The medical literature includes case reports and series of patients with chronic inflammatory demyelinating polyneuropathy (CIDP) plus central nervous system (CNS) demyelination as well as patients with multiple sclerosis (MS) associated with demyelinating neuropathy.1–3 Onset of central and peripheral nervous system symptoms is usually acute in paediatric patients.4 In recent years, the term “combined central and peripheral demyelinisation” (CCPD) has been proposed to describe the occurrence of demyelination in both these locations.5 However, there is still no formal definition for the condition, and its aetiopathogenic mechanisms are yet to be established.
Among the different phenotypes of CIDP, Lewis-Sumner syndrome, also known as multifocal acquired demyelinating sensory and motor neuropathy (MADSAM), is characterised by asymmetry, predominantly affecting the upper limbs. We have found no association between this form and CNS demyelination. We describe the case of a patient diagnosed with a form of CIDP (Lewis-Sumner syndrome or MADSAM) and displaying CNS demyelination, a finding which conditions diagnosis and management.
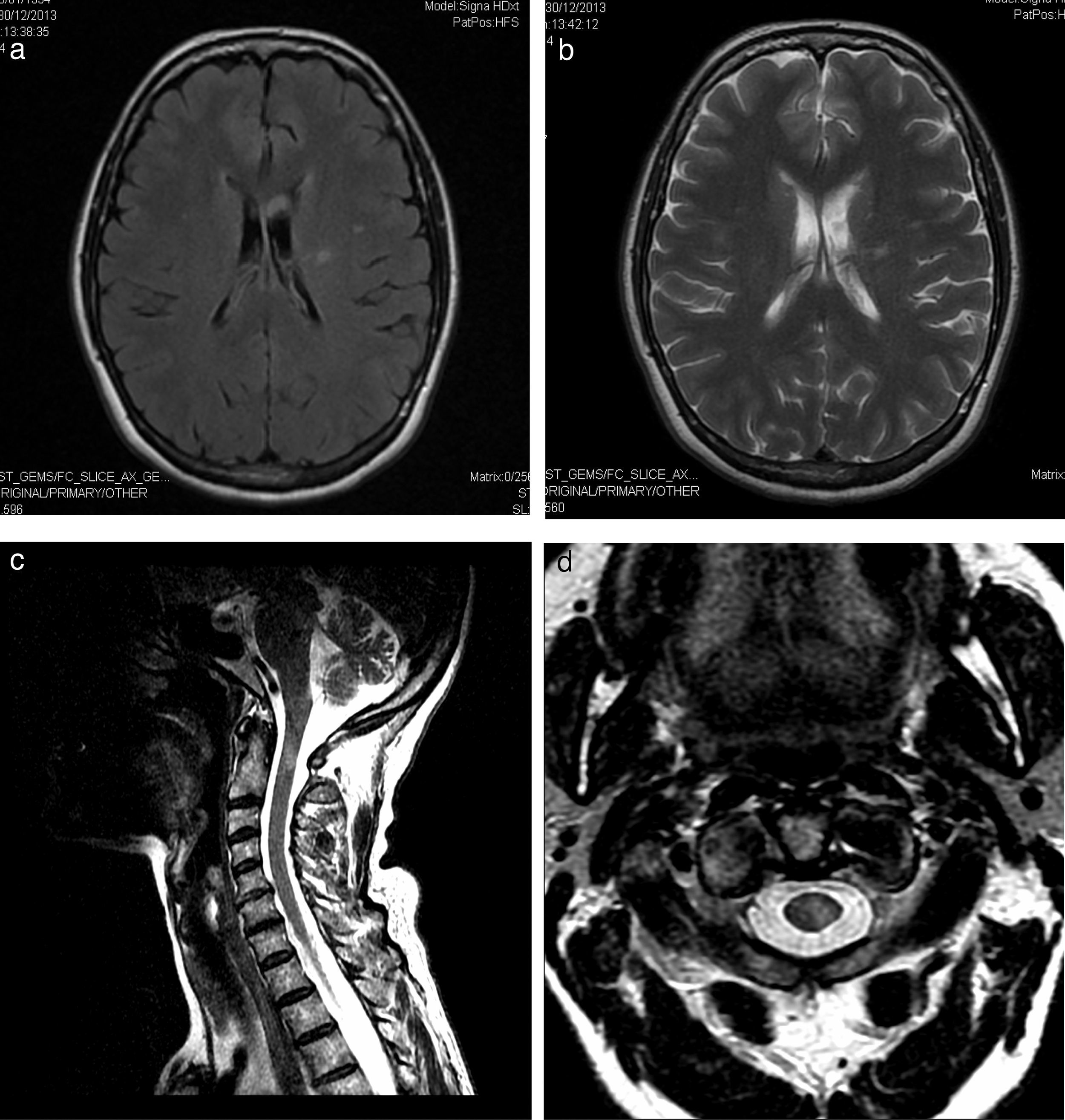
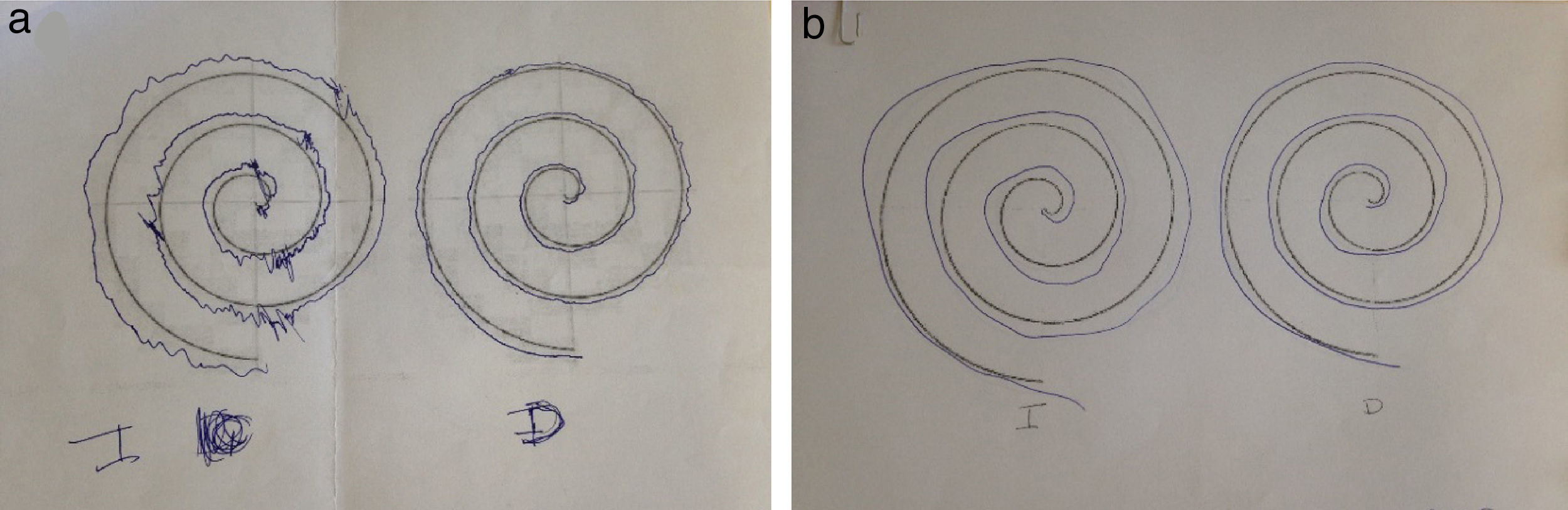
Our patient was a 58-year-old woman with a history of Hashimoto thyroiditis, who was admitted to another hospital due to subacute onset of sensory alterations in her right hand and extending to the other limbs. A neurophysiological study revealed mixed neuropathy which was asymmetrical and predominantly motor. Our patient was diagnosed with multiple neuritis; symptoms improved with administration of immunoglobulins (IG). She came to our department due to a one-month history of dysaesthesia in her right hand, extending to the left hand, the distal region of her left foot, and the left side of her neck and rib cage. The examination revealed 4+/5 muscle balance in the right interossei muscles, 4/5 in the left interossei muscles and left extensor digitorum muscle, 4+/5 in the left tibialis anterior and gastrocnemius muscles, 5−/5 in the right tibialis anterior and gastrocnemius muscles, and abolished stretch reflexes; no sensory alterations were found. Our patient had normal gait and displayed instability during the Romberg test. A complete blood count and serology and autoimmune tests revealed no abnormal findings except for elevated TPO-Ab levels (5631IU/mL). A lumbar puncture disclosed no cells and a protein level of 97mg/dL. A nerve conduction study revealed proximal and distal sensorimotor demyelinating polyneuropathy predominantly affecting the upper limbs, particularly the left upper limb (asymmetrical). A brain MRI scan displayed 2 hyperintense areas on T2-weighted and FLAIR sequences (Fig. 1a and b). Our patient improved after the first cycle of IG. During follow-up, she displayed distal weakness, ataxic gait, and tremor in her hands, especially in the left hand (Fig. 2). A T2-weighted MRI scan of the brachial and lumbosacral plexi revealed no abnormalities except for a hyperintense area in the spinal cord (Fig. 1C and D). Visual evoked potentials (VEP) showed prolonged P100 latency in the left eye. Our patient tested negative for neurofascin-155 antibodies. Symptoms improved after several cycles of IG and propranolol (Fig. 2).
(a) FLAIR sequence showing signal hyperintensity in the left periventricular white matter extending towards the corona radiata and left centrum semiovale. (b) T2-weighted sequence displaying an area of probable malacia-gliosis. (c) Sagittal and (d) axial T2-weighted sequences showing a hyperintense lesion measuring 5mm×4mm×1.3mm in the cervical spinal cord, at the level of the middle third of the odontoid process.
Our patient was diagnosed with Lewis-Sumner syndrome, initially manifesting with relapses. Given the published evidence of the association between MADSAM and brachial plexus signal hyperintensity and/or hypertrophy,5 we requested an MRI scan of the brachial and lumbosacral plexi; this revealed a hyperintense area in the spinal cord, which we interpreted as being inflammatory in nature. To our knowledge, the literature reports no association between this form of CIDP and CNS demyelinating lesions, but it does describe cases of similar cervical lesions in patients with extensive parenchymal lesions, such as ADEM,6 associated with demyelinating polyneuropathy in the form of CIDP or acute inflammatory demyelinating polyradiculoneuropathy.7
Co-presence of central and peripheral nervous system demyelination was first described by Amit et al.8 in 1992 in a paediatric patient. These authors used the term “CCPD”, although several other designations have been proposed, including CIDP with CNS involvement, peripheral neuropathy with MS, and relapsing-remitting disease of the central and peripheral nervous systems.9 Kawamura et al.10 identified neurofascin antibodies in patients with CCPD and hypothesised that central and peripheral demyelination may have common pathophysiological mechanisms. Ogata et al.11 published a study of 40 patients with CCPD, the largest series analysed to date. These authors described the demographic, laboratory, neuroimaging, and VEP features of this condition, and established 2 subtypes, depending on whether symptoms in these 2 locations appeared simultaneously (within 2 months of one another) or at different times throughout disease progression (>2 months). The first subtype (peripheral and central nervous system symptoms appear simultaneously) is associated with severe impairment, respiratory disorders, and more extensive cerebral and spinal cord lesions. On the other hand, the second subtype (symptoms manifest at different stages of the disease, even years apart) is usually associated with optic nerve involvement. Our patient met the criteria proposed by Ogata et al.11 for the second subtype: asynchronous onset of central and peripheral nervous system symptoms and impaired visual evoked potentials. Tremor has been described in patients with CIDP,12 but to our knowledge no studies have linked it to CCPD or MADSAM.
In conclusion, the course of CCPD differs from that of demyelination affecting a single location. Therefore, correctly diagnosing CCPD may guide future immunomodulatory treatment for further relapses.
Please cite this article as: Cuenca Hernández R, Gordo Mañas R, Gredilla Molinero J. Descripción de un caso de Combined central and peripheral demyelinization. Neurología. 2017;32:547–550.
This study was presented in poster format at the 67th Annual Meeting of the Spanish Society of Neurology, 2015.