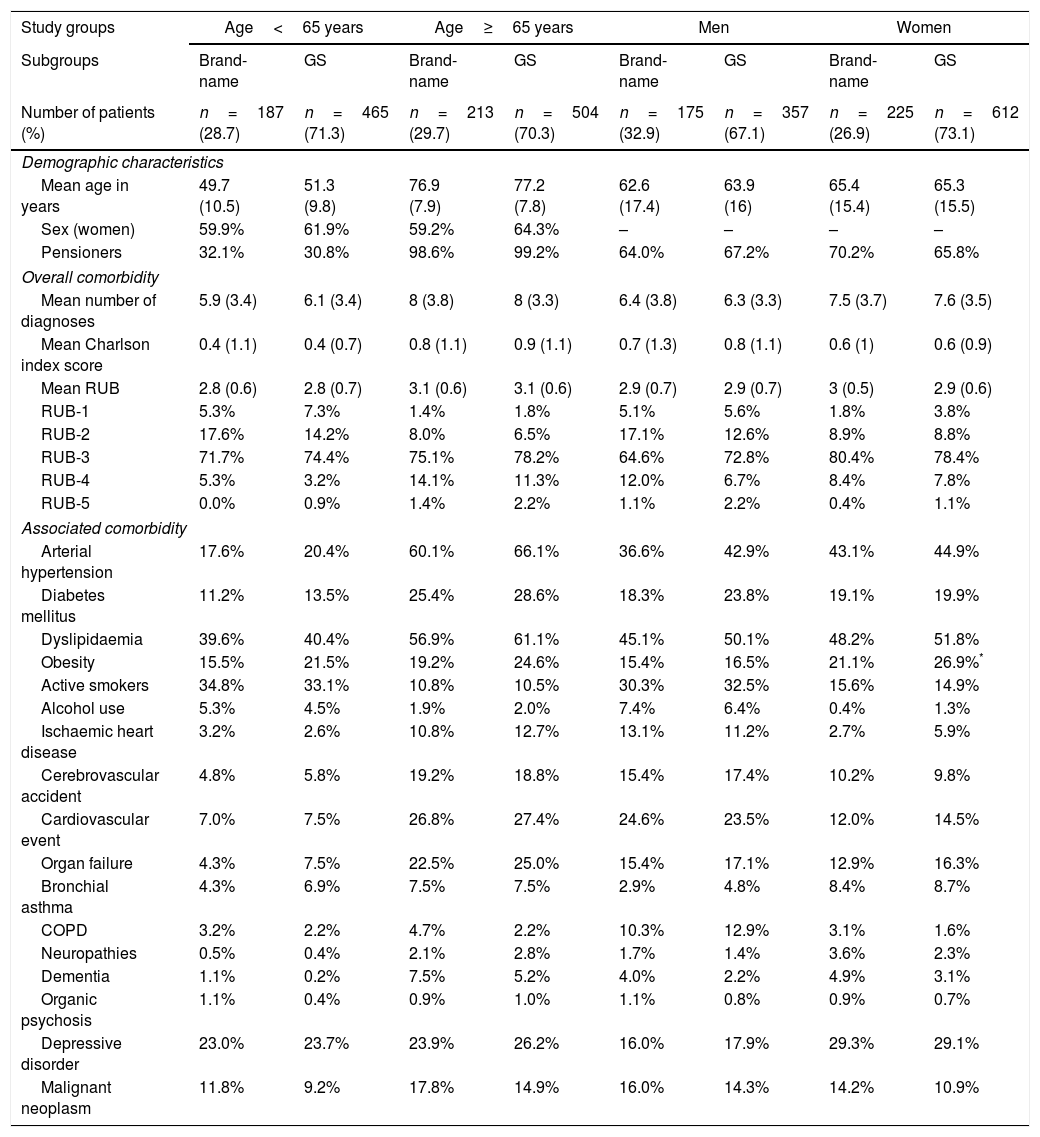
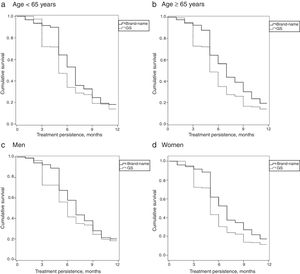
Table 1 describes the baseline characteristics and associated comorbidities of patients with PNP by study group. The baseline characteristics of the 4 study groups were comparable in patients receiving brand-name drug vs GS. Mean treatment duration with brand-name gabapentin was higher than with GS (7.3 vs 6.3 months; P<.001). MPR (86.5% vs 81.3%; P<.001) and mean daily dose (1322.5 vs 1153.5mg; P=.008) were also higher with brand-name gabapentin. Persistence to treatment (in all time ranges: 3, 6, 9, and 12 months) was higher with the brand-name drug than with the GS. The MPRs (by group) comparing brand vs GS were as follows: a) <65 years: 86.3% (91.3–91.2) vs 81.4% (77.8–84.9), no significant differences; b) ≥65 years: 86.6% (82.0–91.2) vs 81.1% (77.6–84.5), P<.05; c) men: 86.8% (81.7–91.8) vs 83.1% (79.2–87.0), no significant differences; and d) women: 86.2% (82.0–91.2) vs 80.2% (77.0–83.4), P<.05. Annual persistence was: a) <65 years: 19.9% vs 14% (P<.05); b) ≥65 years: 21.1% vs 13.9% (P<.01); c) men: 20.7% vs 15.2% (P<.05); and d) women: 18.7% vs 11.4% (P<.01). Fig. 2 shows the treatment persistence curves.
Baseline characteristics (demographic data and comorbidity) of the patient series by study group.
| Study groups | Age<65 years | Age≥65 years | Men | Women | ||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | Brand-name | GS | Brand-name | GS | Brand-name | GS | Brand-name | GS |
| Number of patients (%) | n=187 (28.7) | n=465 (71.3) | n=213 (29.7) | n=504 (70.3) | n=175 (32.9) | n=357 (67.1) | n=225 (26.9) | n=612 (73.1) |
| Demographic characteristics | ||||||||
| Mean age in years | 49.7 (10.5) | 51.3 (9.8) | 76.9 (7.9) | 77.2 (7.8) | 62.6 (17.4) | 63.9 (16) | 65.4 (15.4) | 65.3 (15.5) |
| Sex (women) | 59.9% | 61.9% | 59.2% | 64.3% | – | – | – | – |
| Pensioners | 32.1% | 30.8% | 98.6% | 99.2% | 64.0% | 67.2% | 70.2% | 65.8% |
| Overall comorbidity | ||||||||
| Mean number of diagnoses | 5.9 (3.4) | 6.1 (3.4) | 8 (3.8) | 8 (3.3) | 6.4 (3.8) | 6.3 (3.3) | 7.5 (3.7) | 7.6 (3.5) |
| Mean Charlson index score | 0.4 (1.1) | 0.4 (0.7) | 0.8 (1.1) | 0.9 (1.1) | 0.7 (1.3) | 0.8 (1.1) | 0.6 (1) | 0.6 (0.9) |
| Mean RUB | 2.8 (0.6) | 2.8 (0.7) | 3.1 (0.6) | 3.1 (0.6) | 2.9 (0.7) | 2.9 (0.7) | 3 (0.5) | 2.9 (0.6) |
| RUB-1 | 5.3% | 7.3% | 1.4% | 1.8% | 5.1% | 5.6% | 1.8% | 3.8% |
| RUB-2 | 17.6% | 14.2% | 8.0% | 6.5% | 17.1% | 12.6% | 8.9% | 8.8% |
| RUB-3 | 71.7% | 74.4% | 75.1% | 78.2% | 64.6% | 72.8% | 80.4% | 78.4% |
| RUB-4 | 5.3% | 3.2% | 14.1% | 11.3% | 12.0% | 6.7% | 8.4% | 7.8% |
| RUB-5 | 0.0% | 0.9% | 1.4% | 2.2% | 1.1% | 2.2% | 0.4% | 1.1% |
| Associated comorbidity | ||||||||
| Arterial hypertension | 17.6% | 20.4% | 60.1% | 66.1% | 36.6% | 42.9% | 43.1% | 44.9% |
| Diabetes mellitus | 11.2% | 13.5% | 25.4% | 28.6% | 18.3% | 23.8% | 19.1% | 19.9% |
| Dyslipidaemia | 39.6% | 40.4% | 56.9% | 61.1% | 45.1% | 50.1% | 48.2% | 51.8% |
| Obesity | 15.5% | 21.5% | 19.2% | 24.6% | 15.4% | 16.5% | 21.1% | 26.9%* |
| Active smokers | 34.8% | 33.1% | 10.8% | 10.5% | 30.3% | 32.5% | 15.6% | 14.9% |
| Alcohol use | 5.3% | 4.5% | 1.9% | 2.0% | 7.4% | 6.4% | 0.4% | 1.3% |
| Ischaemic heart disease | 3.2% | 2.6% | 10.8% | 12.7% | 13.1% | 11.2% | 2.7% | 5.9% |
| Cerebrovascular accident | 4.8% | 5.8% | 19.2% | 18.8% | 15.4% | 17.4% | 10.2% | 9.8% |
| Cardiovascular event | 7.0% | 7.5% | 26.8% | 27.4% | 24.6% | 23.5% | 12.0% | 14.5% |
| Organ failure | 4.3% | 7.5% | 22.5% | 25.0% | 15.4% | 17.1% | 12.9% | 16.3% |
| Bronchial asthma | 4.3% | 6.9% | 7.5% | 7.5% | 2.9% | 4.8% | 8.4% | 8.7% |
| COPD | 3.2% | 2.2% | 4.7% | 2.2% | 10.3% | 12.9% | 3.1% | 1.6% |
| Neuropathies | 0.5% | 0.4% | 2.1% | 2.8% | 1.7% | 1.4% | 3.6% | 2.3% |
| Dementia | 1.1% | 0.2% | 7.5% | 5.2% | 4.0% | 2.2% | 4.9% | 3.1% |
| Organic psychosis | 1.1% | 0.4% | 0.9% | 1.0% | 1.1% | 0.8% | 0.9% | 0.7% |
| Depressive disorder | 23.0% | 23.7% | 23.9% | 26.2% | 16.0% | 17.9% | 29.3% | 29.1% |
| Malignant neoplasm | 11.8% | 9.2% | 17.8% | 14.9% | 16.0% | 14.3% | 14.2% | 10.9% |
RUB: resource utilisation band; GS: generic substitutions; COPD: chronic obstructive pulmonary disease.
Pairwise comparisons: *P<.05.
Values are expressed as percentages or means (SD).
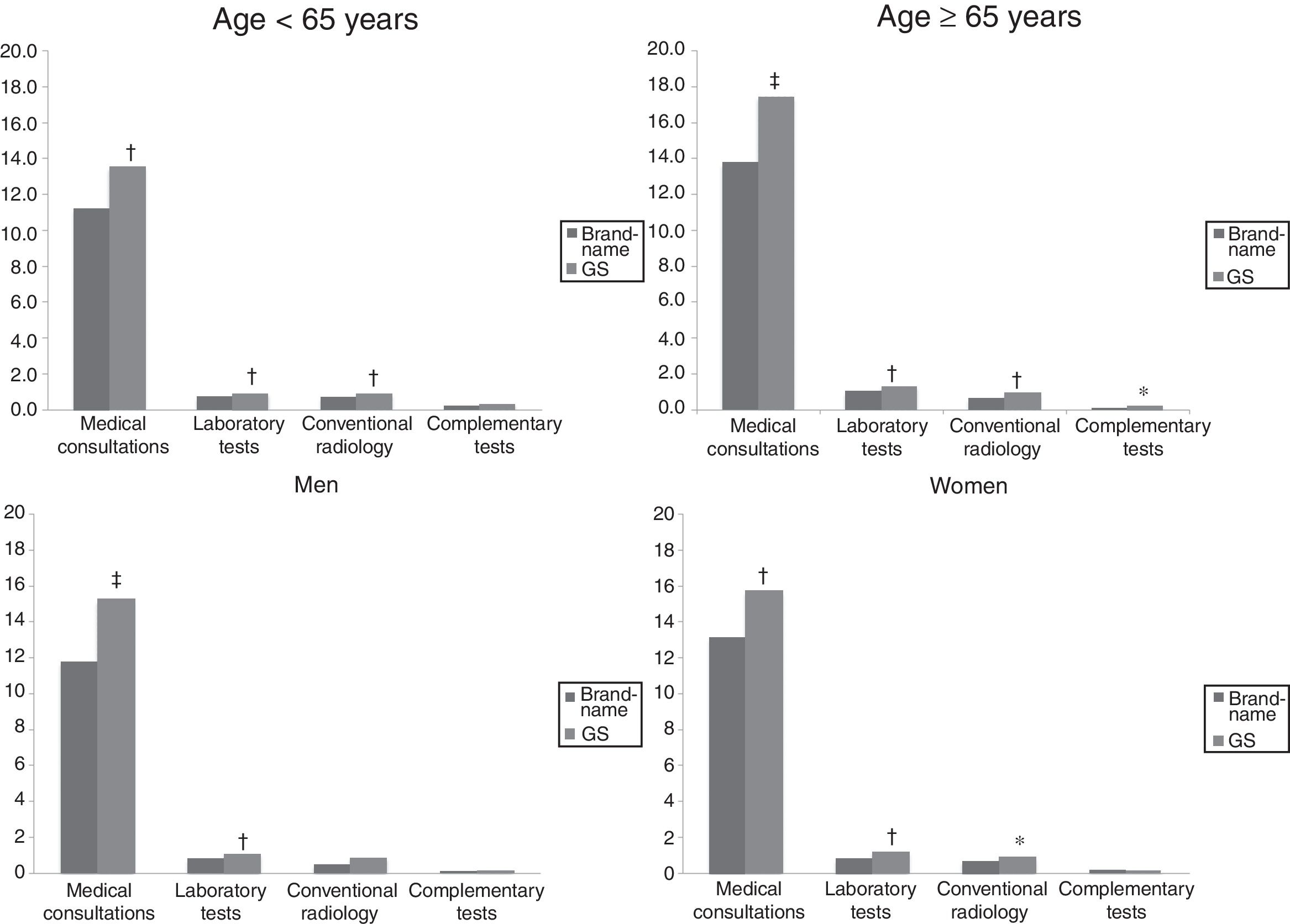
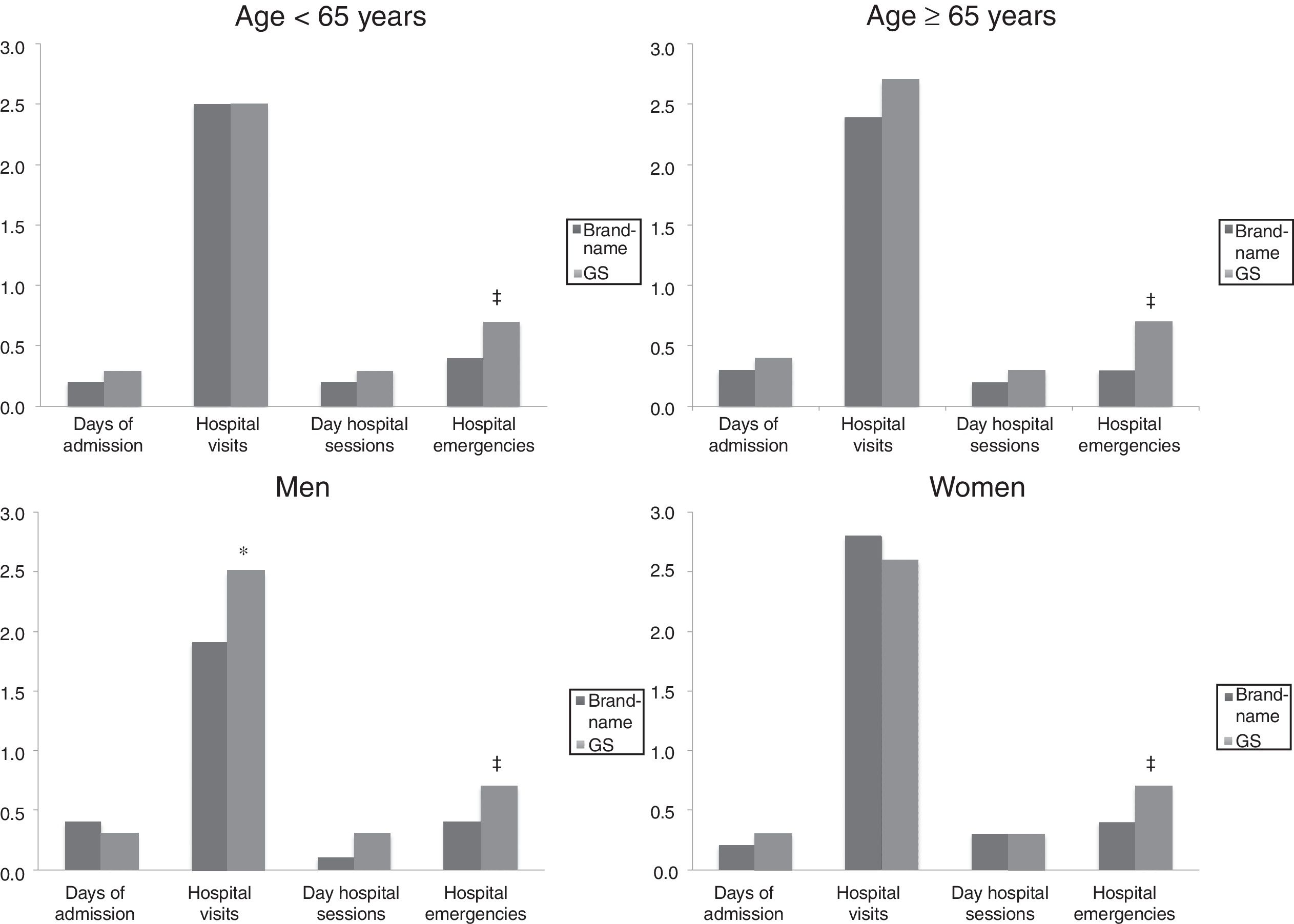
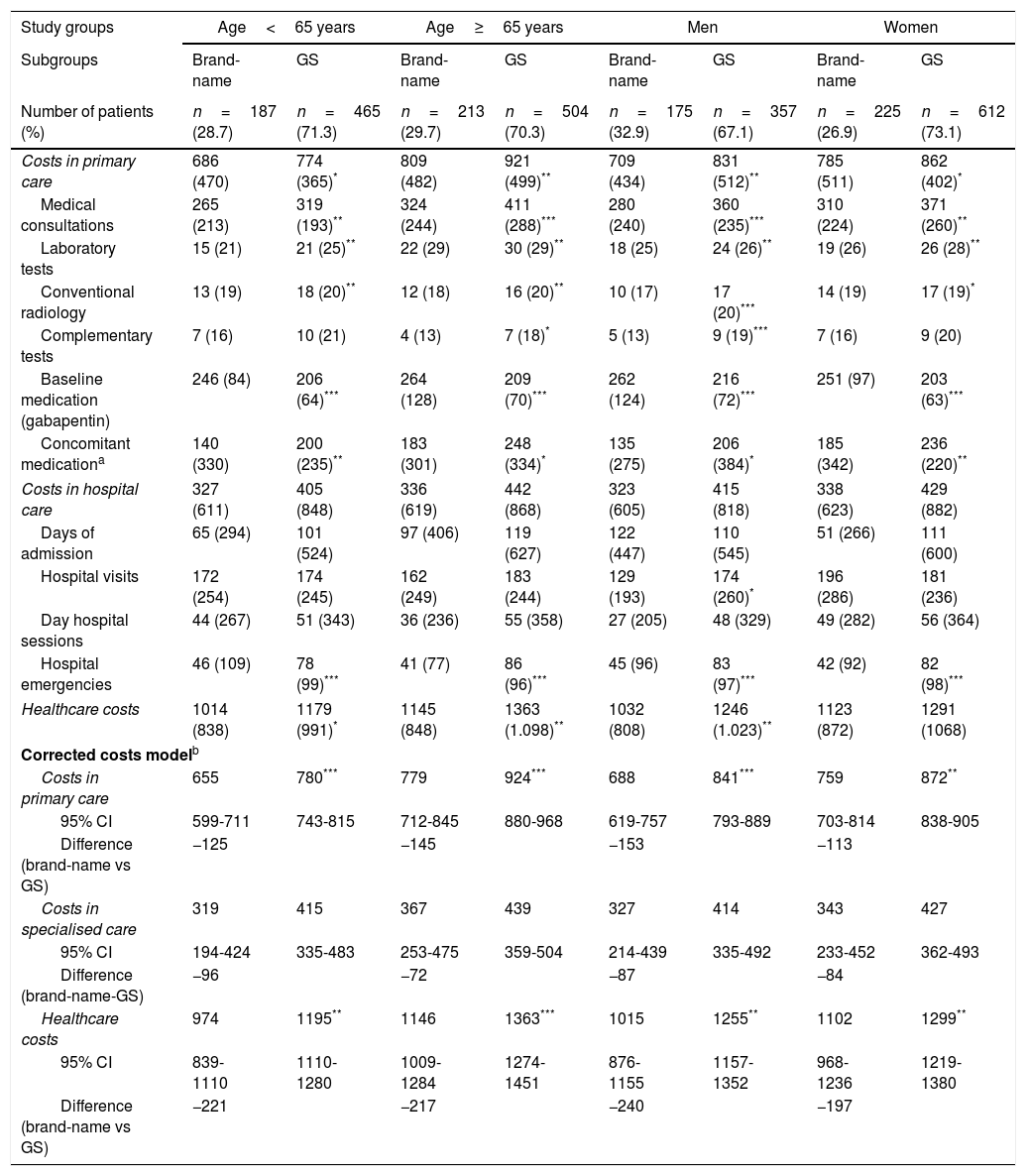
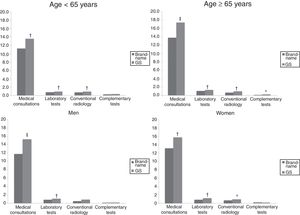
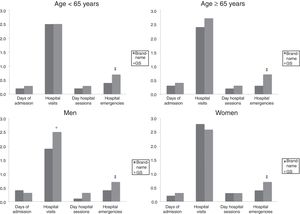
Figs. 3 and 4 compare the use of healthcare resources by the groups of patients receiving the brand-name drug vs the GS. In general, patients treated with brand-name gabapentin used less resources, especially in primary care consultations (12.5 vs 15.5; P=.001) and emergency services (0.4 vs 0.7; P<.001). These differences were identified in all 4 study groups (for age and sex). Table 2 details the different elements of healthcare costs according to study group. The average gross healthcare costs were higher with the administration of GS vs brand-name drugs (€1275 vs €1083; P<.001), whereas the healthcare cost adjusted for covariables was €1277 vs €1057 (P<.001) (a difference of €220). Part of this cost difference between the brand-name drug and the GS corresponded to primary care costs, since the differences observed in specialised care were not statistically significant. These differences were also observed in the different study groups (corrected costs, ANOVA): a) <65 years: €974 vs €1195 (P<.001); b) ≥65 years:, €1146 vs €1363 (P<.001); c) men: €1015 vs €1255 (P<.001); and d) women: €1102 vs €1299 (P<.01).
Healthcare costs by study group.
| Study groups | Age<65 years | Age≥65 years | Men | Women | ||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | Brand-name | GS | Brand-name | GS | Brand-name | GS | Brand-name | GS |
| Number of patients (%) | n=187 (28.7) | n=465 (71.3) | n=213 (29.7) | n=504 (70.3) | n=175 (32.9) | n=357 (67.1) | n=225 (26.9) | n=612 (73.1) |
| Costs in primary care | 686 (470) | 774 (365)* | 809 (482) | 921 (499)** | 709 (434) | 831 (512)** | 785 (511) | 862 (402)* |
| Medical consultations | 265 (213) | 319 (193)** | 324 (244) | 411 (288)*** | 280 (240) | 360 (235)*** | 310 (224) | 371 (260)** |
| Laboratory tests | 15 (21) | 21 (25)** | 22 (29) | 30 (29)** | 18 (25) | 24 (26)** | 19 (26) | 26 (28)** |
| Conventional radiology | 13 (19) | 18 (20)** | 12 (18) | 16 (20)** | 10 (17) | 17 (20)*** | 14 (19) | 17 (19)* |
| Complementary tests | 7 (16) | 10 (21) | 4 (13) | 7 (18)* | 5 (13) | 9 (19)*** | 7 (16) | 9 (20) |
| Baseline medication (gabapentin) | 246 (84) | 206 (64)*** | 264 (128) | 209 (70)*** | 262 (124) | 216 (72)*** | 251 (97) | 203 (63)*** |
| Concomitant medicationa | 140 (330) | 200 (235)** | 183 (301) | 248 (334)* | 135 (275) | 206 (384)* | 185 (342) | 236 (220)** |
| Costs in hospital care | 327 (611) | 405 (848) | 336 (619) | 442 (868) | 323 (605) | 415 (818) | 338 (623) | 429 (882) |
| Days of admission | 65 (294) | 101 (524) | 97 (406) | 119 (627) | 122 (447) | 110 (545) | 51 (266) | 111 (600) |
| Hospital visits | 172 (254) | 174 (245) | 162 (249) | 183 (244) | 129 (193) | 174 (260)* | 196 (286) | 181 (236) |
| Day hospital sessions | 44 (267) | 51 (343) | 36 (236) | 55 (358) | 27 (205) | 48 (329) | 49 (282) | 56 (364) |
| Hospital emergencies | 46 (109) | 78 (99)*** | 41 (77) | 86 (96)*** | 45 (96) | 83 (97)*** | 42 (92) | 82 (98)*** |
| Healthcare costs | 1014 (838) | 1179 (991)* | 1145 (848) | 1363 (1.098)** | 1032 (808) | 1246 (1.023)** | 1123 (872) | 1291 (1068) |
| Corrected costs modelb | ||||||||
| Costs in primary care | 655 | 780*** | 779 | 924*** | 688 | 841*** | 759 | 872** |
| 95% CI | 599-711 | 743-815 | 712-845 | 880-968 | 619-757 | 793-889 | 703-814 | 838-905 |
| Difference (brand-name vs GS) | −125 | −145 | −153 | −113 | ||||
| Costs in specialised care | 319 | 415 | 367 | 439 | 327 | 414 | 343 | 427 |
| 95% CI | 194-424 | 335-483 | 253-475 | 359-504 | 214-439 | 335-492 | 233-452 | 362-493 |
| Difference (brand-name-GS) | −96 | −72 | −87 | −84 | ||||
| Healthcare costs | 974 | 1195** | 1146 | 1363*** | 1015 | 1255** | 1102 | 1299** |
| 95% CI | 839-1110 | 1110-1280 | 1009-1284 | 1274-1451 | 876-1155 | 1157-1352 | 968-1236 | 1219-1380 |
| Difference (brand-name vs GS) | −221 | −217 | −240 | −197 | ||||
GS: generic substitutions; CI: confidence interval.
Values are expressed as percentages or means (SD).
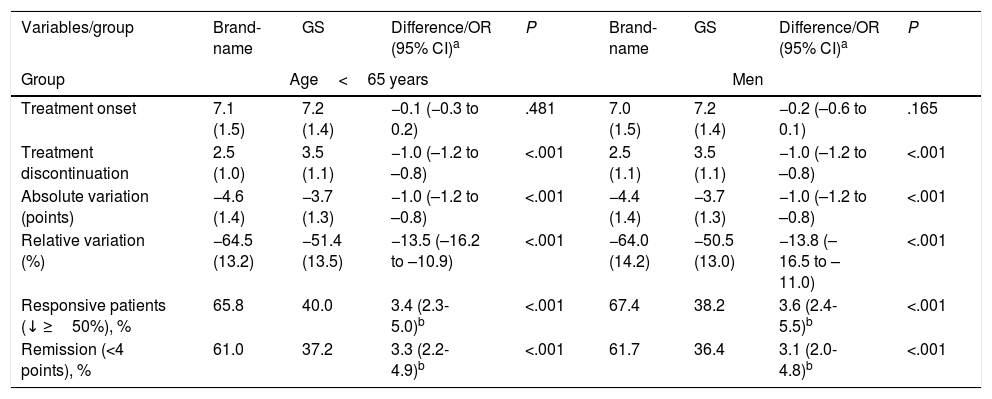
We did not observe statistically significant differences in scores on the pain scales administered at the beginning of the study. Table 3 details the changes in pain intensity at treatment onset and discontinuation by study group. Treatment with brand-name gabapentin was associated with a further decrease from baseline pain levels in comparison with GS: 13.5% (95% CI: 10.9-16.2) and 10.8% (95% CI: 8.2-13.5) in the <65 and ≥65 year groups, respectively (P<.001 in both cases) and 10.7% (95% CI: 8.2-13.2) and 13.8% (95% CI: 11.0-16.5), in women and men, respectively (P<.001 in both cases).
Changes in pain intensity at treatment onset and discontinuation by study group.
| Variables/group | Brand-name | GS | Difference/OR (95% CI)a | P | Brand-name | GS | Difference/OR (95% CI)a | P |
|---|---|---|---|---|---|---|---|---|
| Group | Age<65 years | Men | ||||||
| Treatment onset | 7.1 (1.5) | 7.2 (1.4) | −0.1 (−0.3 to 0.2) | .481 | 7.0 (1.5) | 7.2 (1.4) | −0.2 (–0.6 to 0.1) | .165 |
| Treatment discontinuation | 2.5 (1.0) | 3.5 (1.1) | −1.0 (–1.2 to –0.8) | <.001 | 2.5 (1.1) | 3.5 (1.1) | −1.0 (–1.2 to –0.8) | <.001 |
| Absolute variation (points) | −4.6 (1.4) | −3.7 (1.3) | −1.0 (–1.2 to –0.8) | <.001 | −4.4 (1.4) | −3.7 (1.3) | −1.0 (–1.2 to –0.8) | <.001 |
| Relative variation (%) | −64.5 (13.2) | −51.4 (13.5) | −13.5 (–16.2 to –10.9) | <.001 | −64.0 (14.2) | −50.5 (13.0) | −13.8 (–16.5 to –11.0) | <.001 |
| Responsive patients (↓ ≥50%), % | 65.8 | 40.0 | 3.4 (2.3-5.0)b | <.001 | 67.4 | 38.2 | 3.6 (2.4-5.5)b | <.001 |
| Remission (<4 points), % | 61.0 | 37.2 | 3.3 (2.2-4.9)b | <.001 | 61.7 | 36.4 | 3.1 (2.0-4.8)b | <.001 |
| Groups | Age≥65 years | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Treatment onset | 7.1 (1.5) | 7.2 (1.5) | −0.1 (−0.3 to 0.2) | .516 | 7.2 (1.5) | 7.2 (1.5) | −0.1 (−0.2 to 0.2) | .984 |
| Treatment discontinuation | 2.6 (1.1) | 3.4 (1.2) | −0.7 (−0.9 to −0.5) | <.001 | 2.6 (1.1) | 3.4 (1.1) | −0.7 (−0.9 to −0.5) | <.001 |
| Absolute variation (points) | −4.4 (1.4) | −3.8 (1.4) | −0.7 (−0.9 to −0.5) | <.001 | −4.5 (1.4) | −3.8 (1.5) | −0.7 (−0.9 to −0.5) | <.001 |
| Relative variation (%) | −62.6 (14.0) | −52.1 (14.6) | −10.8 (−13.5 to −8.2) | <.001 | −63.0 (13.3) | −52.5 (14.7) | −10.7 (−13.2 to −8.2) | <.001 |
| Responsive patients (↓ ≥50%), % | 66.2 | 41.9 | 2.8 (2.0-4.1)b | <.001 | 64.9 | 42.3 | 2.7 (1.9-3.9)b | <.001 |
| Remission (<4 points), % | 58.7 | 39.7 | 2.3 (1.6-3.3)b | <.001 | 58.2 | 39.7 | 2.4 (1.7-3.4)b | <.001 |
GS: generic substitutions; CI: confidence interval; OR: odds ratio; P: statistical significance.
Values are expressed as percentages or means (SD).
Whereas in the international scientific literature we can find references to the differential effect of the brand-name drug vs the GS with the same active ingredient, and we have previously published our experience with PNP,9 no studies analysing the effect of sex or age on these results could be found. We therefore consider our study to be necessary and important. The results reveal that compared to the brand-name drug, the GS of gabapentin was associated with poorer treatment persistence and clinical outcomes (pain reduction) in patients with PNP. This leads to greater resource use and healthcare costs. These findings are also observed when analysing data by age range and sex, showing similar cost differences per patient (savings of approximately €200 per patient per year when the original brand is used), and statistically significant additional pain reductions.
A GS has the same qualitative and quantitative composition of the active ingredient and formula as the reference drug, with demonstrated bioequivalence (bioavailability studies). It differs in the composition of excipients and external appearance, which may be responsible for the bioappearance and the potential nocebo effect. The entry into the market of these drugs has helped to reduce the public health system's drug spending, although generic and brand-name drugs currently represent the same cost in Spain. Therefore, and considering current legislation, there are no pharmacological arguments preventing the prescription of brand-name or generic drugs. However, in addition to the already known reasons for non-compliance (which may be intended [sociodemographic factors, adverse effects, price of drugs, lack of understanding of treatment, health status, etc.] or unintended [patients forgetting how to take the medication correctly, etc.]), the study results show that the administration of a GS may be an additional factor to be taken into account. The bioappearance or prescription of different generic gabapentin products which were not included in the study may influence our results (lack of compliance/adherence). These factors include different appearance (colour, shape),24 lack of certain presentations (delayed, slow-release, or absorption),25,26 variability of excipients,27 the effect of copayment,28 and potentially the nocebo effect.29
Given the lack of appropriate standardisation of methodology in terms of patient characteristics and the number and measurement of the variables studied, the external validity of our results must be interpreted with caution. Our results show higher adherence/persistence rates for brand-name drugs than for GS. These data are not generalised in the literature but are consistent with the findings of several other authors.30,31 Our results show that the use of a GS is associated with greater healthcare resource use and higher costs, with a somewhat lower degree of clinical effectiveness than the use of a brand-name drug, regardless of patients’ age group or sex. The temporal correlation between the lack of adherence, decreased clinical effectiveness, and the greater healthcare resource use is unquestionable and highly consistent with findings reported in the literature.9,28–33 These data are difficult to compare due to a shortage of published studies, as mentioned previously. Tran et al.34 report that the use of generic drugs is associated with poorer achievement of therapeutic objectives (clinical effectiveness: LDL-cholesterol) in the treatment of dyslipidaemia. A prospective study by Gagne et al.35 revealed that patients starting treatment with generic statins showed higher rates of non-compliance and cardiovascular events in comparison with brand-name statins; these data cannot be generalised but go in line with other published studies.9,33
Our results also show that the cost of baseline medication was higher in patients treated with brand-name gabapentin, regardless of age or sex. This could be explained by 3 circumstances: a) the patient recruitment period was long (5 years) and the price of the brand-name drug during the first years was higher than that of GS (not at reference price); b) the therapeutic adherence of these patients was also higher; and c) there is a higher proportion of patients (especially in the group with gabapentin) who received higher doses of the drug. However, the most plausible explanation may be higher persistence to the brand-name treatment.9 However, we should stress that in the 4 groups analysed, the sum of the baseline cost of the drug and the concomitant medication for pain was lower (€20, €10, €25, and €3 in the <65 year, ≥65 year, male, and female groups, respectively) when we compared brand-name gabapentin with GS gabapentin.
The results of this study suggest some intervention strategies, particularly for policymakers and healthcare administrators. Changes to the appearance of the drug may lead patients to lose confidence in the safety and effectiveness of the GS, leading to reduced treatment adherence, especially in the case of chronic diseases and polymedicated patients. Decreased variability in the appearance of chemically identical drugs may help promote adherence.13–15
Possible limitations to the study are those typical of retrospective studies, such as the underreporting of diseases; variability in the professionals and patients included, given the observational design; the cost system used; or the possible presence of a classification bias. In this regard, the possible errors in the diagnostic classification of PNP, or the absence of some variable that may affect final results (patients’ socioeconomic status, changes to the prescribed drug dosage, changes to the GS presentations, etc.) must be considered a limitation of the study. Furthermore, we could not obtain data from all patients, such as for measures of clinical effectiveness, especially during the final period; however, this limitation was observed in similar proportions in the different study subgroups. The very conservative imputation made (worst observation carried forward) makes our observations more robust. In our opinion, the main limitations of our study were: a) selection bias on behalf of the physician responsible for starting treatment with brand-name or generic gabapentin, since this was not random, as is usual in everyday clinical practice; and b) the external validity of the study (data generalisation), since the study was conducted in a single healthcare provider, for which reason data must be interpreted with caution.
This study offers new perspectives with a view to its replication in other healthcare institutions and promoting new intervention strategies to encourage treatment adherence. In conclusion, and considering the limitations mentioned, this analysis has shown that patients who started treatment with brand-name gabapentin vs GS showed a higher degree of treatment adherence, leading to lower healthcare costs; better clinical outcomes in pain reduction were also observed. These findings were similar in both men and women and in subjects aged <65 and ≥65 years.
Author contributionsA. Sicras and J. Rejas conceived and designed the study; A. Sicras collected the data and performed the statistical analysis. All authors participated in data interpretation and the drafting, review, and approval of the manuscript.
Conflicts of interestJ. Rejas and M. Pérez are employed by Pfizer S.L.U. The remaining authors have no conflicts of interest to declare.
Please cite this article as: Navarro-Artieda R, Rejas-Gutiérrez J, Pérez-Paramo M, Sicras-Mainar A. Efecto de la edad y el género sobre las consecuencias clínicas y económicas del tratamiento con especialidad farmacéutica de marca o genérica en pacientes con dolor neuropático periférico en práctica clínica habitual. Neurología. 2018;33:141–153.