Drug-resistant epilepsy affects 25% of all epileptic patients, and quality of life decreases in these patients due to their seizures. Early detection is crucial in order to establish potential treatment alternatives and determine if the patient is a surgical candidate.
DevelopmentPubMed search for articles, recommendations published by major medical societies, and clinical practice guidelines for drug-resistant epilepsy and its medical and surgical treatment options. Evidence and recommendations are classified according to the criteria of the Oxford Centre for Evidence-Based Medicine (2001) and the European Federation of Neurological Societies (2004) for therapeutic actions.
ConclusionsIdentifying patients with drug-resistant epilepsy is important for optimising drug therapy. Experts recommend rational polytherapy with antiepileptic drugs to find more effective combinations with fewer adverse effects. When adequate seizure control is not achieved, a presurgical evaluation in an epilepsy referral centre is recommended. These evaluations explore how to resect the epileptogenic zone without causing functional deficits in cases in which this is feasible. If resective surgery is not achievable, palliative surgery or neurostimulation systems (including vagus nerve, trigeminal nerve, or deep brain stimulation) may be an option. Other treatment alternatives such as ketogenic diet may also be considered in selected patients.
La epilepsia resistente al tratamiento médico afecta a una cuarta parte de los pacientes con epilepsia. Como consecuencia de las crisis estos pacientes presentan una peor calidad de vida, por lo que es fundamental su diagnóstico para establecer posibles alternativas terapéuticas e iniciar una valoración prequirúrgica.
DesarrolloBúsqueda de artículos en PubMed y recomendaciones de las Guías de Práctica Clínica (GPC) y Sociedades Científicas más relevantes, referentes a epilepsia refractaria y al tratamiento médico y quirúrgico. Se clasifican las evidencias y recomendaciones según los criterios pronósticos del Oxford Centre for Evidence Based Medicine (2001) y de la European Federation of Neurological Societies (2004) para actuaciones terapéuticas.
ConclusionesLa identificación de los pacientes con epilepsia refractaria es importante para optimizar el tratamiento farmacológico. Se recomienda el empleo de una politerapia racional de fármacos antiepilépticos, buscando combinaciones que aumenten la eficacia y minimicen los efectos adversos. Cuando no se consigue el control adecuado de las crisis es necesario realizar una valoración prequirúrgica en un centro especializado, con el fin de resecar la zona epileptógena sin producir déficits al paciente en los casos en los que sea posible. En caso contrario se recurrirá a procedimientos de cirugía paliativa o sistemas de neuroestimulación (vagal, trigeminal o cerebral). Otras alternativas, como la dieta cetógena, también pueden considerarse en pacientes seleccionados.
Identifying patients with drug-resistant epilepsy is essential in order to optimise drug treatment, start the evaluation process to determine if they are candidates for surgery, and opt for surgery or other non-pharmacological alternatives on a case-by-case basis.
The methodological steps followed in drafting this chapter of the Official Clinical Practice Guidelines for Epilepsy were described in the first chapter of the guidelines, published as an article in Neurología. Classification of the levels of evidence and the grades of recommendation is in accordance with the 2004 European Federation of Neurological Societies guidelines regarding therapeutic actions, and the modified version of the Oxford Centre for Evidence-Based Medicine levels of evidence (2001) for prognostic studies.
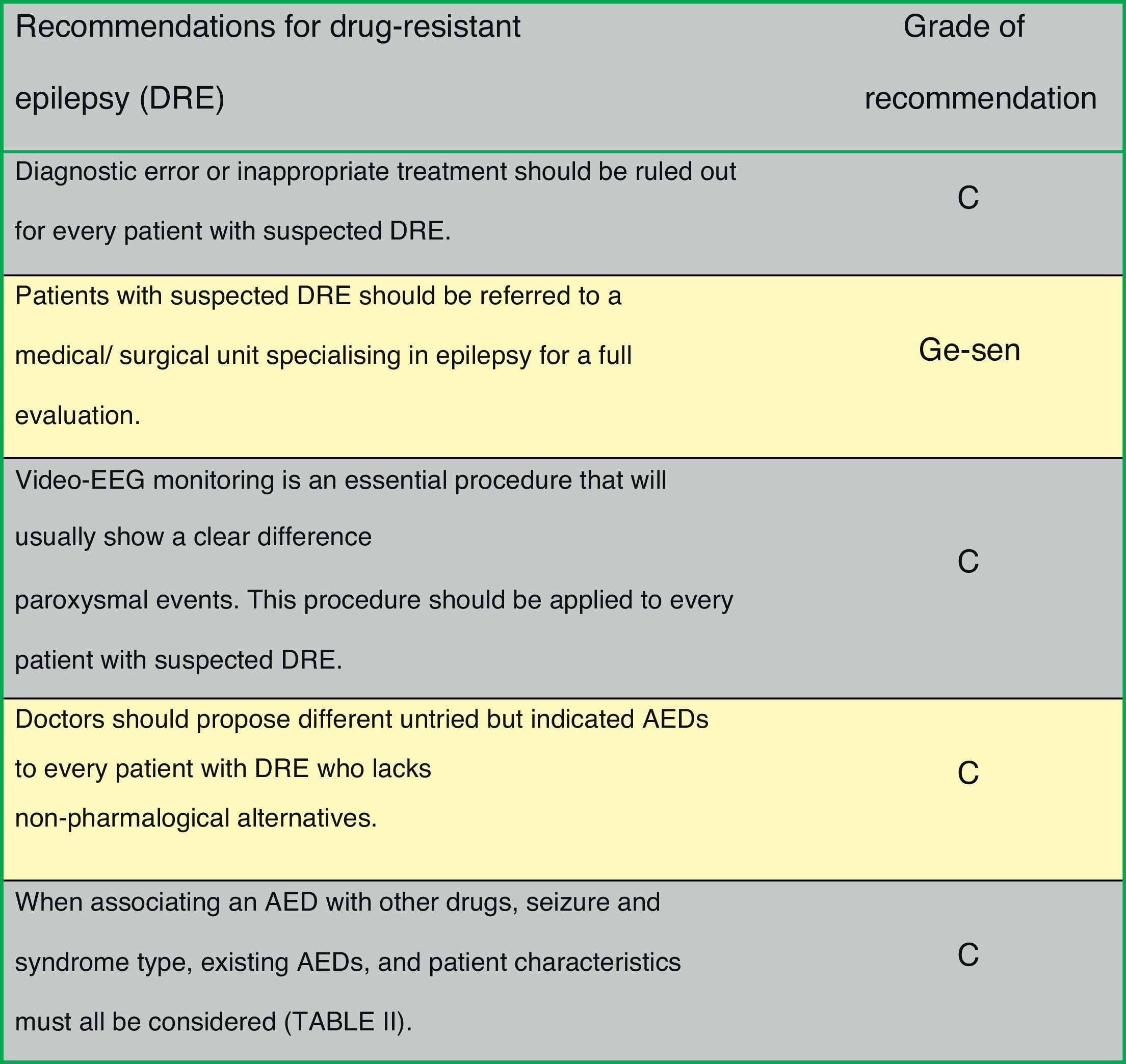
Drug-resistant epilepsyThe International League Against Epilepsy (ILAE) defines drug-resistant epilepsy as “failure of adequate trials of two tolerated, appropriately chosen and used antiepileptic drug schedules (whether as monotherapies or in combination) to achieve sustained seizure freedom”.1 Seizure freedom is considered to be sustained when the patient is seizure-free for more than one year, or has sporadic seizures separated by a period three times the longest interval between seizures prior to the treatment, whichever is longer. About 25% of all patients with epilepsy present drug-resistant epilepsy.2 As a consequence of poor control over their epileptic seizures (ES), they present an increased risk of early death, trauma, and psychosocial alterations, while their quality of life is diminished. Drug-resistant epilepsy may show temporary remission periods (4% of adult cases yearly, with higher rates in children) but ES frequently reappear. Therefore, identifying patients with drug-resistant epilepsy is essential in order to start preparing the presurgical evaluation, and to arrange for possible therapeutic alternatives in specialised units or centres.
Prognostic factors for developing drug-resistant epilepsyThe risk factors associated with a poor prognosis for epilepsy are age-dependent.
- •
In children (prospective study)3: age younger than one year, symptomatic epilepsy, mental retardation or overall developmental delay, pathological neuroimaging study, or a high seizure frequency prior to being diagnosed with drug-resistant epilepsy (level of evidence [LE] II).
- •
In children (prospective study)4: weekly seizures during the first year of treatment, weekly seizures prior to treatment onset, or remote symptomatic epilepsy (LE I).
- •
In adolescents5: focal epilepsy, mental retardation or psychiatric disturbances (LE II).
- •
In adults (prospective study)6: symptomatic focal epilepsy, initial consciousness impairment during seizures, multiple seizure types, tonic-akinetic seizures, or anomalies on the electroencephalogram (EEG) (LE IV).
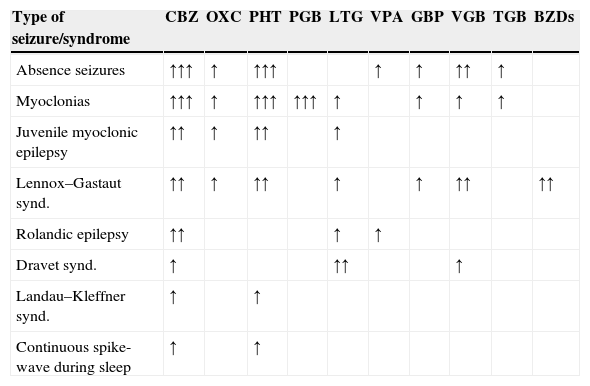
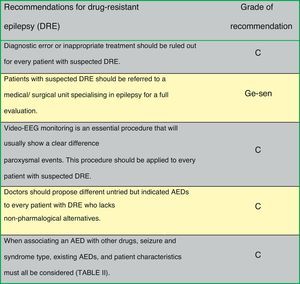
Evaluation of patients with drug-resistant epilepsy in a specialised centre permits exclusion of cases of pseudo-resistance (up to 25% of the patients referred to a specialised centre do not have epilepsy, and their most frequent final diagnoses are syncope, non-epileptic seizures, and sleep disorders).7 Video-EEG monitoring is essential for identifying seizure characteristics. Medical treatment should be optimised since some AEDs are effective against certain types of ES but exacerbate others (Table 1). The patient should receive advice on factors that can make control of ES difficult, such as treatment non-compliance, sleep deprivation, intercurrent diseases, alcohol use, hormonal changes, or drug interactions.8,9
Antiepileptic drugs that can increase seizure frequency or exacerbate seizures in certain types of epilepsy.
| Type of seizure/syndrome | CBZ | OXC | PHT | PGB | LTG | VPA | GBP | VGB | TGB | BZDs |
|---|---|---|---|---|---|---|---|---|---|---|
| Absence seizures | ↑↑↑ | ↑ | ↑↑↑ | ↑ | ↑ | ↑↑ | ↑ | |||
| Myoclonias | ↑↑↑ | ↑ | ↑↑↑ | ↑↑↑ | ↑ | ↑ | ↑ | ↑ | ||
| Juvenile myoclonic epilepsy | ↑↑ | ↑ | ↑↑ | ↑ | ||||||
| Lennox–Gastaut synd. | ↑↑ | ↑ | ↑↑ | ↑ | ↑ | ↑↑ | ↑↑ | |||
| Rolandic epilepsy | ↑↑ | ↑ | ↑ | |||||||
| Dravet synd. | ↑ | ↑↑ | ↑ | |||||||
| Landau–Kleffner synd. | ↑ | ↑ | ||||||||
| Continuous spike-wave during sleep | ↑ | ↑ |
BZD, benzodiazepines; CBZ, carbamazepine; GBP, gabapentin; LTG, lamotrigine; OXC, oxcarbazepine; PGB, pregabalin; PHT, phenytoin; TGB, tiagabine; VGB, vigabatrin; VPA, valproic acid.
There is not enough information for the lastest AEDs: lacosamide (LCM), eslicarbazepine (ESL), retigabine (RTG), perampanel (PER).
Potential for exacerbation: ↑limited; ↑↑moderate; ↑↑↑ significant.
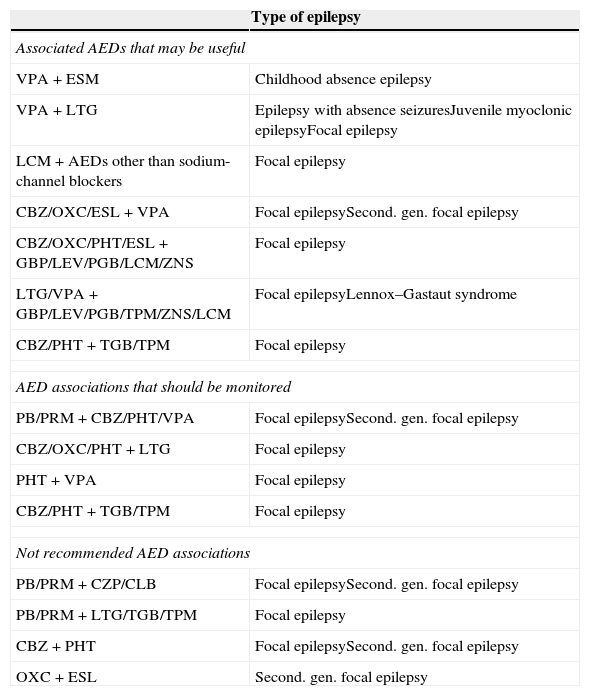
Rational polytherapy is recommended in patients with drug-resistant epilepsy, with the aim of finding AED combinations that increase efficacy (supra-additive effect) and minimise adverse effects (infra-additive effect)10 (Table 2):
- •
Combining AEDs with different action mechanisms to achieve a broad action spectrum intended to cover all types of ES experienced by the patient.11–15
- •
Avoiding AEDs with similar toxicity profiles and adjusting doses according to the patient's characteristics (age, sex, physical condition, and comorbidities).
- •
Considering that using more than 2 AEDs tends to result in more numerous adverse effects rather than a real improvement in seizure control (especially in elderly or polymedicated patients).
- •
Customising treatment for type of ES, type of epilepsy, and even epileptic syndrome by aetiology or gene (avoiding AEDs that block sodium channels in patients with mutations in the sodium channel gene SCN1A that causes Dravet syndrome).
Associated antiepileptic drugs (AED).
| Type of epilepsy | |
|---|---|
| Associated AEDs that may be useful | |
| VPA+ESM | Childhood absence epilepsy |
| VPA+LTG | Epilepsy with absence seizuresJuvenile myoclonic epilepsyFocal epilepsy |
| LCM+AEDs other than sodium-channel blockers | Focal epilepsy |
| CBZ/OXC/ESL+VPA | Focal epilepsySecond. gen. focal epilepsy |
| CBZ/OXC/PHT/ESL+GBP/LEV/PGB/LCM/ZNS | Focal epilepsy |
| LTG/VPA+GBP/LEV/PGB/TPM/ZNS/LCM | Focal epilepsyLennox–Gastaut syndrome |
| CBZ/PHT+TGB/TPM | Focal epilepsy |
| AED associations that should be monitored | |
| PB/PRM+CBZ/PHT/VPA | Focal epilepsySecond. gen. focal epilepsy |
| CBZ/OXC/PHT+LTG | Focal epilepsy |
| PHT+VPA | Focal epilepsy |
| CBZ/PHT+TGB/TPM | Focal epilepsy |
| Not recommended AED associations | |
| PB/PRM+CZP/CLB | Focal epilepsySecond. gen. focal epilepsy |
| PB/PRM+LTG/TGB/TPM | Focal epilepsy |
| CBZ+PHT | Focal epilepsySecond. gen. focal epilepsy |
| OXC+ESL | Second. gen. focal epilepsy |
CLB, clobazam; CZP, clonazepam; ESM, ethosuximide; LEV, levetiracetam; PB, phenobarbital; PRM, primidone; TPM, topiramate; ZNS, zonisamide.
Most authors recommend trying all novel AEDs until achieving control over drug-resistant epilepsy, provided that those drugs are indicated for the patient's ES or epilepsy type.16 A systematic review and meta-analysis of the efficacy of all novel AEDs as adjunctive treatment for drug-resistant epilepsy found total control over ES in about 6% of all patients and a reduction in critical frequency in 21%17 (LE I). Population studies in patients with drug-resistant epilepsy describe long remission periods thanks to successive changes in medication (8%-28%)18,19 (LE III).
Fig. 1 presents the consensus recommendations issued by the authors of the Official Clinical Practice Guidelines for drug-resistant epilepsy.
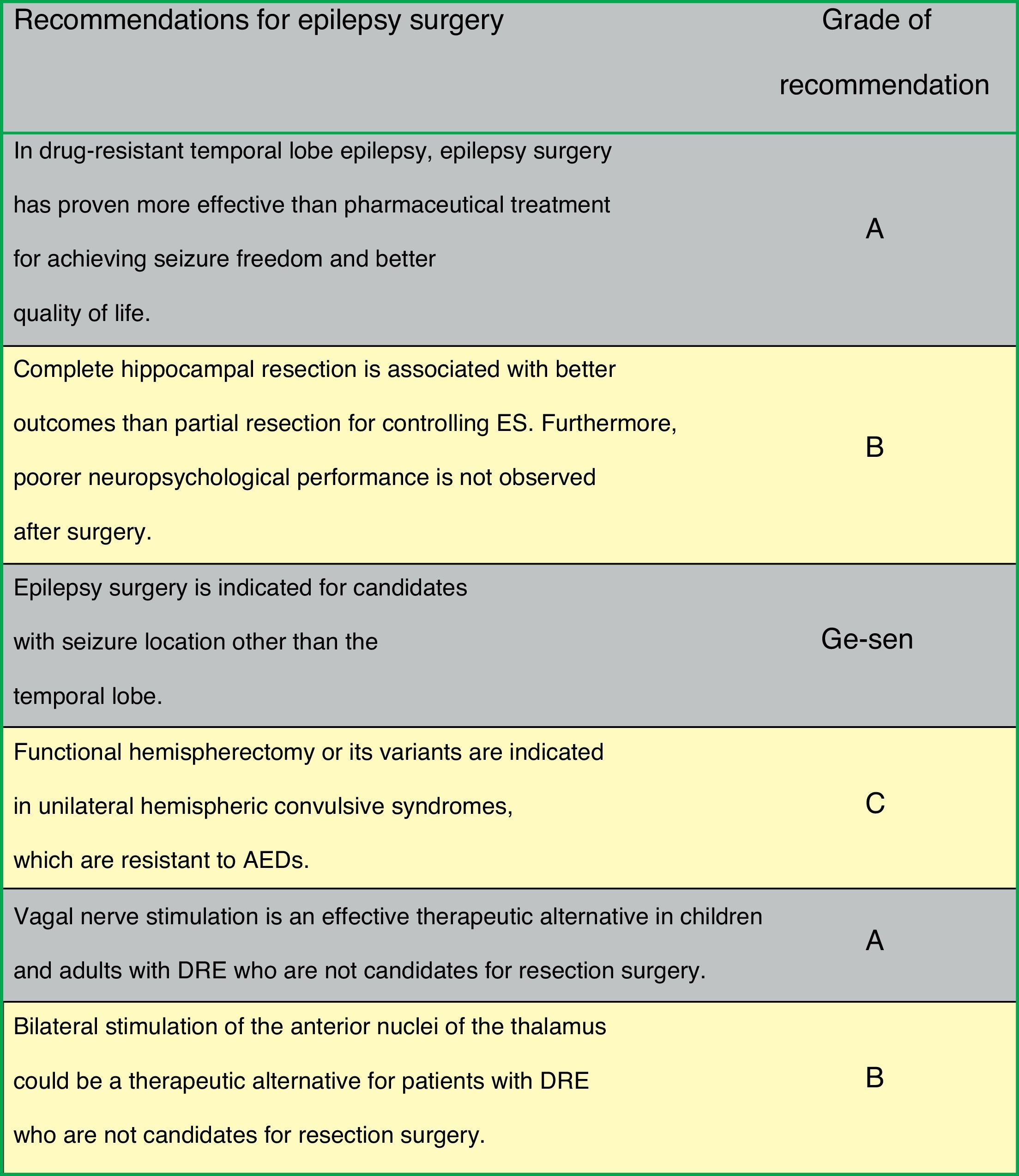
Epilepsy surgeryEpilepsy surgery is aimed at eliminating or reducing ES by excising the epileptogenic zone without causing deficits in the patient (resection surgery). Approximately 5% of all patients may benefit from surgical epilepsy treatment.20 If epilepsy surgery is not possible, disconnective procedures or vagal nerve or brain stimulation will be used.21,22
Indications for surgery include:
- 1.
Patients with drug-resistant epilepsy and seizures that interfere with their daily lives. In children, special attention must be paid to the adverse effects of AEDs.23
- 2.
The progression timeline of epilepsy should have reached at least 2 years, except in patients with life-threatening seizures or in children.
- 3.
Epilepsies that can be treated with surgery: surgically remediable epileptic syndrome (poor control with drugs and good results with surgery), or when surgery can improve epilepsy with acceptable sequelae, in light of the baseline situation and seizure severity.24
Absolute or relative contraindications are listed below.
- •
Age does not represent an absolute contraindication; the risk/benefit ratio in elderly patients should be assessed.
- •
Cause of epilepsy: surgery is not considered when the aetiology is a progressive neurological disease, with the exception of Rasmussen encephalitis.
- •
Concomitant diseases contraindicate surgery if they have significant effects on vital or functional prognosis.
- •
Concomitant psychiatric disorders contraindicate surgery if they may compromise the surgical result or subsequent follow-up.
- •
IQ score below 70 indicates a poorer prognosis, but it is not a contraindication.
Epilepsy surgery currently requires a multidisciplinary team of specialists with specific training, working together in the same unit and equipped with the necessary technological means.25 This team should include epileptologists, neurologists and/or paediatric neurologists, neurosurgeons, structural and functional neuroimaging specialists, neuropsychologists, psychiatrists, nursing staff specifically trained in epilepsy, and EEG technicians. Presurgical evaluation of patients will consist of examinations aimed at identifying the location and extension of the epileptogenic zone, and assessing the potential impact of surgery on the patient's neurological and cognitive functions and the emotional status.26 In addition to clinical assessment, doctors initially order a series of complementary studies: video-EEG, brain MRI with epilepsy protocol, neuropsychological studies, and a psychiatric evaluation. Certain cases will require more specific studies, such as functional MR imaging, Wada test, ictal SPECT, SISCOM images, PET images, magnetoencephalography, electrocorticography, or semi-invasive or invasive electrodes.27 We recommend that the final decision on surgical treatment be made in a meeting of all members of the multidisciplinary team.
Surgically treatable epilepsyEpilepsy treatable with surgical resection procedures- •
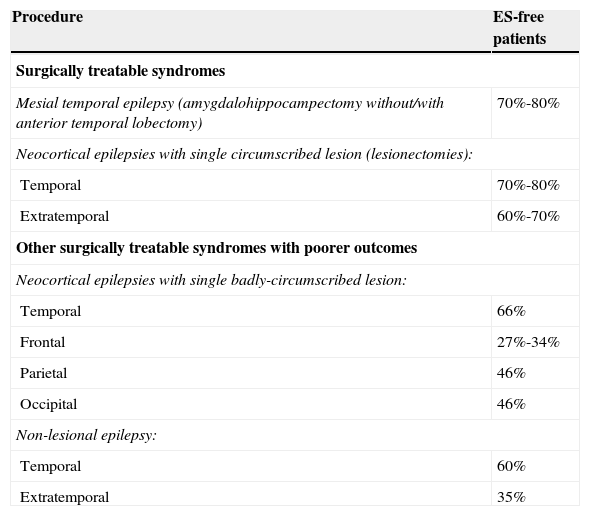
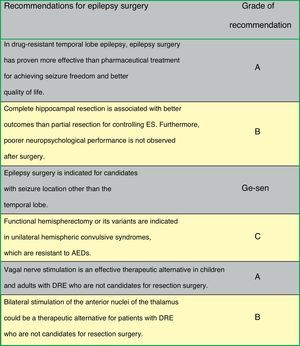
Mesial temporal lobe epilepsy secondary to hippocampal sclerosis. Amygdalohippocampectomy is usually performed along with anterior temporal lobectomy, after which two-thirds of patients normally become seizure free. Surgery and subsequent treatment with AEDs provide better seizure control than medical treatment alone28,29 (LE I). A randomised clinical trial has observed that total hippocampectomy might be associated with a better response regarding ES control, with no increase in associated neuropsychological morbidity30 (LE II). The decision to spare or resect the superior temporal gyrus does not impact surgery outcomes or performance of confrontation naming tasks31 (LE II). Patients with hippocampal sclerosis are generally referred late for surgical assessment, even though early surgery is more effective than AED treatment32,33 (LE II).
- •
Neocortical epilepsies with a single circumscribed lesion. Lesionectomy is the indicated procedure. The epileptogenic zone is located in the immediate vicinity of the structural lesion and MRI scans are usually sufficient to guide surgery. Invasive studies may be necessary to define the limits of the epileptogenic zone. Rates of seizure freedom in series of patients after temporal lobe surgery are similar to those observed in patients treated with temporomesial resection associated with hippocampal sclerosis and slightly higher than results following extratemporal surgery.34 The ILAE recently issued new recommendations for surgery in cases of ES secondary to cavernomas.35
- •
Hemispheric syndromes. Principal types include hemimegalencephaly, Sturge–Weber syndrome, Rasmussen syndrome, and other unilateral catastrophic epilepsies. These syndromes are treated surgically with hemispherectomy or functional hemispherectomy. Some 60% to 80% of patients remain seizure-free after surgery36 (LE III). Candidates are patients with prior hemiparesis and loss of useful function in the hand contralateral to the affected hemisphere.
- •
Other surgically treatable syndromes with poorer outcomes: neocortical epilepsies with no single circumscribed lesion. Surgery involves resecting the neocortical area identified as the zone where the ES begin. It is used in patients with dual pathology, poorly circumscribed lesions and even patients without visible lesions on neuroimaging studies. In epilepsy without lesions, surgery uses the results of functional studies (EEG, SPECT, and PET) as a guide for placing intracranial electrodes. In cases of temporal epilepsy, as many as 60% of all patients attain seizure freedom, but this rate decreases to 35% for extratemporal locations37–39 (LE IV).
Table 3 displays prognostic data for the different surgical procedures.
Results of epilepsy surgery. Seizure-free patients.
| Procedure | ES-free patients |
|---|---|
| Surgically treatable syndromes | |
| Mesial temporal epilepsy (amygdalohippocampectomy without/with anterior temporal lobectomy) | 70%-80% |
| Neocortical epilepsies with single circumscribed lesion (lesionectomies): | |
| Temporal | 70%-80% |
| Extratemporal | 60%-70% |
| Other surgically treatable syndromes with poorer outcomes | |
| Neocortical epilepsies with single badly-circumscribed lesion: | |
| Temporal | 66% |
| Frontal | 27%-34% |
| Parietal | 46% |
| Occipital | 46% |
| Non-lesional epilepsy: | |
| Temporal | 60% |
| Extratemporal | 35% |
Palliative surgery includes procedures aimed at reducing ES so as to improve patients’ quality of life.
- •
Multiple subpial resection. Today this procedure is used exclusively in eloquent areas in such disorders as Landau–Kleffner syndrome. Up to 55% of patients are seizure-free, and 4% display permanent deficits.26
- •
Corpus callosotomy. Partial or total sectioning of the corpus callosum. This procedure is used especially in case of atonic seizures, with 70% of the patients treated surgically displaying seizure reduction.40
- •
Gamma knife radiosurgery. This technique consists of the destruction of a small volume of brain tissue by applying a high dose of radiation (gamma particles emitted by radioactive cobalt). This radiation is applied stereotactically in a single session and any radiation reaching surrounding areas is minimal. It is used in selected patients with mesial temporal epilepsy, hypothalamic hamartoma-related epilepsy, and cavernoma-related epilepsy.
- •
Deep brain stimulation. Deep brain stimulation is based on the potentially regulating role played by subcortical structures in ES genesis and transmission. Stimulation has been targeted at the cerebellum, thalamic and subthalamic nuclei, caudate nucleus, hypothalamic nucleus, and substantia nigra. The SANTE study, which used bilateral stimulation of the anterior nuclei of the thalamus, showed a 56% median reduction in seizure frequency, and 54% of patients had a seizure reduction of at least 50%41 (LE II).
- •
Vagus nerve stimulation. This procedure consists of periodical stimulation of the vagus nerve by placing helical bipolar stimulating leads around the cervical portion of the neck. A wire connects the leads to a pulse generator located on the thoracic wall. The technique reduces ES frequency in patients with drug-resistant epilepsy who are not candidates for resection surgery.42–44 A recently published meta-analysis reviewing the published studies with a level of evidence of I, II, and III, and including a total of 3321 patients, showed a median ES reduction of 44.6% after a mean follow-up period of 10 months. Seizure frequency decreased significantly by the first year of follow-up after surgery (51% if >1 year, P<.001). Of the patient total, 50.6% experienced an ES reduction of >20% (Engel classes I-III), with 4.6% of patients becoming seizure-free45 (LE I). In patients younger than 6 years, ES reduction was even higher (62.0%). ES reduction reached 47.8% in 93 patients with Lennox–Gastaut syndrome.45 It has also been proved effective and well-tolerated in patients with other epileptic encephalopathies, such as Dravet syndrome, and epilepsy with myoclonic-astatic seizures46 (LE IV). Vagus nerve stimulation is now used for more epilepsy types than focal epilepsy, its initial indication. It has also been used in idiopathic generalised epilepsy, patients with repeated episodes of status epilepticus, and in elderly patients47–49 (LE IV).
- •
Trigeminal nerve stimulation. This technique has been recently approved for use in the European Union as adjunctive treatment for drug-resistant epilepsy in adults and children older than 9 years. In a randomised controlled trial, 40.5% of the patients responded to treatment after a follow-up period of 18 weeks.50
Fig. 2 lists the therapeutic actions after a first ES as recommended by consensus of the authors of the guidelines on surgery for epilepsy.
Other non-surgical proceduresKetogenic dietThe ketogenic diet aims to replace carbohydrates with lipids. Metabolism of fatty acids generate ketone bodies (KB) (beta-hydroxybutyric acid, acetoacetic acid, and acetone to a lesser extent), which become the source of energy for the brain. Ketogenic diet promotes synthesis of glutamine (precursor of gamma-aminobutyric acid, an inhibitory neurotransmitter), which is structurally similar to GABA and would therefore have a direct antiepileptic effect. This treatment can be beneficial for children,51,52 and it is the treatment of choice in cases of pyruvate dehydrogenase complex deficiency and lack of glucose transporter (GLUT-1). The treatment should be started at an early stage to allow the brain to use KB as an energy source53 (LE III). In Lennox–Gastaut syndrome, ketogenic diet will be especially effective for reducing myoclonic and atonic seizure, and results in seizure freedom in up to 16% of these patients54 (LE III). Current data suggest that this diet may have a similar effect in adults and in children.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: López González FJ, Rodríguez Osorio X, Gil-Nagel Rein A, Carreño Martínez M, Serratosa Fernández J, Villanueva Haba V, et al. Epilepsia resistente a fármacos. Concepto y alternativas terapéuticas. Neurología. 2015;30:439–446.