Understanding the consumption patterns of antiepileptic drugs (AEDs) is important since epilepsy generates a large percentage of healthcare costs and the adverse effects of antiepileptic drugs may have a significant impact on the patients’ quality of life. This is why it may be beneficial to document consumption patterns among the epileptic population of the Western Málaga district.
Since the only neurology departments in Málaga province are found in the 2 public hospitals in the capital city, we believe that ascertaining AED consumption patterns in our hospital can provide an approximate idea of how they are consumed throughout the Western Málaga district. Data might also be extrapolatable to the province's total population.
We collected data from patients with a definite diagnosis of active epilepsy1 who attended weekly follow-up visits at Hospital Virgen de la Victoria in Málaga for a year. Data were also collected from patients with inactive epilepsy but still taking antiepileptic drugs.
We obtained a sample of 515 patients, including 507 on treatment. Clinical characteristics of our patients resembled those of epileptic patients in Europe, as we amply described in an article published in 2012.2
Of the total patients, 54.3% were on monotherapy, 30.87% were on double therapy, 12.2% received triple therapy, and only about 1% were treated with 4 drugs.
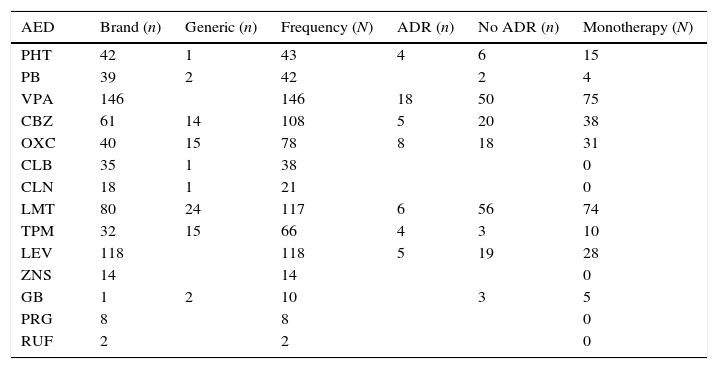
The most widely used drug in the whole sample, whether in monotherapy or combination therapy, was valproic acid (28.3%), followed by levetiracetam and lamotrigine (23% each). In monotherapy only, the most widely used drugs were valproic acid and lamotrigine (14% each), followed by carbamazepine, oxcarbazepine, and levetiracetam in smaller percentages (7%, 6%, and 7%, respectively). Table 1 lists the consumption data for each antiepileptic drug used by patients in our sample.
Consumption pattern of each antiepileptic drug in the sample.
| AED | Brand (n) | Generic (n) | Frequency (N) | ADR (n) | No ADR (n) | Monotherapy (N) |
|---|---|---|---|---|---|---|
| PHT | 42 | 1 | 43 | 4 | 6 | 15 |
| PB | 39 | 2 | 42 | 2 | 4 | |
| VPA | 146 | 146 | 18 | 50 | 75 | |
| CBZ | 61 | 14 | 108 | 5 | 20 | 38 |
| OXC | 40 | 15 | 78 | 8 | 18 | 31 |
| CLB | 35 | 1 | 38 | 0 | ||
| CLN | 18 | 1 | 21 | 0 | ||
| LMT | 80 | 24 | 117 | 6 | 56 | 74 |
| TPM | 32 | 15 | 66 | 4 | 3 | 10 |
| LEV | 118 | 118 | 5 | 19 | 28 | |
| ZNS | 14 | 14 | 0 | |||
| GB | 1 | 2 | 10 | 3 | 5 | |
| PRG | 8 | 8 | 0 | |||
| RUF | 2 | 2 | 0 |
ADR: only patients taking the drug in monotherapy are included.
CBZ, carbamazepine; CLB, clobazam; CLN, clonazepam; AED, antiepileptic drug; GB, gabapentin; LEV, levetiracetam; LMT, lamotrigine; n, patients reporting use of brand name vs generic/patients reporting ADRs vs no ADRs; N, total patients in the sample; OXC, oxcarbazepine; PRG, pregabalin; PHT, phenytoin; PB, phenobarbital; ADR, adverse drug reactions; RUF, rufinamide; VPA, valproic acid; TPM, topiramate; ZNS, zonisamide.
Whereas 12% of the patients stated that they were taking one or more generic drugs, 17% of the patients in the sample did not know the brand of the antiepileptic drug they were taking (reference brand, alternative brand, or generic). Of the total samples, 2.9% of the patients reported a recent seizure and suggested that it might have been caused by changing the brand of antiepileptic drug.
Only 28% of the 407 patients questioned about adverse drug events mentioned having experienced one. We should indicate that in order to list adverse events for each drug separately, we conducted the study only with those patients on monotherapy.
In the total sample, the most frequently reported adverse events were those affecting the cognitive sphere (8.9%), followed by physical adverse events (6.4%), and dizziness of any kind (4.9%). Therefore, and in line with other authors’ findings,3–5 the most frequent adverse events are related to the nervous system. We also observed abnormalities in laboratory analyses in almost half of the patients (48.8%) tested at least once.
Considering only those patients on monotherapy, topiramate was the drug that caused the most adverse events. Among patients treated with topiramate monotherapy, 57% of those questioned reported adverse events, especially weight loss. Topiramate was followed by phenytoin (40% of patients treated in monotherapy and asked about adverse events) and oxcarbazepine (30.7%). These 2 drugs affected concentration and caused sleepiness. Valproic acid was responsible for adverse effects, especially alopecia and/or obesity, in 26.4% of the interviewed patients treated with that drug in monotherapy. Carbamazepine caused adverse events, mainly digestive, in 20% of patients on that treatment, some of whom also presented alterations in laboratory results that might have been related to that drug. A similar percentage presented levetiracetam-related adverse events, most of which affected mood. Lamotrigine (9.67%) was the drug causing the fewest adverse events.
Grouping antiepileptic drugs taken as monotherapy by generation did not show a statistically significant degree of association, although new drugs are associated with fewer adverse events than classic antiepileptic drugs are (18% vs 25%). Detailed data cannot be presented here because our article appears in the Letters to the Editor section, but they can be consulted in Table 1.
Consumption patterns of antiepileptic drugs in our patient sample are similar to those reported in other studies.3,6 However, some changes have been observed: increased consumption of new drugs and decreased use of the classic AEDs phenytoin and phenobarbital. In our sample, 51% of the patients use new drugs and 49% remain on classic AEDs (expressed as percentages of the total sample, including monotherapy and combination therapy). In our study, the most widely used drug was valproic acid, but the literature contains only a few epidemiological studies reporting these data. According to Gallito et al.7 the most widely used drug in Italy is phenobarbital; the Russian study by Ghekht et al.8 and the Icelandic study by Olaffson and Hauser9, cite carbamazepine as the most widely used drug (36%).
The main limitation of our study is its double selection bias. On the one hand, selected patients represent only one hospital, meaning that we did not consider patients whose follow-up visits take place in private clinics or primary care centres. The population may also contain undiagnosed epileptics or patients who do not attend follow-up visits. On the other hand, the search for adverse events was conducted using a clinical interview and not a specific questionnaire. As a result, many events may go undetected even when doctors are specifically searching for them.10,11 We should stress that this study was only able to measure adverse events from drugs that patients are currently taking. As a result, this study collected no information about cases in which adverse events (severe effects or drug intolerance) resulted in withdrawal of the drug in question.
In any case, we would like to highlight the high rate of adverse events related to topiramate, even though the significance of this tendency is questionable since only a few patients were taking this drug in monotherapy (n=7). Other authors, however, have presented similar findings.12,13 In our opinion, the most noteworthy finding from this study is that it does not identify fewer adverse events for new drugs than for classic drugs. It does show different ranges of adverse events for each of the two drug types, and this finding is consistent with results from other studies.4,5,14
Our study is the first large-scale contribution to the knowledge of AED consumption patterns in the south of Spain. Consumption of classic drugs remains high and its pattern is similar to that of new drugs. Although the type of adverse events differs between classic and new drugs, we found no statistically significant differences between the two drug groups with regard to presence or absence of adverse events. Classic drugs therefore remain a feasible option. Furthermore, a small percentage of patients have associated a change in the brand of AED with the occurrence of an epileptic seizure. Considering all of the above, the type and brand of an antiepileptic drug should always be selected on a case-by-case basis, while seeking to ensure rational drug use.
All data from this study are included in a doctoral thesis.15
Please cite this article as: Garcia-Martin G, Chamorro-Muñoz MI, Martin-Reyes G, Dawid-Milner MS, Perez-Errazquin F, Romero-Acebal M. Perfil de consumo de antiepilépticos en los pacientes epilépticos del Área Oeste de Málaga. Neurología. 2015;30:67–69.
Socio-epidemiological study of epilepsy. Western Málaga district. Healthcare management and quality of life.