Guillain-Barré syndrome, an acute polyradiculoneuropathy that presents with weakness and areflexia, is the most common cause of acute flaccid paralysis. In certain patients, respiratory failure is secondary to this disorder, eventually causing patients to require mechanical ventilation and experience additional complications due to diminished respiratory support and related mobility limitations. Prognoses for most of these cases are positive; treatment consists of basic support combined with plasmapheresis or administration of immunoglobulins.
ObjectiveThis study sought to describe the socio-demographic, clinical, laboratory and neurophysiological characteristics of patients with Guillain-Barré syndrome who were hospitalised in the Intensive Care Unit of the Neurological Institute of Colombia between 2006 and 2012.
MethodologyThis study presents a case series.
ResultsWe surveyed 25 patients (32% female and 68% male) with Guillain-Barré syndrome and an average age of 54 years. Sixty per cent of these patients were admitted between days 3 and 7 after symptom onset; 64% had a history of respiratory infection and 20% had a history of intestinal infection. In addition, 84% of the patients presented with albuminocytological dissociation. We observed the following clinical subtypes of Guillain-Barré syndrome: inflammatory demyelinating polyneuropathy in 32%, acute motor-sensory axonal neuropathy in 28%, acute motor axonal neuropathy in 28%, and Miller Fisher syndrome in 12%.
ConclusionsIn this descriptive study of a group of critical care patients with GBS, results depended on patients’ clinical severity at time of admission. Our findings are similar to results published in the international literature.
El síndrome de Guillain-Barré (SGB) es la causa más común de parálisis flácida aguda. En algunos pacientes ocurre falla ventilatoria secundaria a este trastorno, con complicaciones secundarias al soporte ventilatorio y a la movilidad reducida. La mayoría de los casos tienen buen pronóstico; el tratamiento se hace con plasmaferésis o inmunoglobulinas, además del soporte básico.
ObjetivoDescribir las características sociodemográficas, clínicas, de laboratorio y electrofisiológicas de los pacientes con SGB hospitalizados en las Unidades de Cuidado Intensivo (UCI) y Cuidado Especial del Instituto Neurológico de Colombia entre 2006 y 2012.
MetodologíaPresentación de serie de casos.
ResultadosPresentamos a 25 pacientes con SGB; el 68% de los pacientes fueron hombres, con una edad promedio de 54 años. El 60% de los pacientes ingresó entre los días 3 y 7 del inicio del cuadro, el 64% tuvo antecedente de infección respiratoria y el 20% de infección intestinal 20%. La mayoría de los pacientes (84%) presentó disociación albúmino-citológica. El 32% se presentó con polineuropatía inflamatoria desmielinizante aguda, el 28% con polineuropatía axonal motora y sensitiva aguda, el 28% con polineuropatía axonal motora aguda y el 12% con síndrome de Miller-Fisher. Los pacientes de UCI presentaron mayor tiempo de estancia hospitalaria, infecciones y un peor desenlace medido por Rankin modificado al mes.
ConclusionesEsta descripción corresponde a un grupo de pacientes críticos con SGB; su desenlace estuvo determinado por la severidad del cuadro clínico al ingreso. Nuestros hallazgos son comparables con lo publicado en la literatura mundial.
Guillain-Barré syndrome (GBS) is an acute polyneuropathy which affects approximately 0.4 to 4 people per 100000 per year. It has a bimodal distribution, presenting more frequently among young adults and elderly patients; however, most published studies have reported higher rates of GBS only in the population older than 50 years. In two thirds of the patients, symptom onset is preceded by an infection.1 Cross-reaction between antibodies against microbial antigens and neuronal molecules has therefore been suggested as a possible mechanism of autoimmune damage,2 since it would affect nerve conduction and result in motor, sensory, or autonomic symptoms.1,3–5
Diagnosis of GBS is essentially clinical. Increased protein levels in CSF without pleocytosis (albuminocytologic dissociation) support a diagnosis of GBS. However, we should not overlook that this finding may be absent in up to 50% of patients in the first week after symptom onset, and in up to 25% during the third week.6 Therefore, normal CSF findings do not rule out GBS, especially if the analysis is performed in the first few days. Electrodiagnostic studies (nerve conduction studies and electromyography) help doctors classify the GBS type according to the pattern of neuronal damage as one of the following electrophysiological variants: acute inflammatory demyelinating polyradiculoneuropathy (AIDP), acute motor axonal neuropathy (AMAN), and acute motor and sensory axonal neuropathy (AMSAN). These subtypes are associated with different outcomes and degrees of severity.7 AIDP is the most common variant of GBS in the USA and Europe,8 whereas in such countries as China, Japan, and Mexico, the axonal forms account for 65% of the cases.9
Miller Fisher syndrome (MFS) is a rare clinical variant of GBS characterised by ophthalmoplegia, ataxia, and areflexia. Its pathogenesis is associated with the presence of IgG antibodies to ganglioside GQ1b, which can be found in more than 80% of all cases. Electrophysiological abnormalities are variable and usually mild, except for absence of soleus H-reflex, a fairly consistent finding in patients with MFS which may therefore constitute a reliable diagnostic indicator of the disease.10
Nearly 50% of all patients need to be hospitalised in an intensive care unit (ICU), mainly because of respiratory disease (in 17% to 30%) and autonomic involvement (20%).11,12 Several clinical factors have been associated with a higher risk of requiring mechanical ventilation: rapidly progressing symptoms, simultaneous onset of weakness in the upper and lower limbs, brachial diplegia (3/5 or lower), cephaloplegia, facial weakness, and bulbar involvement. On the contrary, preservation of deep tendon reflexes in the upper limbs was associated with a lower risk of requiring mechanical ventilation.13
Several factors have been associated with respiratory insufficiency in these patients. A prospective study14 of 154 patients with GBS found that the independent risk factors for mechanical ventilation were decreased forced vital capacity and the proximal/distal compound muscular amplitude potential ratio of the common peroneal nerve. Another prospective study of 397 patients found that the main indicators of respiratory insufficiency were a shorter time interval between onset of weakness and admission (faster disease progression), more pronounced motor involvement, and presence of facial and/or bulbar weakness.15
Hyponatraemia secondary to syndrome of inappropriate antidiuretic hormone secretion (SIADH) is frequent in patients with GBS and has been associated with poor outcomes. In a prospective study of 50 patients with GBS, 48% had SIADH, most of them moderate to severe. The patients with SIADH showed higher rates of bulbar weakness, respiratory insufficiency, mortality, and long-stay hospitalisation, as well as more severe motor impairment at discharge.16
During ICU hospitalisation, these patients have an increased risk of such complications as pneumonia (54%), sepsis (24%), cardiac arrhythmias (22%), ileus and/or intestinal perforation (17%), deep venous thrombosis, pulmonary thromboembolism, gastrointestinal bleeding, and pseudomembranous colitis, among others. They also remain functionally dependent for extended periods, and the mortality rate among patients requiring mechanical ventilation (8.5%) exceeded the average mortality rate of all patients with GBS at one year of follow-up (5-6.5%).17
Pharmacological treatment for GBS is based on immunomodulation, which includes 2 options: plasmapheresis,18 aimed at removing circulating antibodies and complement, and immunoglobulin,19 which interferes with T and B cell activation, modulates the expression of Fc receptors, and inhibits complement activation, among other proposed action mechanisms.20 While the benefits of both therapies have already been demonstrated by placebo-controlled studies, current evidence is not sufficient to support either one as superior to the other in terms of efficacy and cost-effectiveness. Treatment decisions should be based on such other variables as availability of treatment, safety profile of the treatment in each clinical context, and the experience of the medical team.21
Materials and methodsWe conducted a retrospective observational study between 2006 and 2012 which included all the patients admitted to either the ICU or the special care unit (SCU) with a diagnosis of acute polyneuropathy, GBS, or MFS.
Admission to one critical care unit or the other is determined based on severity. The ICU manages patients with indicators of respiratory insufficiency who will require mechanical ventilation and/or invasive monitoring, whereas the SCU manages those patients with a lower degree of severity but whose risk of haemodynamic or respiratory impairment is beyond the scope of a general admission ward.
We assessed the following variables: demographic, clinical, laboratory, and electrophysiological characteristics; score on the Hughes GBS disability scale1; respiratory involvement; treatment; complications (dysautonomia, infection, hyponatraemia); and short-term prognosis (at 1 and 3 months) measured with the modified Rankin Scale (mRS).
Results describe our patients’ characteristics broken down by level of severity and the critical care unit where they were treated. We used SPSS statistical software, version 21, for data processing and analysis.
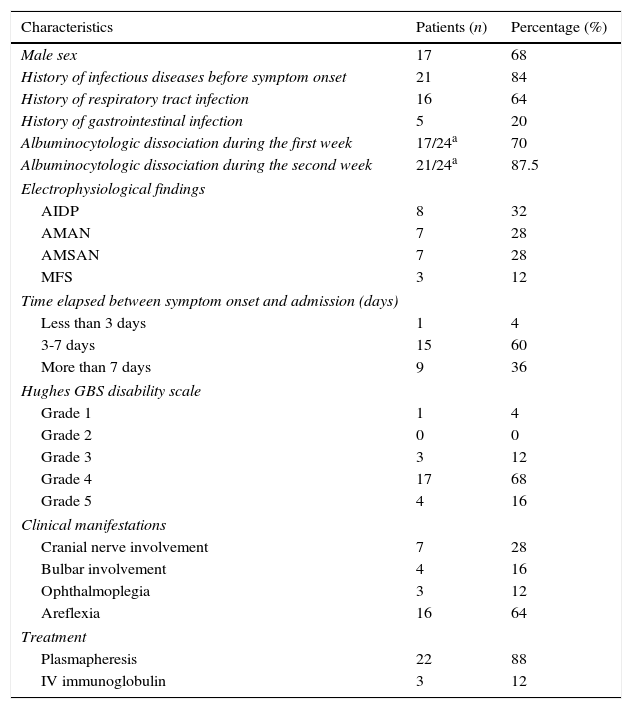
ResultsA total of 25 patients with a diagnosis of GBS were admitted to the ICU and SCU between January 2006 and December 2012. Most of the patients were men (68% vs 32% women), with a mean age±SD of 54±19.7 years. Eighty-four percent of the patients had an infectious disease prior to onset of neurological symptoms. Baseline characteristics of our patients are listed in Table 1. Table 2 shows patients’ characteristics broken down by unit of admission (ICU or SCU).
Patient characteristics at baseline.
| Characteristics | Patients (n) | Percentage (%) |
|---|---|---|
| Male sex | 17 | 68 |
| History of infectious diseases before symptom onset | 21 | 84 |
| History of respiratory tract infection | 16 | 64 |
| History of gastrointestinal infection | 5 | 20 |
| Albuminocytologic dissociation during the first week | 17/24a | 70 |
| Albuminocytologic dissociation during the second week | 21/24a | 87.5 |
| Electrophysiological findings | ||
| AIDP | 8 | 32 |
| AMAN | 7 | 28 |
| AMSAN | 7 | 28 |
| MFS | 3 | 12 |
| Time elapsed between symptom onset and admission (days) | ||
| Less than 3 days | 1 | 4 |
| 3-7 days | 15 | 60 |
| More than 7 days | 9 | 36 |
| Hughes GBS disability scale | ||
| Grade 1 | 1 | 4 |
| Grade 2 | 0 | 0 |
| Grade 3 | 3 | 12 |
| Grade 4 | 17 | 68 |
| Grade 5 | 4 | 16 |
| Clinical manifestations | ||
| Cranial nerve involvement | 7 | 28 |
| Bulbar involvement | 4 | 16 |
| Ophthalmoplegia | 3 | 12 |
| Areflexia | 16 | 64 |
| Treatment | ||
| Plasmapheresis | 22 | 88 |
| IV immunoglobulin | 3 | 12 |
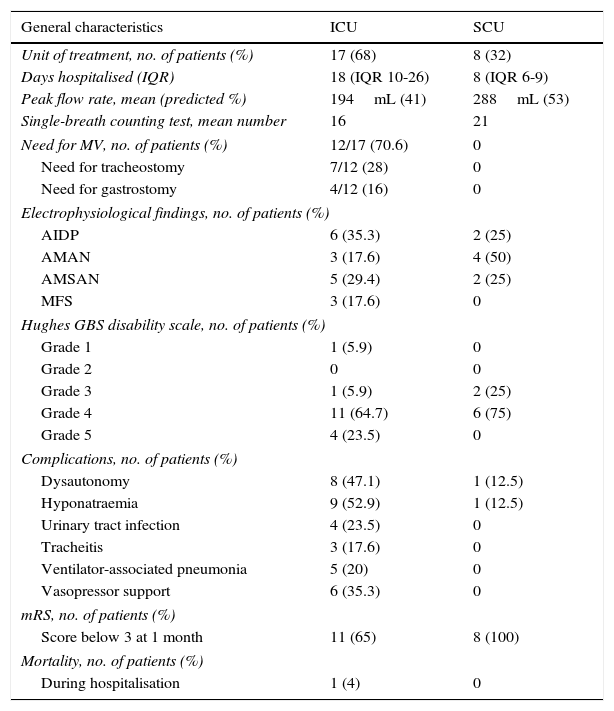
Patient characteristics by critical care unit.
| General characteristics | ICU | SCU |
|---|---|---|
| Unit of treatment, no. of patients (%) | 17 (68) | 8 (32) |
| Days hospitalised (IQR) | 18 (IQR 10-26) | 8 (IQR 6-9) |
| Peak flow rate, mean (predicted %) | 194mL (41) | 288mL (53) |
| Single-breath counting test, mean number | 16 | 21 |
| Need for MV, no. of patients (%) | 12/17 (70.6) | 0 |
| Need for tracheostomy | 7/12 (28) | 0 |
| Need for gastrostomy | 4/12 (16) | 0 |
| Electrophysiological findings, no. of patients (%) | ||
| AIDP | 6 (35.3) | 2 (25) |
| AMAN | 3 (17.6) | 4 (50) |
| AMSAN | 5 (29.4) | 2 (25) |
| MFS | 3 (17.6) | 0 |
| Hughes GBS disability scale, no. of patients (%) | ||
| Grade 1 | 1 (5.9) | 0 |
| Grade 2 | 0 | 0 |
| Grade 3 | 1 (5.9) | 2 (25) |
| Grade 4 | 11 (64.7) | 6 (75) |
| Grade 5 | 4 (23.5) | 0 |
| Complications, no. of patients (%) | ||
| Dysautonomy | 8 (47.1) | 1 (12.5) |
| Hyponatraemia | 9 (52.9) | 1 (12.5) |
| Urinary tract infection | 4 (23.5) | 0 |
| Tracheitis | 3 (17.6) | 0 |
| Ventilator-associated pneumonia | 5 (20) | 0 |
| Vasopressor support | 6 (35.3) | 0 |
| mRS, no. of patients (%) | ||
| Score below 3 at 1 month | 11 (65) | 8 (100) |
| Mortality, no. of patients (%) | ||
| During hospitalisation | 1 (4) | 0 |
Of the patient total, 60% visited the hospital between 3 and 7 days after symptom onset. Upon their admission to the ICU, we assessed the degree of motor impairment on the Hughes functional grading scale: 17 patients (68%) had grade 4; 4 patients (16%) had grade 5; 3 patients (12%) had grade 3, and only one patient (4%) had the lowest degree of motor impairment. This last patient was admitted due to involvement of the lower cranial nerves.
Seventeen patients (68%) were admitted to the ICU and 8 patients (32%) to the SCU. The reasons for admission to the critical care units were respiratory failure (60%), need for orotracheal intubation (16%), and need for plasmapheresis (20%).
Albuminocytologic dissociation was detected in 21 patients (87.5%) during the second week of progression, 3 patients (12%) showed no albuminocytologic dissociation, and CSF analysis was not performed for one patient. CSF tests completed during the first week showed no albuminocytologic dissociation in 4 patients; however, dissociation was observed in all follow-up analyses completed during the second week. One of the 3 patients showing no dissociation underwent CSF testing during the first week only (no follow-up CSF analyses were performed); the other 2 patients repeated CSF tests during the second week (on day 15 in a patient with MFS and on day 10 in a patient with AMAN).
Regarding GBS subtypes according to electrodiagnostic findings, the axonal forms were the most common variants: 7 patients (28%) had AMAN and another 7 patients had AMSAN. Eight patients (32%) had ADIP and 3 patients (12%) MFS. Tests for anti-GQ1b antibodies yielded positive results in 2 patients.
Twelve patients (48% of the total sample) required mechanical ventilation; this figure corresponds to 70.6% of the patients admitted to the ICU. Of these patients, 6 (35.3%) were already intubated upon admission to the unit, 3 were intubated immediately upon admission, and the remaining 3 required intubation on their first day after admission. Average peak flow rate in these patients was 194mL, which corresponds to 41% of the predicted value, and patients were able to count to a mean of 16 in the single-breath counting test. Mean hospitalisation time in these units was 11 days (IQR: 8-20): 18 days in the ICU (IQR: 10-26), and 8 days in the SCU (IQR: 6-9). Hospitalisation times were longer for patients who required ventilatory support, averaging 19 days (IQR: 13-37).
Blood sodium level was measured at admission; 40% of the patients had hyponatraemia (sodium levels below 135mOsm/L), and the mean sodium level was 126.87±7.5mOsm/L. Only one patient with hyponatraemia (12.5%) was admitted to the SCU while 9 patients (52.9%) with this disorder were admitted to the ICU; 72.7% of the patients with hyponatraemia required mechanical ventilation.
Patients admitted to the ICU presented complications related to hospitalisation: 5 patients (20%) presented ventilator-associated pneumonia, 4 patients (23.4%) had urinary tract infections, 3 patients (17.6%) had tracheitis, 8 (47.1%) presented dysautonomia, and 6 patients (35.6%) required vasopressor support. Only one of the patients admitted to the SCU presented dysautonomia (12.5%). Plasmapheresis was the most frequent treatment (22 patients); only 3 patients received immunoglobulin.
One month after discharge, 72% of the patients had an mRS score of 3 or lower. The percentage of patients with an mRS score of 3 or lower one month after discharge was higher in the SCU group than in the ICU group (100% vs 64%). All patients who underwent a follow-up assessment at 3 months (a total of 17) showed an mRS score of 3 or lower.
One patient (4%) died due to ventilator-associated pneumonia in the ICU: a 77-year-old woman with severe axonal damage.
DiscussionOur study describes a subgroup of patients with severe GBS who were admitted to neurointensive care units (ICU and SCU).
In a previous study conducted in Colombia including a series of 46 patients with GBS, 50% of the total sample were admitted to an ICU. Of these patients, 54% were women and 71.11% showed albuminocytologic dissociation; regarding electrophysiological patterns, 24 had ADIP, 14 AMAN, and 5 AMSAN. Three patients died.22
In our study sample, GBS was more frequent in men (68%) and in the sixth decade of life (mean age, 52.8 years). Our findings are in line with the results published by Sejvar et al.23 According to their meta-analysis of studies from North America and Europe, GBS incidence increased with age and the disease was more frequent in men.
A history of respiratory tract infection was found in 64% of the patients, whereas a history of gastrointestinal infection was found in 20%. Another review article including studies of North American and European populations24 found similar results: up to 53% of the patients had a history of upper respiratory tract infection, and between 6% and 26% had a history of gastrointestinal infection.
Regarding the electrophysiological classification, we found more cases of the axonal variants (AMAN and AMSAN), which were identified in 56% of the patients, followed by AIDP in 32%. MFS was diagnosed in 12% of the cases. Our results agree with other studies stating that the axonal forms of GBS are more frequent in South and Central America, Japan, and China.24
Albuminocytologic dissociation has been reported in up to 50% of all patients with GBS during the first week, and up to 75% during the third week1; in our study. albuminocytologic dissociation was found in 70% of the patients during the first week, and 87.5% during the second week. Performing CSF analyses to detect albuminocytologic dissociation 7 days after symptom onset is undoubtedly essential; this test is of great clinical value, especially if conducting electrophysiological studies at early stages is not an option.
Mean time in the ICU in our case series was 21 days and 48% of the patients required mechanical ventilation, including 4 (20%) presenting ventilator-associated pneumonia. These results are similar to those previously reported in the international literature. In the group of 114 patients studied by Henderson et al.,11 mean time in the ICU was approximately 30 days and 53% of the patients required mechanical ventilation. Eighty-two per cent of the patients presented complications, mainly pneumonia and tracheobronchitis associated with mechanical ventilation (50%) and sepsis (20%). Another study17 described a group of 76 patients with GBS who required ICU stays lasting a mean time of 21 days, and 78% of them required invasive respiratory support.
In our sample, the patients with GBS who required intubation were those with higher severity levels at admission, hyponatraemia, and those with low peak flow rates compared to the predicted value and who were unable to count to 20 in one breath. This coincides with the results of some prospective studies identifying such independent risk factors for mechanical ventilation as reduced forced vital capacity,14 shorter times between onset of weakness and hospital admission, greater severity at admission (assessed with the Medical Research Council sum score2), and presence of facial and/or bulbar weakness.15
Other complications detected during ICU stays were dysautonomia (47.1%) and hyponatraemia (52.9%). In our sample, patients with hyponatraemia had a mean sodium level of 126.87±7.5mOsm/L; 28% of these patients had sodium levels below 133mOsm/L, which is within the range described in previous studies. Hyponatraemia (usually mild to moderate) in these studies affected between 25%11 and 46% of the patients with GBS admitted to the ICU.17 As reported by earlier studies,16 hyponatraemia appeared to predict poor outcome given that 72.7% of the patients with this disorder required mechanical ventilation.
Plasmaphereris was the treatment of choice in 22 patients (88%); the remaining 3 patients, who had MFS, were treated with immunoglobulin. The reason for this choice is that plasmapheresis is more readily available in our hospital, and immunoglobulin therapy is therefore reserved for specific cases, such as patients with MFS. Retrospective studies have shown that immunoglobulin therapy reduces symptom duration somewhat in these patients.25
According to different studies, mortality rates range from 4% to 12.1%17,26,27; our results are within this range (4%). A prospective study of a cohort of 527 patients with GBS found a low mortality rate (2.5%) after 6 months of follow-up. However, the authors mentioned that this low mortality rate was likely due to the fact that most of their patients’ clinical symptoms were not severe enough for ICU admission. Most of the deaths occurred during the recovery phase. Risk factors mentioned by these authors included age (older than 40 years), severe weakness at admission, use of mechanical ventilation, longer delays between onset of weakness and admission, and time to peak disability.28 These same factors were also present in the patient in our series who died.
ConclusionGuillain-Barré syndrome is an important cause of acute disability and a significant percentage of the patients affected may require mechanical ventilation and treatment in an ICU. Several factors have been related to the course of the disease and are able to predict better or poorer clinical outcomes.
The aim of our study was to describe certain clinical, laboratory, and electrophysiological findings in a specific population of patients with GBS who required treatment in neurointensive care units, and to report the treatment used and the main complications.
The factors we identified as being related to respiratory failure and need for intubation were higher severity levels at admission, hyponatraemia, and low peak flow rate compared to the predicted value and inability to count to 20 in one breath. The mortality rate was low (4%) and 100% of the patients evaluated at 3 months had a positive functional outcome according to the modified Rankin scale.
FundingThis study received no funding from any sources.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: González P, García X, Guerra A, Arango JC, Delgado H, Uribe CS, et al. Experiencia del síndrome de Guillain-Barré en una Unidad de Cuidados Intensivos neurológicos. Neurología. 2016;31:389–394.
Functional scale with grades of 0 (healthy), 1 (symptomatic but capable of running), 2 (incapable of running), 3 (unable to walk without assistance), 4 (confined to bed), 5 (requiring assisted ventilation), and 6 (dead).