Hospital on-call neurology shifts are frequently on-site, but some on-call services may be off-site or mixed. Intravenous tissue plasminogen activator (tPA) is one of the main reperfusion treatments for acute ischaemic stroke (AIS). This study assesses door-to-needle times (DNT) when the neurologist is on-site or off-site.
MethodsWe performed a prospective, observational study from 2012 to 2017, including patients with AIS and treated with tPA. Data were collected on sex, age, door-to-scan time, scan-to-needle time, and DNT. The on-duty neurologist was on-site from 08:00 to 20:00, and on call but off-site from 20:00 to 8:00. Three groups were formed: on-site, off-site, and off-site with resident present.
ResultsOur sample included 138 patients. The mean age was 69.7 years, and 45.7% of patients were women. Ninety-six patients were admitted during the on-site shift, 25 during the off-site shift, and 17 during the off-site–resident present shift. Patients admitted during the on-site and off-site shifts presented DNTs of 59 and 72 minutes, respectively (P = .003). DNTs were 59, 74, and 68 minutes (P = .001), respectively, for the on-site, off-site, and off-site–resident present shifts; the difference between DNTs for on-site and off-site shifts was statistically significant. No differences were observed between DNTs according to time of day (morning, afternoon, or night), or between weekdays and weekends.
ConclusionDNT is influenced by whether the on-duty neurologist is on- or off-site at the time of code stroke activation. The presence of a neurology resident can reduce DNT.
La guardia de Neurología es frecuentemente presencial; es decir, con el neurólogo presente en el centro hospitalario; pero en algunos centros, puede ser localizada o mixta. La Fibrinólisis Intravenosa (FIV) es uno de los principales tratamientos reperfusores en el Ictus Isquémico Agudo (IIA). El objetivo del siguiente trabajo es comparar el Tiempo Puerta-Aguja (TPA) durante la guardia presencial y la guardia localizada.
Material y MétodosEstudio prospectivo, observacional, desde el año 2012 hasta el 2017, en el que se incluyeron pacientes con IIA y FIV. Se recogieron datos como sexo, edad, hora del inicio de los síntomas, hora de llegada al hospital, hora de TAC, hora de inicio de FIV. Se consideró guardia de «Presencia» desde las 8:00 hasta las 20:00 y «Localizado» desde las 20:00 hasta las 08:00, dividido en 3 grupos: guardia «Presencial», guardia «Localizada» y guardia «Localizada con Residente».
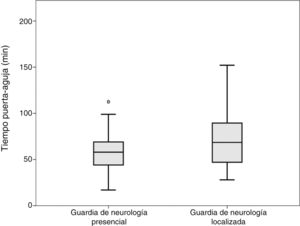
ResultadosN = 138. Edad media 69,7 años; mujeres 45,7%. Pacientes en guardia de «Presencia» 96, «Localizada» 42, de los cuales 17 con Residente. Ambos grupos presentaban características basales similares. Los TPA de los grupos «Presencia» y «Localizado» fueron 59 y 72 minutos respectivamente (p = 0,003). Los TPA de grupos «Presencia», «Localizada» y «Localizada con Residente» respectivamente 59; 74 y 68 minutos (p = 0,001), con significación entre «Presencia» y «Localizado». No se observaron diferencias entre el TPA dependiendo de la franja horaria de mañana, tarde y noche ni entre días laborales y días de fin de semana.
ConclusiónLa presencia o ausencia del neurólogo en el centro hospitalario en el momento del código ictus, influye en la demora de administración de tratamiento fibrinolítico. La presencia de residente de Neurología puede acelerar la realización del proceso.
Code stroke is an emergency protocol for immediate assessment of patients with suspected cerebrovascular events, aimed at increasing and optimising the chances of brain reperfusion. The reperfusion therapies currently available for ischaemic stroke are intravenous fibrinolysis (IVF) with alteplase1,2 and mechanical thrombectomy (MT) via catheterisation, which has been shown to be effective in the treatment of large-vessel occlusion.2–9
Early treatment with reperfusion therapy is essential to achieving satisfactory outcomes.1,10–15 Treatment delays depend on such factors as clinical diagnosis of the cerebrovascular event, transport times, and time from hospital arrival to administration of IVF (door-to-needle time).
Most hospitals have on-call neurologists, who will either be on-site (neurologists physically present at the hospital 24 hours a day), off-site (neurologists not physically present at the hospital at all times, but ready to attend hospital when needed), or mixed (neurologists on-site or off-site, depending on the time of day).
The main purpose of this study is to analyse the differences in door-to-needle times between patients attended by on-site and off-site on-call neurologists. As a secondary objective, we analysed the impact on door-to-needle times of the presence of neurology residents during on-call shifts and the time of day and day of the week when code stroke is activated.
Material and methodsWe conducted a prospective, observational study of patients attended following in-hospital or pre-hospital code stroke activation and treated with IVF with or without MT at a secondary hospital between 2012 and 2017. We excluded all patients whose medical history did not allow for accurate reconstruction of the timeline of stroke management. We also excluded patients who were already hospitalised or transferred from other centres and for whom neurological assessment or complementary test results were available, and those in whom code stroke had been reactivated (in the event of transient ischaemic attack or stroke improvement), as these patients had been assessed prior to code stroke activation.
Data were gathered on sex, age, date of stroke, day of the week when code stroke was activated, baseline NIHSS score, baseline ASPECT score, NIHSS score at 24 hours, ASPECT score at 24 hours, and mRS score at 3 months. We also gathered data on the time of symptom onset (or time when the patient was last known to be asymptomatic), time of hospital arrival, time when CT was performed, and time of onset of IVF (time when the first bolus was administered).
Our hospital has an off-site on-call neurology service: an on-call neurologist is physically present at the hospital between 08:00 and 20:00; between 20:00 and 08:00, the on-call neurologist must be reachable by telephone and available to travel to the hospital if code stroke is activated. Neurology residents work 24-hours on-call shifts (8:00-8:00) several days per week. Therefore, on some days both an off-site on-call neurologist and an on-site resident are available, whereas other days only an off-site on-call neurologist is available. Residents attend code stroke patients but do not start IVF until the off-site neurologist arrives at the hospital and assesses the patient. We classified patients into 3 groups, according to whether they were attended by an on-site neurologist, an off-site neurologist, or an off-site neurologist plus an on-site neurology resident.
The study was approved by the local ethics committee. Statistical analysis was conducted using SPSS®. Normality was tested with the Kolmogorov-Smirnov test; data were analysed with ANOVA and the t test. The study was approved by the local ethics committee.
ResultsOver 600 code stroke activations were recorded between 2012 and 2017, with a total of 174 patients receiving IVF. We excluded 18 patients due imprecise temporal data, 15 patients due to code stroke reactivation (following clinical worsening or transient ischaemic attack), 2 cases of in-hospital code stroke activation, and one patient who underwent clinical assessment and complementary tests at another centre. A total of 138 patients were finally included in the study. All patients were managed in accordance with the currently available treatment guidelines.1,2
Mean age (standard deviation [SD]) in our sample was 69.7 (12.1) years; 63 patients were women (46.3%). In our sample, arterial hypertension was recorded in 65.9% of patients, dyslipidaemia in 39.9%, emboligenic heart disease in 36.6%, diabetes mellitus in 29.7%, and history of cerebrovascular events in 12.5%; 12% of patients were active smokers and 63.4% had never smoked. Baseline mRS score was ≤ 1 in 94.4% of patients. The mean (SD) NIHSS score upon arrival at hospital was 15 (6) points, with a mean ASPECT score of 9.6. Mean door-to-needle time was 63 (24) minutes. Mean NIHSS score at 24 hours was 9 (7) points, with an ASPECT score of 7 (2) at 24 hours. Stroke was atherothrombotic in 42.8% of cases, cardioembolic in 32.6%, lacunar in 3.6%, of other determined aetiology in 7.2%, and of undetermined aetiology (cryptogenic) in 8.3%. At 3 months, 41.4% of patients scored ≤ 2 on the mRS. Table 1 presents the main baseline characteristics of our sample, by group.
Demographic data, risk factors, and clinical characteristics of patients with acute ischaemic stroke treated with intravenous fibrinolysis, by type of on-call neurologist.
| Variable | On-site on-call neurologist (n = 96) | Off-site on-call neurologist (n = 42) |
|---|---|---|
| Age (years) | 70 (12) | 70 (12) |
| Women, n (%) | 44 (45) | 19 (45.2) |
| Hypertension, n (%) | 61 (63.5) | 30 (71.4) |
| Diabetes mellitus | 28 (29.2) | 13 (31) |
| Baseline glucose level (mg/dL) | 127 (38) | 131 (50) |
| Baseline NIHSS score | 15 (6) | 14 (5) |
| Baseline ASPECT score | 9.68 | 9.55 |
| Time to fibrinolysis | 141 (45) | 168 (61) |
| NIHSS score at 24 h | 9 (7) | 9 (8) |
| ASPECT score at 24 h | 7 (2) | 7 (3) |
| mRS score at 3 months | 3 (2) | 3 (2) |
ASPECT: Alberta Stroke Program Early CT score; mRS: modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale.
A total of 96 patients (70.7%) were attended by an on-site on-call neurologist and 42 by an off-site on-call neurologist (17 [12.2%] with a resident and 25 without a resident).
Door-to-CT, CT-to-needle, and door-to-needle times were calculated for all 3 groups (“on-site,” “off-site,” and “off-site plus resident” groups). Door-to-needle time was similar in all groups. CT-to-needle times in the on-site, off-site, and off-site plus resident groups were 36, 52, and 45 minutes, respectively. The Kolmogorov-Smirnov test confirmed normal distribution in all groups, and ANOVA revealed statistically significant differences between the 3 groups (P = .02).
Door-to-needle times were 59, 74, and 68 minutes, respectively. Again, the Kolmogorov-Smirnov test confirmed the normality of the data distribution, and ANOVA revealed significant differences (P = .01). Results are shown in Table 2. The post-hoc analysis of both comparisons revealed that differences corresponded to the comparison between the on-site and the off-site groups exclusively, with no significant differences in the comparisons with the off-site plus resident group.
Stroke management times, by type of on-call neurologist.
| Variable | On-site on-call neurologist (n = 96) | Off-site on-call neurologist (n = 25) | Off-site on-call neurologist + neurology resident (n = 17) | P |
|---|---|---|---|---|
| Door-to-CT time (min) | 22 (12) | 20 (9) | 24 (11) | .7 |
| CT-to-needle time (min) | 36 (19) | 52 (31) | 45 (20) | .02 |
| Door-to-needle time (min) | 59 (20) | 74 (32) | 68 (24) | .01 |
| Variable | On-site on-call neurologist (n = 96) | Off-site on-call neurologist (w/ or w/o neurology resident) (n = 42) | P |
|---|---|---|---|
| Door-to-CT time (min) | 22 (12) | 21 (10) | .98 |
| CT-to-needle time (min) | 36 (19) | 49 (27) | .008 |
| Door-to-needle time (min) | 59 (20) | 72 (29) | .003 |
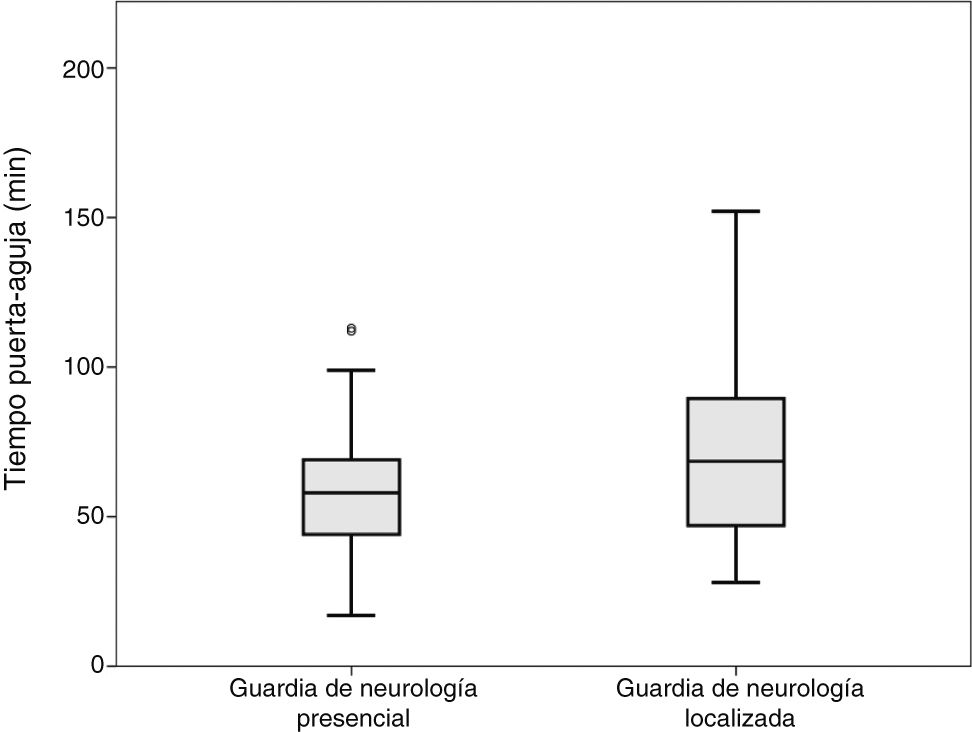
Comparisons were performed between the on-site and the off-site groups; patients attended by both the off-site neurologist and a resident were included in the off-site group. No differences were observed in door-to-CT time. Mean CT-to-needle times were 36 and 49 minutes, respectively (P = .008; t test). Mean door-to-needle times were 59 and 72 minutes, respectively (P = .003; t test) (Fig. 1).
We also analysed the impact of the time of the day when code stroke was activated (morning [8:00-15:00], evening [15:00-22:00], or night [22:00-8:00]) on management times. Mean (SD) door-to-needle times were 60 (17) minutes in the morning shift, 61 (26) minutes in the evening shift, and 71 (35) minutes in the night shift (P = .15).
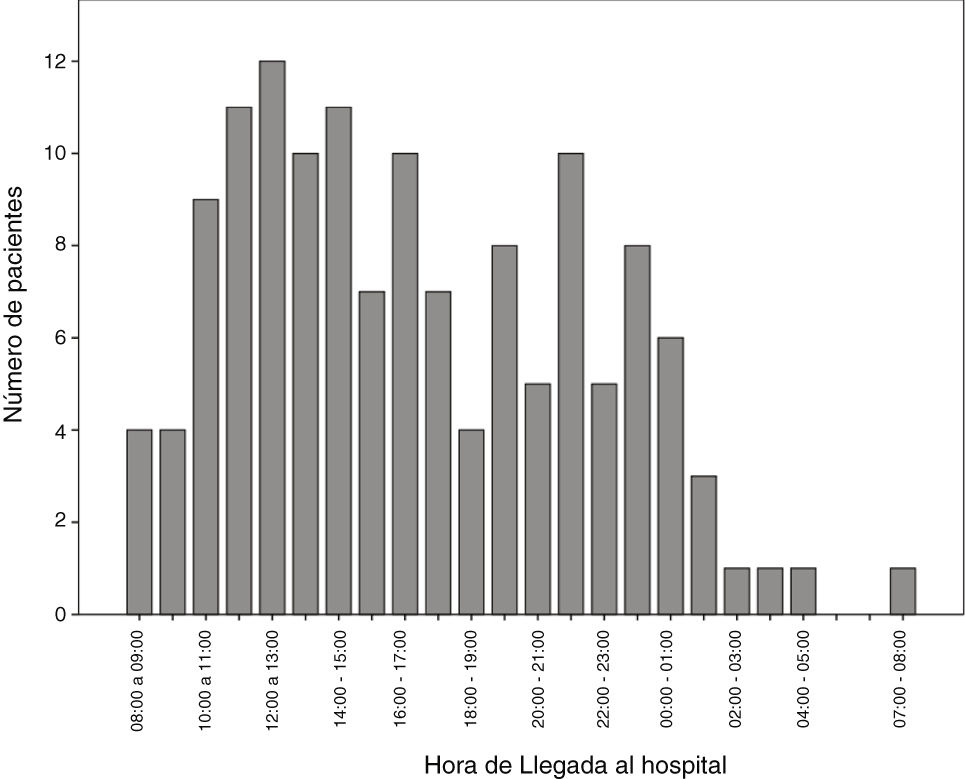
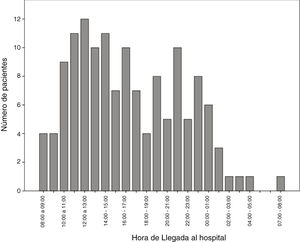
The number of code stroke activations leading to administration of IVF was higher in the morning shift, particularly between 12:00 and 14:00 (Fig. 2).
We also evaluated the effect of the day of the week (weekdays [Monday to Friday] or weekend [Saturday and Sunday]) on management times. The mean door-to-needle time was 64 (22) minutes on weekdays and 57 (22) on weekends (P = .48).
DiscussionWe observed significant differences in code stroke management times between patients attended by on-site and by off-site on-call neurologists. Our results show that absence of an on-site neurologist upon arrival of the patient at the hospital results in longer management times, and consequently in delayed treatment administration (Fig. 1). Likewise, our data suggest that presence of a neurology resident may speed up the process.
In our autonomous community, only reference hospitals have an on-call neurologist available on-site 24 hours a day, 7 days a week. Other hospitals may or may not have an on-call neurologist, who may be on-site or off-site depending on the time of the day. This may be due to the fact that code stroke is more frequently activated in the morning and less frequently at night (Fig. 2). In recent decades, the number of neurological emergencies has increased both at neurovascular reference centres and at other types of centres, probably due to greater public awareness, more neurology training at emergency departments, and especially due to the emergence of new reperfusion therapies, which have widened the therapeutic window. This underscores the need for emergency, specialised stroke care.
One of the limitations of analysing management times is the lack of consensus on how they should be defined. Should “door” be defined as arrival at the healthcare centre or the activation of code stroke at any time? These concepts are difficult to establish when the initial assessment and complementary tests are performed at other centres before admission to the hospital where the patient will receive treatment. Should “needle” be considered the moment when the bolus is infused or when perfusion is started? Some studies even consider “needle” as the moment when the decision is made to administer IVF, rather than the time when it is actually administered. Kruyt et al.16 propose a definition for “door” and “needle.” According to these authors, “door” is the moment when the patient arrives at the hospital (or the first consultation with the neurology department, in the case of hospitalised patients), and “needle” is the moment when the intravenous bolus of alteplase is administered, provided that this is followed immediately by administration of the continuous infusion. In our study, we aimed to homogenise our sample, establishing “door” as the moment when the patient arrived at the hospital (we excluded the patients previously assessed or undergoing complementary tests at other centres, or for whom code stroke was reactivated due to clinical worsening) and “needle” as the moment when the intravenous bolus was administered. While this reduced the size of our sample, our results are conclusive.
Treatment options for acute ischaemic stroke are time-dependent; therefore, many studies have focused on optimising door-to-needle times. Advani et al.17 demonstrated that training primary care physicians, nurses, and paramedics significantly reduced door-to-needle times. Van Dishoeck et al.18 report an unexplained improvement in door-to-needle times between 2008 and 2012, which may be due to increased awareness of the importance of acting quickly in the event of stroke, both among the general population and in hospital staff. Fonarow et al.19 showed that a national training initiative improved door-to-needle times and decreased the incidence of haemorrhagic complications. Sauser et al.20 compared door-to-CT time against CT-to-needle time, and found greater delays in the latter (60 [32] minutes, vs 22 [15]), which suggests greater room for improvement in management times after imaging studies; these results are similar to our own. Lorenzano et al.21 analysed management delays at different times of day and days of the week, observing longer door-to-needle times and total management times in patients attended at night and during the weekend. In our study, management times were also longer at night and during the weekend, although these differences were not statistically significant. Xian et al.22 described and analysed the strategies used in a group of hospitals from the United States to improve door-to-needle times: fast assessment by the neurovascular team, a single-call code stroke activation system, and stocking alteplase in the emergency department; cumulative improvements in management times were observed for each strategy implemented.
The order of the steps involved in the code stroke protocol has been studied extensively, as it is considered a fundamental aspect of optimising stroke management times. Recent studies, including the one by Iglesias Mohedano et al.,23 have evaluated treatment times following protocol optimisation, including such measures as activation of code stroke and collection of patient data before the patient arrives at the hospital, avoiding early complementary tests except for electrocardiography and coagulation study (if the patient is taking vitamin K antagonists), administration of IVF in the radiology room, and transfer to the vascular medicine department if needed. Schrock et al.24 found that performing electrocardiography and chest radiography studies before the neuroimaging study increased door-to-needle time by 6 and 13 minutes, respectively, showing that the order of complementary tests has an impact on door-to-needle times. Hsieh et al.25 describe several strategies, such as moving the CT room nearer to the emergency department, using special plastic bags to label blood samples as top priority in the laboratory, and video-assisted therapeutic risk communication, which have reduced door-to-needle times from 93 to 57 minutes. More recently, Tan et al.26 reported a decrease in management times, from 84 to 59 minutes, after the implementation of a series of changes in their protocol: prehospital code stroke activation, direct transfer to the radiology room, and management by neurologists and stroke nurses. Van Schaik et al.27 decreased door-to-needle times from 60 to 25 minutes after introducing a standardised action protocol and raising awareness of the importance of intravenous thrombolysis among the healthcare professionals involved. Bhatt et al.28 observed an improvement in management times following the implementation of prehospital code stroke activation, stocking and administering alteplase in the radiology room, and prompt data analysis and feedback for continuous improvement. Ajmi et al.29 achieved a door-to-needle time of only 13 minutes after reviewing their action protocol and implementing simulation-based training sessions for the stroke team.
Therefore, some of the strategies proposed for optimising door-to-needle times are prehospital code stroke activation, collection of personal data before the patient arrives at hospital, direct transfer to the radiology room, prioritising CT, starting administration of alteplase before performing other complementary tests, stocking and administering alteplase in the radiology room, and constant analysis of patient data at regular team sessions.
Given that the effectiveness of brain reperfusion therapy for acute ischaemic stroke largely depends on the time elapsed before its administration, reducing door-to-needle times must be a top priority in all centres. Delays in administering IVF negatively impact patient outcomes, with a time-dependent decrease in their likelihood of recovering from stroke.30 In any case, healthcare centres should study the possibility of implementing on-site on-call neurology services, given that neurologists’ physical absence from the hospital results in delayed administration of reperfusion therapy and probably in poorer functional prognosis.
ConclusionsThe presence or absence of an on-call neurologist at the hospital when code stroke is activated significantly delays administration of fibrinolytic therapy. Availability of an on-site on-call neurologist improved stroke management times. Presence of a neurology resident advanced the management process while the off-site on-call neurologist travelled to the hospital. No significant differences in stroke management times were observed between different times of day or days of the week.
FundingThe authors have received no funding for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Gallardo-Tur A, Carazo-Barrios L, de la Cruz-Cosme C. Tiempo puerta-aguja entre neurólogo presencial y localizado en ictus isquémico tratado con alteplasa. Estudio PRISA. Neurología. 2022;37:543–549.