Although Guillain-Barré syndrome is rare, a marked increase in incidence was observed during the 2016 Zika outbreak in the Americas, with no direct causal relationship being apparent in all cases.
MethodsCases of febrile exanthematous disease and acute flaccid paralysis were reviewed during the period from 1 August to 15 December, 2016; clinical and biochemical assessment was performed for each case to identify Zika-related Guillain-Barré syndrome.
ResultsDuring the study period, we identified 8 patients (6 men and 2 women) with Zika-related Guillain-Barré syndrome, with the most affected age group being 50-59 years. The highest incidence was in the month of September, with 7 cases. The most frequent symptoms were rash and conjunctivitis, followed by fever. The most common presentation was symmetrical ascending paralysis, present in 7 cases. Cranial nerve VII was the most frequently involved nerve. Only 2 patients presented albuminocytologic dissociation. RT-PCR returned negative results for Zika virus in all patients. All patients received intravenous immunoglobulin and all had a good prognosis. None warranted mechanical ventilation. No deaths were recorded. The cumulative incidence rate was 3.9%, monthly incidence was between 0.04 and 2 cases/month.
ConclusionsCases of Guillain-Barré syndrome increased during the Zika outbreak, with an increase in incidence and number of cases per month; however, a direct causal relationship between the 2 conditions could not be demonstrated.
El síndrome de Guillain-Barré es poco frecuente, sin embargo, durante la oleada de zika en América se vio un marcado incremento en la incidencia del síndrome, sin poder denotarse una relación causal directa en todos los casos.
MétodosSe revisaron los casos de enfermedad febril exantemática y parálisis flácida aguda durante el periodo del 1 de agosto a 15 de diciembre del 2016, realizando caracterización clínica y bioquímica de cada caso para ser incluidos como síndrome de Guillain-Barré asociado a zika.
ResultadosDurante el periodo comentado, se encontró una cohorte de 8 pacientes, 6 hombres y 2 mujeres, grupo etario más afectado: 50-59 años. La mayor incidencia fue en el mes de septiembre, con 7 casos. Los síntomas más frecuentes fueron exantema y conjuntivitis, seguidos de fiebre. La presentación más común fue parálisis simétrica ascendente con 7 casos. La afectación de pares craneales más frecuente fue la del vii par craneal. Solo 2 pacientes presentaron disociación albumino-citológica. Todos presentaron PCR-RT negativa a zika. Todos recibieron inmunoglobulina por vía intravenosa y todos tuvieron buen pronóstico. Ninguno ameritó ventilación mecánica. No se registraron defunciones. La tasa de incidencia acumulada es del 3,9%, la incidencia de casos/mes fue de 0,04 casos/mes a 2 casos/mes.
ConclusionesExiste un incremento en los casos de Guillain-Barré durante la oleada de zika, con un incremento en la incidencia y el número de casos/mes; sin embargo, no se pudo demostrar relación causal directa entre estas 2 entidades.
Landry-Guillain-Barré-Strohl syndrome is an acute autoimmune polyradiculopathy in which the peripheral nerves and nerve roots of the spinal cord are affected by molecular mimicry between microbial antigens and nerve antigens. The genetic and environmental factors that increase the likelihood of developing the syndrome remain unknown. The disorder usually manifests days or weeks after the onset of respiratory or gastrointestinal symptoms of viral infection; on occasion, it may be triggered by pregnancy, surgery, or vaccination. The World Health Organization and the Pan American Health Organisation have issued guidelines containing specific standards for the diagnosis and treatment of the syndrome, especially for cases associated with Zika.1,2
The following microorganisms have been detected in patients with Guillain-Barré syndrome (GBS) and history of infection: Campylobacter jejuni (20-50%), cytomegalovirus (5-22%), Haemophilus influenzae (2-13%), Epstein–Barr virus (10%), and Mycoplasma pneumoniae (5%). Other less frequent infectious diseases include Lyme disease; hepatitis A, B, C, D, and E; typhoid fever; dengue; influenza A; Zika virus disease; and human immunodeficiency virus infection. Other factors have also been reported, including surgeries, vaccines, and trauma.3
Before the advent of the new arboviruses (chikungunya and Zika), dengue sporadically caused acute neurological symptoms of GBS4,5; however, during the 2014 and 2015 outbreaks, the incidence of neurological symptoms increased, mainly as a result of Zika virus infection. Zika is currently considered a neurotropic virus, as it has caused cases of Zika-associated congenital syndrome (predominantly microcephaly) and GBS.6,7
Between 1 April 2015 and 31 March 2016, 164237 confirmed and suspected cases of Zika virus infection and 1474 cases of GBS were reported in Bahia (Brazil), Colombia, the Dominican Republic, El Salvador, Honduras, Suriname, and Venezuela. Our analysis suggests that the changes in the reported incidence of Zika virus disease in 2015 and early 2016 are closely related to changes in the incidence of GBS. During the weeks of Zika transmission, there were significant increases in the incidence of GBS as compared to the previous incidence rate, with increases of 172% in the state of Bahia, 211% in Colombia, 150% in the Dominican Republic, 100% in El Salvador, 144% in Honduras, 400% in Suriname, and 877% in Venezuela. Incidence of GBS was reported at 28%.4,8,9
Despite the higher incidence of GBS during the Zika virus outbreak, Zika virus infection could not be always confirmed by serology. The only study that reported serological confirmation was performed between 2013 and 2014 in French Polynesia; in that study, 41 out of 42 patients with GBS (98%) presented positive serological findings for Zika.4 A Colombian study identified 68 patients with GBS, 42 of whom underwent serological testing; 17 patients (40%) presented positive reverse transcriptase polymerase chain reaction (RT-PCR) for Zika virus.9 In the Brazilian state of Bahia, 42 of the 76 patients identified with suggestive symptoms presented GBS; of them, only 10 tested positive for Zika virus. In Rio de Janeiro (Brazil), incidence was reported to increase from 0.67 cases/month to 5.4 cases/month.5,10
ObjectiveThe aims of our study are to identify the incidence of GBS during the 2016 Zika virus outbreak, to characterise the cohort of patients by using clinical and biochemical analyses and imaging studies, and to identify potential causes of each case and analyse the direct relationship with Zika virus.
DesignWe performed a retrospective, descriptive, observational study.
MethodsAt Hospital General de Zona 71 (HGZ 71), belonging to the Mexican Institute of Social Security, the incidence of GBS is relatively low, with an average of one case every 2 years. However, an increase in the number of cases of GBS was observed during the 2016 Zika virus outbreak; in this study, we reviewed the reports of probable cases of febrile and exanthematous illness and cases of acute flaccid paralysis attended at HGZ 71 between 1 August and 15 December 2016. We identified the cases diagnosed with GBS and probable concomitant Zika virus infection that fulfilled the WHO criteria for a probable case of Zika virus disease (patient with maculopapular and pruriginous exanthema and at least 2 of the following symptoms: conjunctivitis, fever, headache, arthralgia, myalgia, periarticular oedema, pruritis, and retro-ocular pain). We used the Brighton criteria to establish a more precise diagnosis of GBS and the Hughes scoring system to measure clinical and functional improvement after treatment with intravenous immunoglobulins (IVIg) and physiotherapy.
ResultsA total of 1881 probable cases of Zika were reported in the Veracruz Norte delegation of the Mexican Institute of Social Security; 243 cases were confirmed by RT-PCR. At our hospital (HGZ 71), we assessed 201 probable cases of Zika virus infection (168 women and 33 men); 29 cases were confirmed by RT-PCR (28 women and one man). Ninety-eight women were pregnant, and Zika virus infection was confirmed by RT-PCR in 26 of these; no case of Zika-associated congenital syndrome was reported.
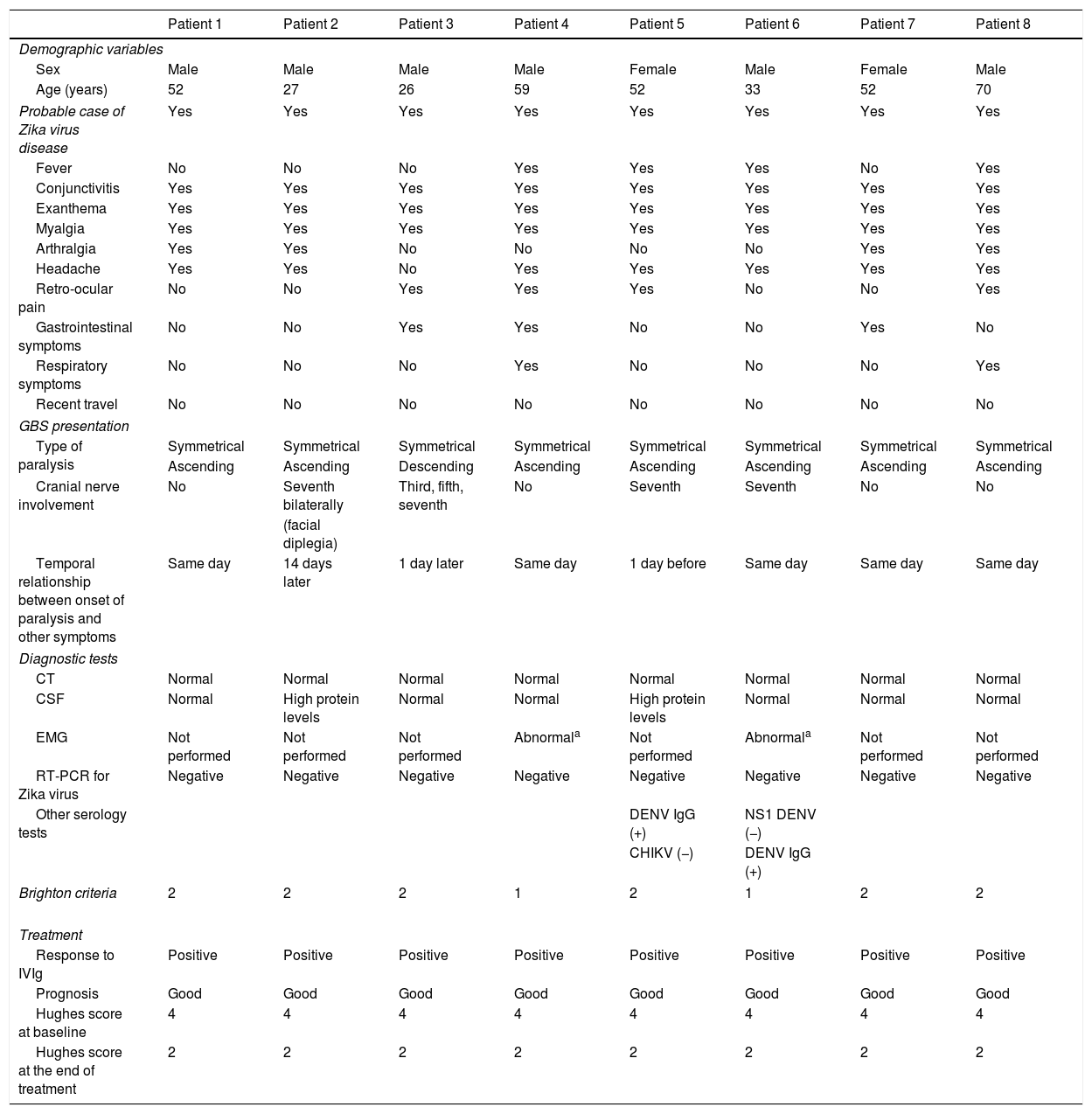
In terms of GBS incidence, 8 patients (6 men and 2 women) were admitted to hospital between August and November 2016; the 50-59 age group was the most widely affected. The highest incidence rate was observed in September, with 7 cases. The clinical characteristics associated with probable Zika virus infection in patients with GBS, in decreasing order of frequency, were exanthema, conjunctivitis, fever, arthralgia, and retro-ocular pain, fulfilling criteria for probable cases of Zika virus disease (Table 1); in 62.5% of the cases, paralysis started the same day as exanthema, conjunctivitis, or fever. GBS presented as symmetrical ascending paralysis in 7 patients (87.5%) and Miller-Fisher syndrome (descending paralysis) in one (12.5%); no patient presented respiratory paralysis. Half of the patients presented involvement of the cranial nerves; the seventh cranial nerve was the most frequently involved (4 patients), with one patient presenting bilateral involvement of this nerve (Table 1).
Clinical characteristics and diagnostic test results of GBS patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | |
|---|---|---|---|---|---|---|---|---|
| Demographic variables | ||||||||
| Sex | Male | Male | Male | Male | Female | Male | Female | Male |
| Age (years) | 52 | 27 | 26 | 59 | 52 | 33 | 52 | 70 |
| Probable case of Zika virus disease | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Fever | No | No | No | Yes | Yes | Yes | No | Yes |
| Conjunctivitis | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Exanthema | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Myalgia | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Arthralgia | Yes | Yes | No | No | No | No | Yes | Yes |
| Headache | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Retro-ocular pain | No | No | Yes | Yes | Yes | No | No | Yes |
| Gastrointestinal symptoms | No | No | Yes | Yes | No | No | Yes | No |
| Respiratory symptoms | No | No | No | Yes | No | No | No | Yes |
| Recent travel | No | No | No | No | No | No | No | No |
| GBS presentation | ||||||||
| Type of paralysis | Symmetrical | Symmetrical | Symmetrical | Symmetrical | Symmetrical | Symmetrical | Symmetrical | Symmetrical |
| Ascending | Ascending | Descending | Ascending | Ascending | Ascending | Ascending | Ascending | |
| Cranial nerve involvement | No | Seventh bilaterally | Third, fifth, seventh | No | Seventh | Seventh | No | No |
| (facial diplegia) | ||||||||
| Temporal relationship between onset of paralysis and other symptoms | Same day | 14 days later | 1 day later | Same day | 1 day before | Same day | Same day | Same day |
| Diagnostic tests | ||||||||
| CT | Normal | Normal | Normal | Normal | Normal | Normal | Normal | Normal |
| CSF | Normal | High protein levels | Normal | Normal | High protein levels | Normal | Normal | Normal |
| EMG | Not performed | Not performed | Not performed | Abnormala | Not performed | Abnormala | Not performed | Not performed |
| RT-PCR for Zika virus | Negative | Negative | Negative | Negative | Negative | Negative | Negative | Negative |
| Other serology tests | DENV IgG (+) | NS1 DENV (−) | ||||||
| CHIKV (−) | DENV IgG (+) | |||||||
| Brighton criteria | 2 | 2 | 2 | 1 | 2 | 1 | 2 | 2 |
| Treatment | ||||||||
| Response to IVIg | Positive | Positive | Positive | Positive | Positive | Positive | Positive | Positive |
| Prognosis | Good | Good | Good | Good | Good | Good | Good | Good |
| Hughes score at baseline | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Hughes score at the end of treatment | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
Albuminocytologic dissociation was observed in the cerebrospinal fluid analysis of 2 patients (25%). Electromyography was only possible in 2 patients, who showed signs of polyradiculopathy and acute demyelination. RT-PCR for Zika was performed within 7 days of diagnosis in all cases, both in serum and urine; findings were negative for all patients. Two patients tested positive in the IgG serology for dengue. Only 2 cases fulfilled Brighton criterion 1 (those who underwent a neurophysiological study), whereas 6 fulfilled Brighton criterion 2.
TreatmentAll patients received IVIg (0.4g/kg/day) for 5 days; treatment was started as soon as possible.
All patients progressed favourably: despite presenting a Hughes score of 4 at symptom onset, they showed a Hughes score of 2 at the end of treatment during their stay at hospital. After discharge, patients continued to receive physiotherapy and rehabilitation.
Cumulative incidence or risk amounts to 3.9% and the incidence rate is 0.039 cases per year. The monthly incidence rate increased from 0.041 to 2 cases/month during the Zika virus outbreak.
DiscussionOur study illustrates the increase in the incidence of GBS during the 2016 Zika virus outbreak in Veracruz (specifically, in the Veracruz Norte delegation), meeting the operational definition of probable case of Zika virus or other arbovirus infection (dengue, chikungunya). However, no direct causal relationship between GBS and Zika virus disease could be established in any patient; our results are consistent with those from large series from Brazil, Colombia, and other Central and South American countries where increases in incidence rates were as high as 877% and 400% (Venezuela and Suriname, respectively). The only cohort of patients with GBS associated with positive results for Zika virus, in 98% of the cases (41/42), is the study conducted in French Polynesia in 2013-2014, before the American Zika virus outbreak.8,9
Since the outbreak, such findings have not been replicated and the incidence of Zika virus–positive patients varies between regions. In Mexico, during the 2015–2016 outbreak, 802 cases of Zika virus–associated GBS were reported, but only 15 of these cases were confirmed with positive RT-PCR findings. In our study, the incidence of GBS increased but we observed no positive results for Zika virus infection, which is consistent with the trends seen in Mexico and other American countries. Considering the above, we believe that a comprehensive diagnostic strategy should be established that includes testing for bacteria and viruses, mainly potential neurotropic viruses, with the aim of accurately identifying the infectious agent. We also recommend performing serology studies for dengue virus, chikungunya virus, Zika virus, human immunodeficiency virus, influenza virus, the TORCH panel, Epstein–Barr virus, West Nile virus, hepatitis B and C viruses, and the Campylobacter jejuni, Haemophilus influenzae, and Mycoplasma pneumoniae bacteria.
In addition to our recommendation regarding accurate causal diagnosis, we recommend that in cases of probable GBS, emergency departments should follow a diagnostic protocol including a cranial computed tomography and/or magnetic resonance imaging study followed by lumbar puncture if such other alterations as tumours or abscesses are ruled out. However, lumbar puncture is recommended after the fifth day of symptom onset, as it may yield normal results in early stages. Once these clinical and biochemical data are obtained, we recommend starting IVIg therapy (0.4mg/kg/day for 5 days) and/or plasmapheresis, with the aim of limiting demyelination and achieving a better functional prognosis, or even full recovery. In case of uncertainty, we also recommend assessment by a neurologist or referral to tertiary care.
We should highlight that 2 patients tested positive in the IgG serology for dengue virus, which suggests immunological memory for this arbovirus; unfortunately, however, comprehensive viral and bacterial screening was not performed to rule out the potential neurotropic agents. To date, no study has aimed to identify a precise aetiological agent for each case of GBS; therefore, research protocols are currently addressing this line of research. Likewise, it would be useful to determine whether the virus is present in other fluids from patients with GBS. In addition to serum and urine tests, new possibilities are being studied, such as cerebrospinal fluid, saliva, or seminal fluid analyses, with the aim of detecting any viral material using RT-PCR or ELISA. Similarly, it has been suggested that Zika virus may act as a commensal, favouring coinfections or viral or bacterial symbioses, with the latter being the causes of GBS.
ConclusionThe incidence of GBS increased during the Zika virus outbreak; however, no direct causal relationship could be established. We recommend following a specific protocol for the identification of causal agents (both bacterial and viral) and for prompt diagnosis and treatment. In order to limit the sequelae of the disease, applicable protocols should be implemented in the emergency department, where cranial computed tomography and lumbar puncture can be performed, immunoglobulin treatment started, or referral to tertiary care determined.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: del Carpio Orantes L, Juárez Rangel FJ, García-Méndez S. Incidencia de síndrome de Guillain-Barré durante la oleada de zika del 2016 en un hospital de segundo nivel. Neurología. 2020;35:160–164.