Numerous cases have been reported of patients with symptoms of Guillain-Barré syndrome associated with COVID-19, but much information is still lacking on this association and its implications. The objective of this review is to analyse the available evidence on this topic in the adult population.
Material and methodsA systematic review was conducted of studies published on scientific databases: PubMed, Cochrane, Science Direct, MEDLINE, and WHO COVID-19 database.
ResultsWe identified 45 studies, which were analysed and completed using the Covidence platform; the final analysis included 24 articles, with a total of 30 patients.
ConclusionsWe found a strong association between both conditions; furthermore, the studies analysed highlight differences in the presentation of the disease, with greater severity of symptoms in Guillain-Barre syndrome associated with COVID-19.
Se han reportado distintos casos de pacientes con cuadro de síndrome de Guillain-Barré asociado a COVID-19, pero falta mucha información aún sobre esta asociación y sus implicancias, el objetivo de esta revisión es analizar la evidencia disponible en esta temática en la población adulta.
Material y MétodosSe realizó una revisión sistemática de estudios publicados en buscadores científicos: PubMed, Cochrane, Science direct, Medline, OMS COVID-19.
ResultadosSe identificaron 45 estudios, los cuales se analizaron y completaron utilizando la plataforma Covidence, incluyendo para el análisis final 24 artículos y sumando un total de 30 pacientes.
ConclusionesSe demuestra una asociación fuerte entre ambas patologías, además los estudios analizados recalcan diferencias en la presentación de la enfermedad con mayor gravedad en los cuadros de Síndrome de Guillain-Barre asociados a COVID-19.
The pandemic we are facing in 2020 is unprecedented. The full effects of infection with the SARS-CoV-2 coronavirus, which causes COVID-19, are still to be determined, and new findings are coming to light every day regarding its transmission, symptoms, progression, new strains, immunity, and association with other pathologies.1 On the latter point, researchers have observed that this virus not only harms the respiratory system, but also affects other systems such as the vascular, renal, and central nervous systems.2
Cases have been reported at several healthcare centres of patients with Guillain-Barré syndrome (GBS) and active or resolved COVID-19; therefore, an association between both conditions has been suggested. Some authors report that COVID-19–associated GBS presents more acute symptom onset, which is suggestive of a form of pre-pandemic GBS.3
Other types of coronaviruses have been reported to infect the central nervous system, and are therefore considered potentially neuroinvasive viruses; central nervous system involvement leads to different manifestations and sequelae affecting neurons and glial cells.4 These manifestations may be classified into 3 types: viral encephalitis, described as inflammatory lesions of the brain parenchyma; acute/toxic infectious encephalopathy, described as a reversible brain dysfunction syndrome caused by systemic toxic symptoms, metabolic disorders and hypoxia during the period of acute infection; and lastly, acute cerebrovascular disease (cerebrovascular accident), which in recent months has been widely attributed to SARS-CoV-2, as the virus triggers a proinflammatory cytokine cascade, as well as high levels of D-dimer and low platelet levels, which can cause a cerebrovascular accident.4
GBS is a severe condition that manifests when the immune system attacks the patient’s own body, and specifically the cells of the peripheral nervous system. The cause is not clear, but this immune disorder is frequently associated with other viral or bacterial pathologies, including influenza, HIV, and herpes virus infection; an association with COVID-19 is currently being studied.5 While GBS may manifest at any age, its incidence is highest among individuals aged 30-50 years. It is considered a demyelinating disease because the lesions mainly affect myelin in the peripheral nerves, leading to paresis, muscle weakness, and even bilateral ascending paralysis. If nerve damage reaches the diaphragmatic nerves, the patient may present respiratory symptoms ranging from mild respiratory failure to the need for invasive ventilatory support. Unfortunately, GBS is incurable; treatment focuses on symptom management and ventilatory support, when needed.5
Several pathological studies, including one by Hamming at al.,6 have shown that angiotensin-converting enzyme 2 (ACE2) acts as a functional receptor of SARS-CoV-2 in human tissues. Considering the similarity in the sequencing of the SARS-CoV and SARS-CoV-2 spike proteins, it has been suggested that SARS-CoV-2 also uses ACE2 as a functional receptor; this was confirmed by other studies in early 2020.7,8
The potential mechanisms by which SARS-CoV-2 may cause neurological damage include the binding of the virus to ACE2 in the blood-brain barrier, enabling it to enter the central nervous system, as well as the existence of haematogenous, transcribrial, and neuronal retrograde dissemination pathways.9,10
The aim of this review is to analyse the available evidence on the associations described between COVID-19 and GBS in the adult population, in order to communicate the clinical manifestations and progression of the reported cases and therefore provide relevant information for preventing the condition.
Material and methodsThis study is a systematic review of articles containing the following MeSH terms: COVID-19, SARS-CoV-2, Guillain-Barré, neuropathies, and demyelinating disease. We included systematic review articles, randomised controlled trials, controlled clinical trials, and observational studies, case series, and case reports. The review includes studies of adult populations published between December 2019 and June 2020. The search tools used were PubMed, Cochrane, Science Direct, MEDLINE, and the World Health Organization search tool for COVID-19 studies. We excluded non-systematic literature reviews and expert opinion articles.
The protocol followed in this systematic review was based on the Preferred Reporting Items for Systematic Review and Meta-Analysis statements, conducted through the Covidence® platform. Two reviewers independently assessed the bias of the studies included. Disagreements were resolved by consensus of the researchers. We used the quality assessment tools published on the National Heart, Lung and Blood Institute website (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools).
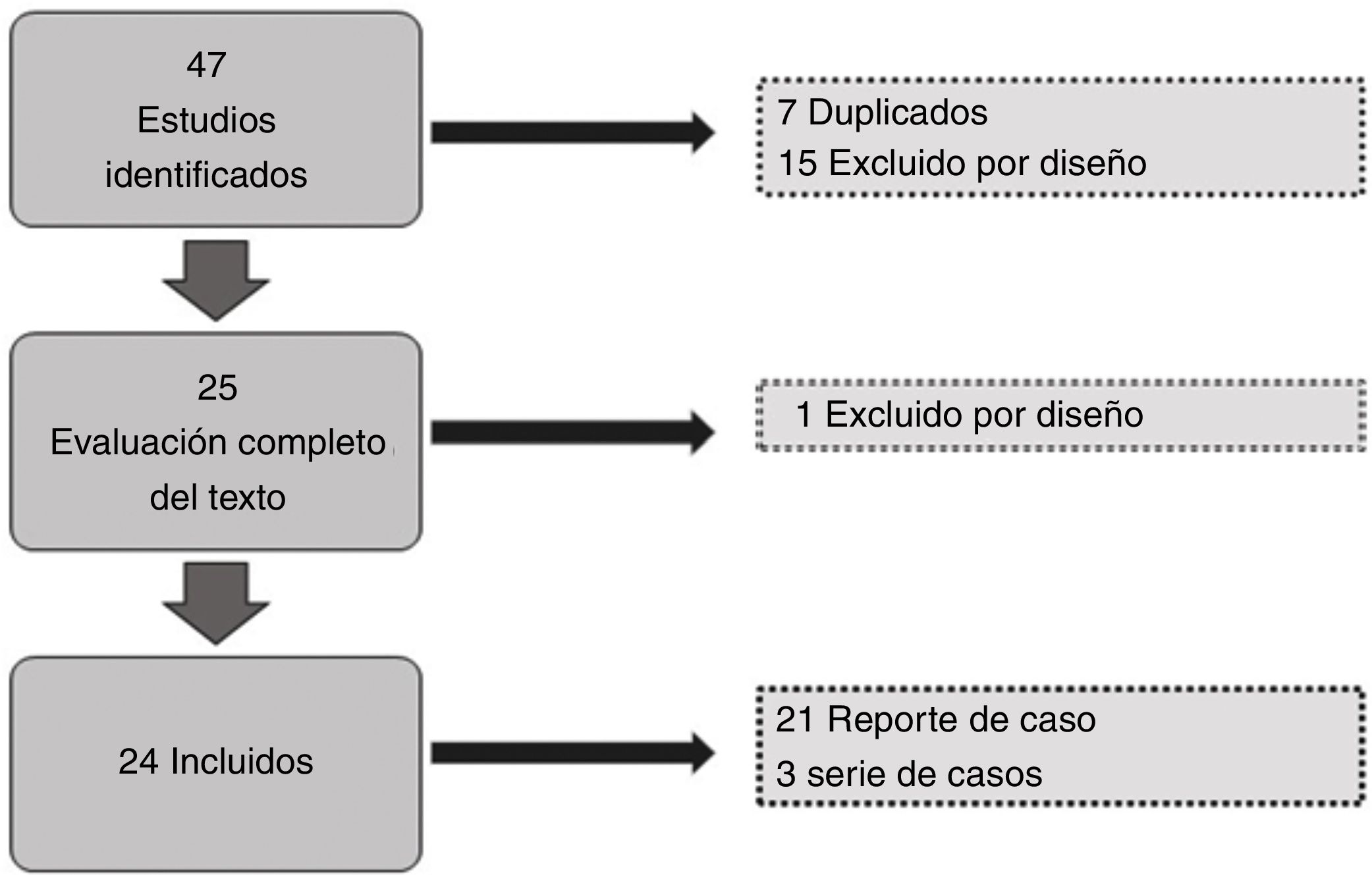
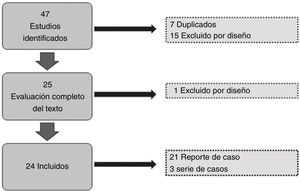
ResultsOf the 47 articles identified in the literature search, we excluded 7 duplicate studies, and a further 15 were excluded during the review of titles and abstracts; of the remaining 25, we excluded one during the review of full texts due to the study design. We finally included 24 studies for data gathering, analysis of results, and quality assessment (Fig. 1). A total of 30 patients were included. Sex and age were not reported in 5 cases (16.6%); and of the remaining 25, 14 were men (46.6%) and 11 women (36.6%).
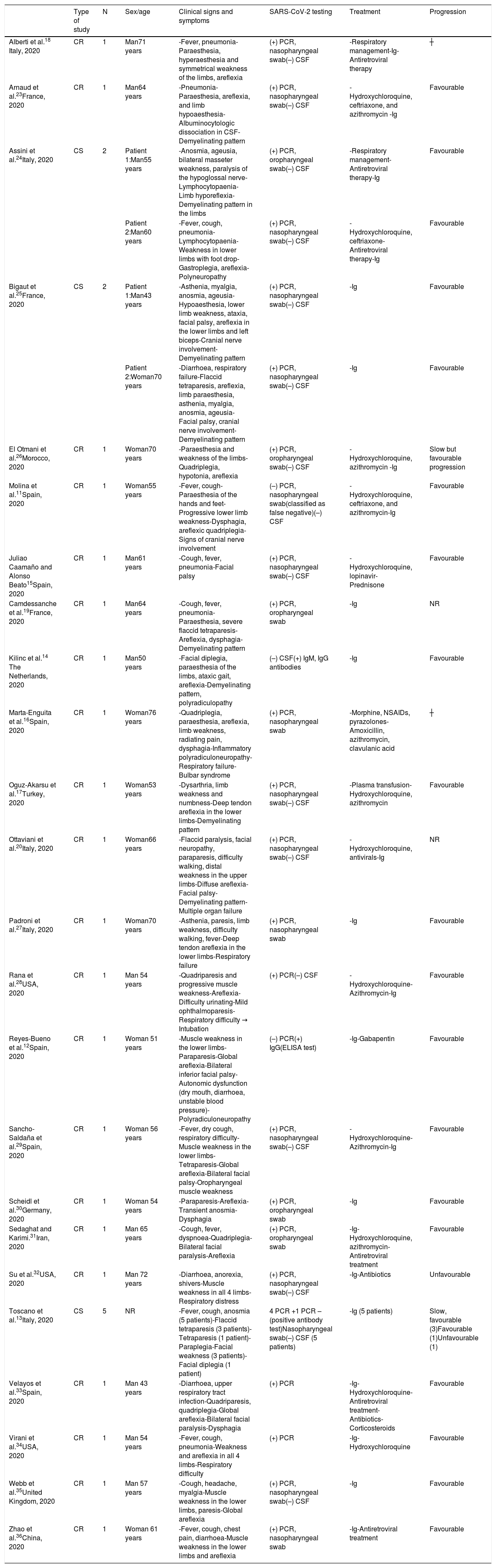
The majority of studies included used PCR testing to detect SARS-CoV-2, with positive results in most of the patients; only 3 studies reported negative PCR tests, including the study by Molina et al.,11 who classified the results as a false negative due to the presence of confirmed cases of COVID-19 among the patient’s family members. The study by Reyes-Bueno et al.12 reported negative results in the PCR test but positive IgG test results; the study by Toscano et al.13 reported 5 patients, only one of whom presented negative results in the PCR test. The study by Kilinc et al.14 is also noteworthy: it does not report PCR testing but rather IgM and IgG tests only, both with positive results.
All patients tested negative for SARS-CoV-2 in the cerebrospinal fluid (CSF).
Regarding the clinical manifestations reported in the studies, the most frequent were lower limb muscle weakness, areflexia, involvement of cranial nerves (facial palsy, facial diplegia, dysphagia, etc), paraparesis, and quadriparesis; slightly less frequent signs included cough, fever, diarrhoea, respiratory failure, anosmia, and ageusia.
Fever was present in most of the reported cases; however, we must consider that several patients presented active COVID-19 at the time of admission; in the case of patients without fever, this was due to the fact that GBS manifested 10, 14, and 21 days after diagnosis of COVID-19.
In terms of treatment, most patients were treated with immunoglobulins (90%), as well as antiretroviral therapy (27%) and hydroxychloroquine (40%) in some studies, with the exception of the study by Juliao et al.,15 who used hydroxychloroquine, prednisone, and antiretroviral therapy but not immunoglobulins; Marta-Enguita et al.,16 who prescribed morphine, NSAIDs, pyrazolones, amoxicillin, and clavulanic acid; and Oguz-Akarsu et al.,17 who used blood plasma transfusion, hydroxychloroquine, and azithromycin.
Two deaths were reported among the patients included in our review, one in the study by Alberti et al.16 and another in the study by Marta-Enguita et al.18 Two studies (Camdessanche et al.19 and Ottaviani et al.20) did not provide data on patient progression; however, outcomes were classified as favourable in 80% of cases.
The main patient characteristics are summarised in Table 1.
Clinical characteristics of the patients included in the studies of our systematic review.
| Type of study | N | Sex/age | Clinical signs and symptoms | SARS-CoV-2 testing | Treatment | Progression | |
|---|---|---|---|---|---|---|---|
| Alberti et al.18 Italy, 2020 | CR | 1 | Man71 years | -Fever, pneumonia-Paraesthesia, hyperaesthesia and symmetrical weakness of the limbs, areflexia | (+) PCR, nasopharyngeal swab(–) CSF | -Respiratory management-Ig-Antiretroviral therapy | ┼ |
| Arnaud et al.23France, 2020 | CR | 1 | Man64 years | -Pneumonia-Paraesthesia, areflexia, and limb hypoaesthesia-Albuminocytologic dissociation in CSF-Demyelinating pattern | (+) PCR, nasopharyngeal swab(–) CSF | -Hydroxychloroquine, ceftriaxone, and azithromycin -Ig | Favourable |
| Assini et al.24Italy, 2020 | CS | 2 | Patient 1:Man55 years | -Anosmia, ageusia, bilateral masseter weakness, paralysis of the hypoglossal nerve-Lymphocytopaenia-Limb hyporeflexia-Demyelinating pattern in the limbs | (+) PCR, oropharyngeal swab(–) CSF | -Respiratory management-Antiretroviral therapy-Ig | Favourable |
| Patient 2:Man60 years | -Fever, cough, pneumonia-Lymphocytopaenia-Weakness in lower limbs with foot drop-Gastroplegia, areflexia-Polyneuropathy | (+) PCR, nasopharyngeal swab(–) CSF | -Hydroxychloroquine, ceftriaxone-Antiretroviral therapy-Ig | Favourable | |||
| Bigaut et al.25France, 2020 | CS | 2 | Patient 1:Man43 years | -Asthenia, myalgia, anosmia, ageusia-Hypoaesthesia, lower limb weakness, ataxia, facial palsy, areflexia in the lower limbs and left biceps-Cranial nerve involvement-Demyelinating pattern | (+) PCR, nasopharyngeal swab(–) CSF | -Ig | Favourable |
| Patient 2:Woman70 years | -Diarrhoea, respiratory failure-Flaccid tetraparesis, areflexia, limb paraesthesia, asthenia, myalgia, anosmia, ageusia-Facial palsy, cranial nerve involvement-Demyelinating pattern | (+) PCR, nasopharyngeal swab(–) CSF | -Ig | Favourable | |||
| El Otmani et al.26Morocco, 2020 | CR | 1 | Woman70 years | -Paraesthesia and weakness of the limbs-Quadriplegia, hypotonia, areflexia | (+) PCR, oropharyngeal swab(–) CSF | -Hydroxychloroquine, azithromycin -Ig | Slow but favourable progression |
| Molina et al.11Spain, 2020 | CR | 1 | Woman55 years | -Fever, cough-Paraesthesia of the hands and feet-Progressive lower limb weakness-Dysphagia, areflexic quadriplegia-Signs of cranial nerve involvement | (–) PCR, nasopharyngeal swab(classified as false negative)(–) CSF | -Hydroxychloroquine, ceftriaxone, and azithromycin-Ig | Favourable |
| Juliao Caamaño and Alonso Beato15Spain, 2020 | CR | 1 | Man61 years | -Cough, fever, pneumonia-Facial palsy | (+) PCR, nasopharyngeal swab(–) CSF | -Hydroxychloroquine, lopinavir-Prednisone | Favourable |
| Camdessanche et al.19France, 2020 | CR | 1 | Man64 years | -Cough, fever, pneumonia-Paraesthesia, severe flaccid tetraparesis-Areflexia, dysphagia-Demyelinating pattern | (+) PCR, oropharyngeal swab | -Ig | NR |
| Kilinc et al.14 The Netherlands, 2020 | CR | 1 | Man50 years | -Facial diplegia, paraesthesia of the limbs, ataxic gait, areflexia-Demyelinating pattern, polyradiculopathy | (–) CSF(+) IgM, IgG antibodies | -Ig | Favourable |
| Marta-Enguita et al.16Spain, 2020 | CR | 1 | Woman76 years | -Quadriplegia, paraesthesia, areflexia, limb weakness, radiating pain, dysphagia-Inflammatory polyradiculoneuropathy-Respiratory failure-Bulbar syndrome | (+) PCR, nasopharyngeal swab | -Morphine, NSAIDs, pyrazolones-Amoxicillin, azithromycin, clavulanic acid | ┼ |
| Oguz-Akarsu et al.17Turkey, 2020 | CR | 1 | Woman53 years | -Dysarthria, limb weakness and numbness-Deep tendon areflexia in the lower limbs-Demyelinating pattern | (+) PCR, nasopharyngeal swab(–) CSF | -Plasma transfusion-Hydroxychloroquine, azithromycin | Favourable |
| Ottaviani et al.20Italy, 2020 | CR | 1 | Woman66 years | -Flaccid paralysis, facial neuropathy, paraparesis, difficulty walking, distal weakness in the upper limbs-Diffuse areflexia-Facial palsy-Demyelinating pattern-Multiple organ failure | (+) PCR, nasopharyngeal swab(–) CSF | -Hydroxychloroquine, antivirals-Ig | NR |
| Padroni et al.27Italy, 2020 | CR | 1 | Woman70 years | -Asthenia, paresis, limb weakness, difficulty walking, fever-Deep tendon areflexia in the lower limbs-Respiratory failure | (+) PCR, nasopharyngeal swab | -Ig | Favourable |
| Rana et al.28USA, 2020 | CR | 1 | Man 54 years | -Quadriparesis and progressive muscle weakness-Areflexia-Difficulty urinating-Mild ophthalmoparesis-Respiratory difficulty → Intubation | (+) PCR(–) CSF | -Hydroxychloroquine-Azithromycin-Ig | Favourable |
| Reyes-Bueno et al.12Spain, 2020 | CR | 1 | Woman 51 years | -Muscle weakness in the lower limbs-Paraparesis-Global areflexia-Bilateral inferior facial palsy-Autonomic dysfunction (dry mouth, diarrhoea, unstable blood pressure)-Polyradiculoneuropathy | (–) PCR(+) IgG(ELISA test) | -Ig-Gabapentin | Favourable |
| Sancho-Saldaña et al.29Spain, 2020 | CR | 1 | Woman 56 years | -Fever, dry cough, respiratory difficulty-Muscle weakness in the lower limbs-Tetraparesis-Global areflexia-Bilateral facial palsy-Oropharyngeal muscle weakness | (+) PCR, nasopharyngeal swab(–) CSF | -Hydroxychloroquine-Azithromycin-Ig | Favourable |
| Scheidl et al.30Germany, 2020 | CR | 1 | Woman 54 years | -Paraparesis-Areflexia-Transient anosmia-Dysphagia | (+) PCR, oropharyngeal swab | -Ig | Favourable |
| Sedaghat and Karimi.31Iran, 2020 | CR | 1 | Man 65 years | -Cough, fever, dyspnoea-Quadriplegia-Bilateral facial paralysis-Areflexia | (+) PCR, oropharyngeal swab | -Ig-Hydroxychloroquine, azithromycin-Antiretroviral treatment | Favourable |
| Su et al.32USA, 2020 | CR | 1 | Man 72 years | -Diarrhoea, anorexia, shivers-Muscle weakness in all 4 limbs-Respiratory distress | (+) PCR, nasopharyngeal swab(–) CSF | -Ig-Antibiotics | Unfavourable |
| Toscano et al.13Italy, 2020 | CS | 5 | NR | -Fever, cough, anosmia (5 patients)-Flaccid tetraparesis (3 patients)-Tetraparesis (1 patient)-Paraplegia-Facial weakness (3 patients)-Facial diplegia (1 patient) | 4 PCR +1 PCR – (positive antibody test)Nasopharyngeal swab(–) CSF (5 patients) | -Ig (5 patients) | Slow, favourable (3)Favourable (1)Unfavourable (1) |
| Velayos et al.33Spain, 2020 | CR | 1 | Man 43 years | -Diarrhoea, upper respiratory tract infection-Quadriparesis, quadriplegia-Global areflexia-Bilateral facial paralysis-Dysphagia | (+) PCR | -Ig-Hydroxychloroquine-Antiretroviral treatment-Antibiotics-Corticosteroids | Favourable |
| Virani et al.34USA, 2020 | CR | 1 | Man 54 years | -Fever, cough, pneumonia-Weakness and areflexia in all 4 limbs-Respiratory difficulty | (+) PCR | -Ig-Hydroxychloroquine | Favourable |
| Webb et al.35United Kingdom, 2020 | CR | 1 | Man 57 years | -Cough, headache, myalgia-Muscle weakness in the lower limbs, paresis-Global areflexia | (+) PCR, nasopharyngeal swab(–) CSF | -Ig | Favourable |
| Zhao et al.36China, 2020 | CR | 1 | Woman 61 years | -Fever, cough, chest pain, diarrhoea-Muscle weakness in the lower limbs and areflexia | (+) PCR, nasopharyngeal swab | -Ig-Antiretroviral treatment | Favourable |
CR: case report; CS: case series; CSF: cerebrospinal fluid; Ig: immunoglobulins; N: number of patients; NR: not reported; NSAID: non-steroidal anti-inflammatory drug; PCR: polymerase chain reaction; ┼ deceased.
It should be noted that all the hospitals where the studies were conducted have experienced an abnormal increase in the number of patients admitted with GBS, with higher prevalence among older patients (mean age of 60 years) than in the cases reported before the pandemic (mean age of 40 years).21 Therefore, it is important to analyse the 2 deceased patients, both aged older than 70 years, as well as the patient with a poor outcome. Considering that older age is an independent risk factor for mortality due to COVID-19,22 further studies (randomised control trials) are needed to establish more accurate results and conclusions about the causal relationship.
The most common symptoms appearing before onset of GBS were ageusia and hyposmia; COVID-19–associated symptoms, such as pneumonia, were also observed to be more severe.23 We should also emphasise the findings of Assini et al.24 regarding the differences in the manifestations of GBS before and after the pandemic. According to these authors, impairment of several cranial nerves in association with a demyelinating peripheral neuropathy is a very infrequent characteristic that only affected 5% of previously reported cases; however, in the context of COVID-19, cranial nerve involvement was observed in 47% of the patients included in this review, and represents a distinctive characteristic of the cases associated with the disease.
The first neurological manifestations presented between 5 and 21 days after onset of COVID-19 symptoms.
Strikingly, CSF analysis was not performed for a considerable percentage of patients (26.6%); this is a relevant limitation as this assessment is very important for correct diagnosis and subsequent treatment, especially in critically ill patients.7
The studies analysed are case reports or case series with small samples, and are therefore considered to present a high risk of bias; however, despite this limitation, it is important to underscore that an association between the 2 pathologies, with a new form of presentation, is increasingly being observed.
ConclusionThe studies analysed show a clear association between both pathologies, with SARS-CoV-2 potentially triggering GBS. Further studies providing higher levels of evidence and including more representative samples are needed for a conclusive analysis of this topic; however, these cases seem to differ from those reported before the pandemic in the older age of onset and more severe clinical manifestations and cranial nerve involvement. In any case, appropriate treatment achieves favourable outcomes.
We hope that this review will lead to future studies that may help professionals and the general population to be more alert to the presence of this condition in their social circles.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThis study received no funding of any kind.
Please cite this article as: Trujillo Gittermann LM, Valenzuela Feris SN, von Oetinger Giacoman A. Relación entre COVID-19 y síndrome de Guillain-Barré en adultos. Revisión sistemática. Neurología. 2020;35:646–654.