Several therapeutic options are available for the symptomatic treatment of Parkinson's disease (PD). There is no reliable information about which factors are involved in the choice of treatment.
ObjectiveTo identify factors contributing to the decision to start treatment with levodopa/carbidopa/entacapone (LCE) in patients with PD.
Patients and methodsWe completed a descriptive cross-sectional retrospective multicentre study of patients with idiopathic PD receiving LCE. Clinical data were collected with special attention to factors that could potentially determine when to initiate treatment with LCE in normal clinical practice.
ResultsWe studied 1050 patients with a mean age of 71.3±8.7 years (58.2% men). Average time from the onset of symptoms to diagnosis was 13.8±12.9 months, with a latency time of 74.5±53.6 months before starting LCE treatment. The most common initial symptoms were tremor (70.6%), reduced dexterity (43.2%) and slowness of movement (41.5%). At the start of LCE treatment, most patients were in Hoehn and Yahr stage 2 (57.5%), with an average rating of 73.4% on the Schwab and England scale. Eight hundred twenty-two patients (78.3%) received treatment with other drugs before starting LCE (mean time between starting any PD treatment and starting LCE was 40.5±47.2 months). Clinical factors with a moderate, marked, or crucial effect on the decision to start LCE treatment were bradykinesia (84.7%), daytime rigidity (72.2%), general decline (72.2%), difficulty walking (66.4%), tremor (62.7%), nocturnal rigidity (56.1%), and postural instability (53%). Difficulty performing activities of daily living was the only psychosocial factor identified as having an influence on the decision (84.3%).
ConclusionsThe decision to start patients with idiopathic PD on LCE treatment is mainly determined by motor deficits and disabilities associated with disease progression.
La enfermedad de Parkinson (EP) cuenta con un tratamiento sintomático amplio. No existe información fidedigna sobre los factores que influyen en la elección del tratamiento.
ObjetivoIdentificar los factores que determinan el inicio del tratamiento con levodopa/carbidopa/entacapona (LCE) en pacientes con EP.
Pacientes y métodosEstudio observacional, transversal retrospectivo y multicéntrico en pacientes con EP idiopática en tratamiento con LCE. Se recogieron datos sobre factores potencialmente implicados, como determinantes del inicio del tratamiento con LCE en la práctica clínica habitual.
ResultadosSe estudió a 1.050 pacientes (edad media 71,3±8,7 años; 58,2%, hombres), con 13,8±12,9 meses de evolución hasta el diagnóstico y 74,5±53,6 meses hasta el momento del inicio del tratamiento con LCE. Los síntomas iniciales incluyeron: temblor (70,6%), reducción de destreza (43,2%) y lentitud de movimientos (41,5%). El estadio de Hoehn y Yahr mayoritario al inicio de LCE fue 2 (57,5%), mientras que la escala de Schwab y England presentó una puntuación media de 73,4%. Ochocientos veintidós pacientes (78,3%) recibieron otros fármacos antes de LCE (tiempo medio entre inicio de tratamiento e inicio con LCE: 40,5±47,2 meses). Los factores clínicos determinantes para iniciar el tratamiento con LCE fueron la presencia de bradicinesia (84,7%), rigidez diurna (72,2%), empeoramiento general (72,2%), dificultad marcha (66,4%), temblor (62,7%), rigidez nocturna (56,1%) e inestabilidad postural (53%). El único factor psicosocial determinante identificado fue la dificultad para realizar las actividades habituales de la vida diaria (84,3%).
ConclusionesEn la EP, el inicio del tratamiento con LCE viene determinado fundamentalmente por los déficits motores y la discapacidad asociada.
Parkinson's disease (PD) presents with both motor symptoms (resting tremor, bradykinesia and rigidity) and nonmotor symptoms. In Spain, PD prevalence is estimated at 1.7% among patients older than 65 years,1 although there are significant regional variations.2
Idiopathic PD accounts for approximately 85% of all the cases of parkinsonism diagnosed annually.3 Its complex aetiology is linked to environmental and genetic factors, which probably explains the disease's heterogeneous progression profiles.4 Within this context, patient subgroups with different pathogenic mechanisms may exist, and each group may require a personalised treatment approach.5 While there is currently no proven neuroprotective treatment, powerful and efficient symptomatic treatments are available,6 but there is no consensus on when or how to start pharmacological treatment.6,7 Some studies suggest that early onset of treatment is beneficial to the patient's quality of life,8,9 while most clinical practice guidelines recommend starting therapy when the first symptoms affecting daily life appear.10 Furthermore, treatment choice is based on several factors that are related to the drug itself (efficacy, complications, safety, etc.), the patient profile (symptoms, age, occupation, comorbidity, etc.) and other circumstances (e.g., socioeconomic status).11–13 This situation promotes uncertainty among patients and variability in clinical practice which has to do with lack of evidence.
Since not enough information is available on the factors influencing some therapeutic decisions made during the course of PD,14 performing studies to identify such influences seems to be justified. There is currently no consensus on which factors are involved in the decision to start combination treatment with set doses of levodopa/carbidopa/entacapone (LCE), which is one of the more frequently used PD treatments. This retrospective study aims to identify the factors affecting the decision by Spanish neurologists to prescribe this treatment in normal clinical practice. To our knowledge, no other study with a similar aim has been carried out in our setting, and the results of our study may be useful for increasing knowledge in this field.
Patients and methodsWe carried out an observational, cross-sectional, retrospective multicentre study in Spain under normal clinical practice conditions. Each participating neurologist recorded data from at least 10 patients treated with LCE until a total of 1050 eligible patients had been included. This sample size was calculated based on the precision considered acceptable for the worst-case scenario (from a precision of ±3% points for a proportion of 50%, to a precision of ±1.8% points for a proportion of 10% in one response category). We included patients aged 18 years and older with idiopathic PD treated with LCE during a minimum of 3 months before inclusion in the study. Patients were excluded if they were participating in a clinical trial during treatment with LCE and if their state of health, in the researcher's opinion, would not permit them to be included in the study.
We collected clinical data from the patients by reviewing their medical histories, paying special attention to the factors determining when LCE treatment was started in normal clinical practice. The study protocol was approved by the Ethics Committee at Hospital Clínic in Barcelona.
In addition to the clinical symptoms specific to PD, we recorded time since diagnosis, initial symptoms, Hoehn and Yahr scale score,15 parts 2 (activities of daily living) and 3 (motor examination) of the Unified Parkinson Disease Rating Scale (UPDRS),16 Schwab and England Activities of Daily Living Scale,17 any previous treatments, and the start date for the different treatments. Furthermore, we analysed associations between the demographic and clinical variables recorded and pathological factors linked to disease progression and to onset of LCE treatment. A clinical or psychosocial factor was considered a determinant in the decision to start LCE treatment if the sum of 2% of patients in the categories ‘moderate influence’ and ‘crucial and determining’ represented more than 50% of the total patients presenting that factor.
Typical descriptive statistics were used according to the characteristics of the variables (categorical or continuous). Parametric (t-test or ANOVA) or non-parametric tests (Mann–Whitney or Kruskal–Wallis) were used to make comparisons. The Chi-square test was used with qualitative variables.
After identifying the clinical and psychosocial determinants, we established the association between these factors and the sociodemographic characteristics of the patients. To do so, we applied a logistic regression model for each determinant (as the dependent variable) using backward stepwise selection of the non-significant sociodemographic variables (independent variables). Results were expressed as odds ratios with their respective 95% confidence intervals.
Statistical analyses were performed using SAS statistical software, version 9.1.3 or later.
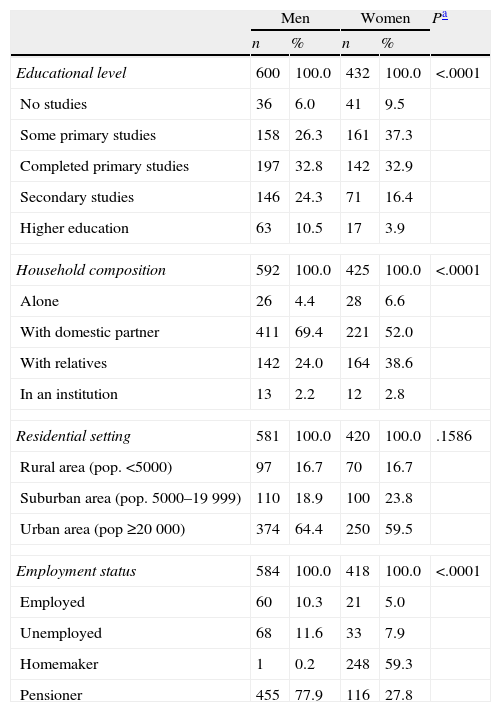
ResultsThe sample consisted of 1050 patients (58% men; mean age: 71.3±8.7 years), assessed by 102 neurologists. The baseline condition of the patients at the start of LCE treatment was as follows. Hoehn and Yahr scale (available for 97.1% of patients): stage 1, 14.9%; stage 2, 57.5%; stage 3, 23.1%; and stage 4, 4.5%. Mean score on UPDRS part II (ADL): 12.8±7.0; UPDRS part III motor examination, 25.7±13.2 (UPDRS parts II and III available for 62.7%). Mean score on the Schwab and England scale (available for 76.5%): 73.4±15.7. Table 1 displays other sociodemographic and medical history data for the sample.
Sociodemographic characteristics of patients at the start of LCE treatment.
| Men | Women | Pa | |||
| n | % | n | % | ||
| Educational level | 600 | 100.0 | 432 | 100.0 | <.0001 |
| No studies | 36 | 6.0 | 41 | 9.5 | |
| Some primary studies | 158 | 26.3 | 161 | 37.3 | |
| Completed primary studies | 197 | 32.8 | 142 | 32.9 | |
| Secondary studies | 146 | 24.3 | 71 | 16.4 | |
| Higher education | 63 | 10.5 | 17 | 3.9 | |
| Household composition | 592 | 100.0 | 425 | 100.0 | <.0001 |
| Alone | 26 | 4.4 | 28 | 6.6 | |
| With domestic partner | 411 | 69.4 | 221 | 52.0 | |
| With relatives | 142 | 24.0 | 164 | 38.6 | |
| In an institution | 13 | 2.2 | 12 | 2.8 | |
| Residential setting | 581 | 100.0 | 420 | 100.0 | .1586 |
| Rural area (pop. <5000) | 97 | 16.7 | 70 | 16.7 | |
| Suburban area (pop. 5000–19999) | 110 | 18.9 | 100 | 23.8 | |
| Urban area (pop ≥20000) | 374 | 64.4 | 250 | 59.5 | |
| Employment status | 584 | 100.0 | 418 | 100.0 | <.0001 |
| Employed | 60 | 10.3 | 21 | 5.0 | |
| Unemployed | 68 | 11.6 | 33 | 7.9 | |
| Homemaker | 1 | 0.2 | 248 | 59.3 | |
| Pensioner | 455 | 77.9 | 116 | 27.8 | |
LCE: levodopa/carbidopa/entacapone.
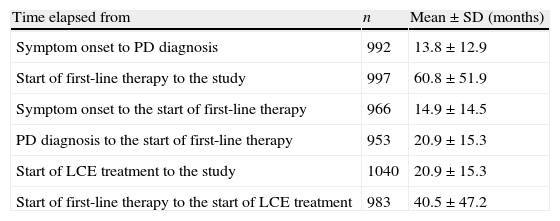
The time elapsed between the onset of typical PD symptoms and the time of the study was 6.21±4.47 years and 5.18±4.40 years from time of diagnosis. Table 2 displays other time data having to do with medical and treatment history.
Relevant periods in patient's medical and treatment history.
| Time elapsed from | n | Mean±SD (months) |
| Symptom onset to PD diagnosis | 992 | 13.8±12.9 |
| Start of first-line therapy to the study | 997 | 60.8±51.9 |
| Symptom onset to the start of first-line therapy | 966 | 14.9±14.5 |
| PD diagnosis to the start of first-line therapy | 953 | 20.9±15.3 |
| Start of LCE treatment to the study | 1040 | 20.9±15.3 |
| Start of first-line therapy to the start of LCE treatment | 983 | 40.5±47.2 |
LCE: levodopa/carbidopa/entacapone.
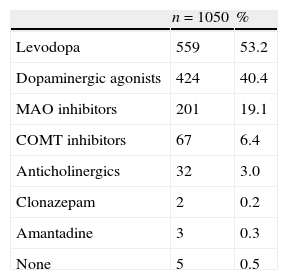
Initial characteristic symptoms included tremor (70.6%), decreased ability and dexterity (43.2%), slow movement (41.5%), rigidity (17.6%) and impaired balance (12.1%). As shown in Table 3, most patients started treatment with levodopa (53%), a dopaminergic agonist (40%), and/or MAO inhibitors (19%).
First-line therapy for Parkinson's disease in the sample.
| n=1050 | % | |
| Levodopa | 559 | 53.2 |
| Dopaminergic agonists | 424 | 40.4 |
| MAO inhibitors | 201 | 19.1 |
| COMT inhibitors | 67 | 6.4 |
| Anticholinergics | 32 | 3.0 |
| Clonazepam | 2 | 0.2 |
| Amantadine | 3 | 0.3 |
| None | 5 | 0.5 |
More than one treatment may be specified for the same patient.
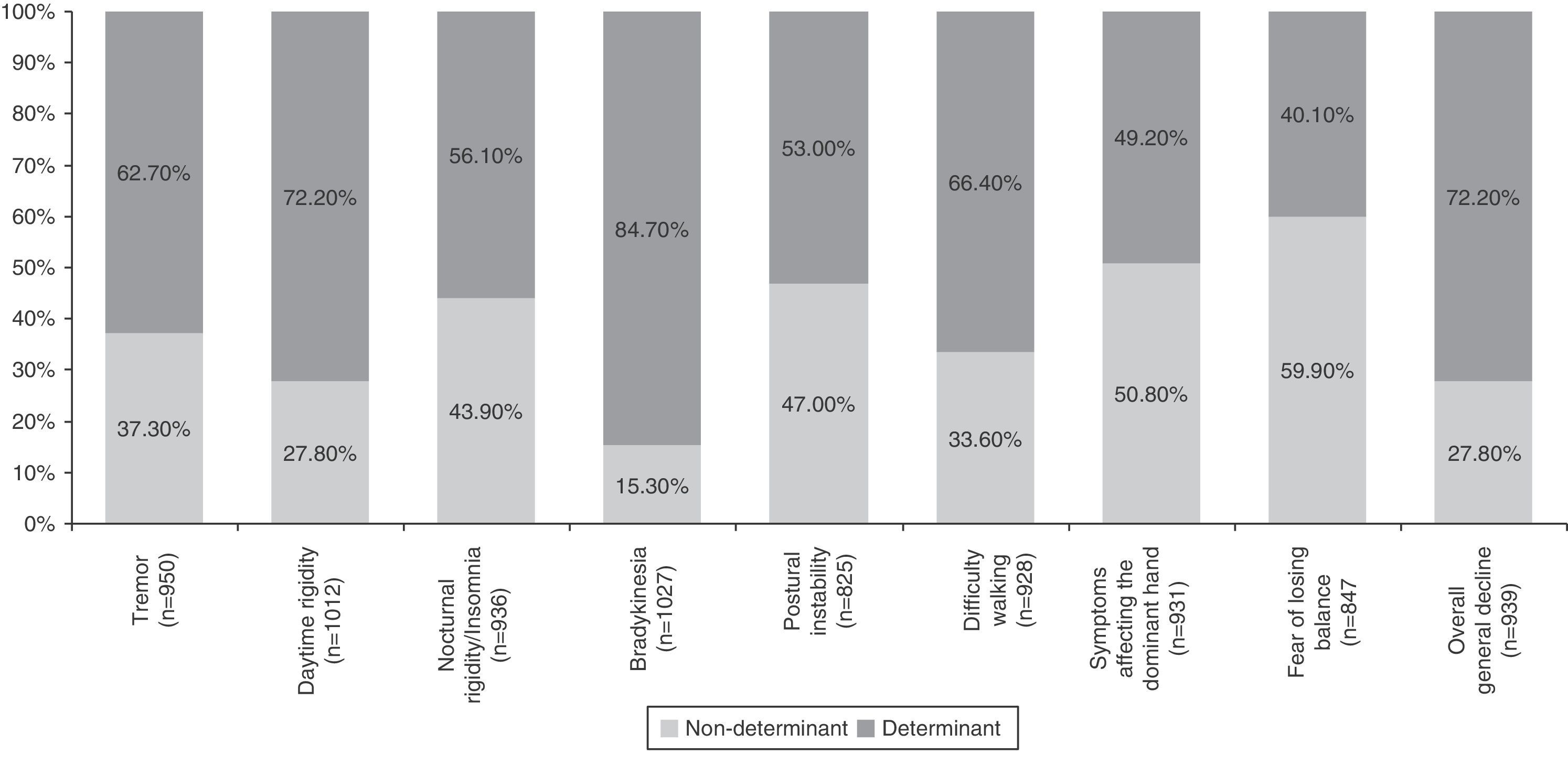
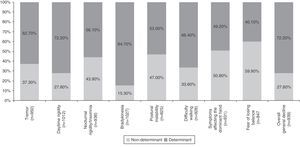
Table 4 summarises all the clinical and psychosocial factors assessed at the start of LCE treatment. The assessed clinical factors included typical signs of PD (tremor, rigidity, and bradykinesia), difficulty walking, loss of manual dexterity, motor fluctuations, and general clinical decline. Some of the most important clinical factors affecting the decision to start LCE treatment were bradykinesia (84.7%), general clinical decline (72.2%), and daytime rigidity (72.2%) (Table 4 and Fig. 1). Other clinical factors were decisive for some patients (wearing-off: 96%; motor fluctuations: 88%), but due to their limited representation in the sample (<5%), they have not been studied exhaustively or taken into account in secondary analyses.
Factors influencing the decision to start LCE treatment.
| Presence (%)a | Influence on the decision (%)b | |||||
| None | Slight | Moderate | Considerable | Decisive | ||
| Main clinical factors | ||||||
| Tremor | 90.5 | 15.4 | 21.9 | 27.2 | 21.2 | 0.0 |
| Daytime rigidity | 96.4 | 9.2 | 18.6 | 32.2 | 23.2 | 16.8 |
| Nocturnal rigidity/insomnia | 89.1 | 16.8 | 27.1 | 26.4 | 19.1 | 10.6 |
| Bradykinesia | 97.8 | 4.7 | 10.6 | 31.5 | 28.5 | 24.6 |
| Postural instability | 78.6 | 20.1 | 26.9 | 25.7 | 17.1 | 10.2 |
| Difficulty walking | 88.4 | 15.3 | 18.3 | 28.6 | 21.0 | 16.8 |
| Symptoms affecting the dominant hand | 88.7 | 24.4 | 26.4 | 25.0 | 13.7 | 10.4 |
| Fear of losing balance | 80.7 | 29.3 | 30.6 | 24.2 | 10.7 | 5.2 |
| Overall clinical decline | 89.4 | 12.9 | 14.9 | 25.8 | 20.8 | 25.7 |
| Motor fluctuations | 2.4 | 0.0 | 0.0 | 0.0 | 12.0 | 88.0 |
| Wearing-off | 2.4 | 0.0 | 0.0 | 0.0 | 4.0 | 96.0 |
| Other | 3.0 | 0.0 | 0.0 | 0.0 | 23.3 | 76.7 |
| Psychosocial factors | ||||||
| Difficulty with activities of daily living | 98.4 | 4.1 | 11.6 | 24.4 | 25.4 | 34.6 |
| Difficulty driving | 69.5 | 44.8 | 19.9 | 17.5 | 11.6 | 6.2 |
| Difficulty with job performance | 65.6 | 45.6 | 20.2 | 15.1 | 9.0 | 10.2 |
| Symptoms are embarrassing for the patient | 77.9 | 34.5 | 31.3 | 21.4 | 10.0 | 2.8 |
| Altered mood | 82.6 | 24.9 | 33.7 | 27.7 | 11.2 | 2.5 |
| Limitations on leisure activities | 80.7 | 35.2 | 29.3 | 22.1 | 9.8 | 3.7 |
| Impact on relationships | 80.1 | 34.0 | 31.2 | 23.3 | 9.9 | 1.7 |
| Secondary sexual dysfunction | 66.7 | 61.6 | 23.1 | 10.7 | 3.1 | 1.4 |
| Requested by patient | 65.2 | 47.6 | 24.2 | 14.6 | 8.5 | 5.1 |
| Other factors linked to PD | ||||||
| Intolerance to other drugs | 0.8 | 0.0 | 0.0 | 0.0 | 50.0 | 50.0 |
| Simplification of treatment | 0.4 | 0.0 | 0.0 | 25.0 | 0.0 | 75.0 |
| Side effects | 0.3 | 0.0 | 0.0 | 0.0 | 33.3 | 66.7 |
| Lack of efficacy of other treatments | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
| Difficulty managing treatment | 0.1 | 0.0 | 0.0 | 0.0 | 100.0 | 0.0 |
Among the psychosocial factors considered, clumsiness and difficulty performing activities of daily living were determinants in the decision to start LCE treatment in 84.4% of the cases.
Association between sociodemographic characteristics and determinantsThe summary of the association between the identified determinants and the sociodemographic characteristics is as follows. (a) No demographic characteristics linked to bradykinesia were identified as determinants in the decision to start LCE treatment; (b) the probability of presenting postural instability as the determinant was lower in patients younger than 65 years than in those older than 75 (OR: 0.48; CI 95%, 0.31-0.74); (c) the probability of presenting difficulty walking as the determinant was higher among patients younger than 65 years than in those older than 75 years (OR: 2.24; CI 95%, 1.49-3.35); (d) the probability of presenting a general clinical decline as the determinant in the decision to start treatment was lower among rural patients than among urban patients (OR: 0.57; CI 95%, 0.39-0.85); and (e) the probability of presenting difficulty with activities of daily living as the determinant was significantly lower among unemployed patients than among pensioners (OR: 0.29; CI 95%, 0.17-0.48).
DiscussionThis study enables us to define the baseline clinical profile of Spanish PD patients started with LCE treatment. The main aim of the study included identifying clinical and psychosocial factors with a potentially significant effect on the neurologist's decision to begin LCE treatment for PD.
Most of the PD patients who started LCE treatment presented motor symptoms at the approximate age of 65 years. They were mainly men with a primary or secondary education, retired, and living with a domestic partner. This demographic profile coincides with profiles described by other series on specialised care.18,19 The mean time elapsed from diagnosis to the first PD treatment was approximately 1.5 months, with a high variability (SD=6.6 months); mean time elapsed between first-line treatment and the start of LCE treatment was approximately 3.5 years.
Levodopa (excluding LCE) and dopaminergic agonists were the drugs most frequently used as first-line treatment. In 40% of the cases, the dopaminergic agonist selected as first-line therapy coincides with the criteria established by most clinical guidelines. Although levodopa is effective for improving motor performance,20 doctors prescribing this drug should be aware that it is associated with motor complications in more than half of the patients after the first 5 years of treatment.21–23 First-line treatment with a dopaminergic agonist aims to lower the risk of such complications.23 However, the development of new levodopa formulations, particularly the LCE combination studied here, has allowed us to optimise the pharmacodynamic profile and the bioavailability of the active ingredient (levodopa). Consequently, these formulations have become useful alternatives for treating motor manifestations and subsequent disability, and generally speaking, they show better tolerability than dopaminergic agonists.22 Nevertheless, use of the LCE formulation as first-line treatment is limited due to the recommendations listed above and the results of studies such as STRIDE-PD. This study demonstrated that starting levodopa treatment with LCE did not delay or decrease the frequency of dyskinesias (and may even increase them).24
Among those patients for whom we have data on the start of LCE treatment, 80.6% presented a Hoehn and Yahr stage of 2.0 or 3.0, with mean scores on the Schwab and England scale and the UPDRS (parts II and III) that indicate general moderate impairment. Most of these patients were not fully independent for activities of daily living or else performed tasks slowly and with difficulty.17 These findings provide evidence that Spanish patients with PD begin LCE treatment relatively late, despite the proven efficacy of LCE in both stable and fluctuating patients25,26 and the demonstrated improvement in some areas of quality of life.27
Analysis of clinical factors involved in the start of LCE treatment found that the main determinants were bradykinesia, general clinical decline, and daytime rigidity, followed by difficulty walking, tremor, nocturnal rigidity, and postural instability. Wearing-off and other motor fluctuations were determining factors for the patients presenting them. The only determinant among the psychosocial factors was difficulty performing activities of daily living. These factors coincide with the areas that improve the most with LCE therapy in patients experiencing the wearing-off effect, according to a recent post hoc analysis.28
However, the decision of when to start this treatment depends on the highly idiosyncratic relationship between the patient and the disease. Once doctors decide to start treatment for PD, they must choose among different strategies (MAO inhibitors, dopaminergic agonists, and levodopa in combination with a decarboxylase inhibitor). The most suitable treatment must be chosen based on the patient's characteristics (age, level of disability, occupation, etc.), the expected benefit (i.e., functional improvement) and the potential risks (i.e., development of dyskinesias and motor fluctuations).29 In any case, it should be noted that levodopa as first-line treatment not only increases the risk of motor complications in the short and medium term, but also involves a higher functional gain. This circumstance must be considered alongside the patient's age and functional requirements.30
The different regression models developed indicated that age, residential setting, and employment status were associated with different clinical determinants. This creates a complex framework of individual, clinical, and social interrelationships in which overall mobility and functional capacity differ between subgroups and are relevant when making treatment decisions.
Of the study's limitations, the following are especially important: (a) its retrospective character; (b) the small percentage of patients presenting wearing-off in this series, a phenomenon whose cause could not be determined due to the study design; (c) the fact that influence of nonmotor symptoms on the decision to prescribe LCE was not taken into account (based on data in the ‘Psychosocial factors’ section in Table 4, these symptoms seem to have a low relevance); and (d) lack of analysis of the potential differences in the prescription of drugs due to socioeconomic factors linked to geographical and political situations.
The main contribution of this study, despite the limitations mentioned above, is that it demonstrates that LCE treatment onset is linked to functional decline and more noticeable signs of neurological impairment in the patient. Future studies focusing on quality of life may be able to evaluate the benefits of LCE treatment27,31 and the possible decrease of that benefit if the start of the appropriate therapy is delayed.8,9
Conflicts of interestThis study received financial support from Novartis Farmacéutica, S.A. PMM received professional fees (as speaker or collaborator in different studies) from Novartis, Britannia, Orion Pharma, Abbott, UCB, and the Movement Disorder Society. He also received research grants from the FIS-ISCIII, IMSERSO, Université Clermont-Ferrand (France), the Movement Disorder Society, Michael J. Fox Foundation (USA), and Queen Sofía Foundation.
BH and JR are employed by Novartis Farmacéutica, S.A.
We thank Emili González-Pérez (Scientific Department at TFS Develop) for his assistance with the manuscript, and Pedro García Ruiz-Espiga (Department of Neurology, Fundación Jiménez Díaz, Madrid) for reviewing the text and making suggestions.
Vicente Bertol Alegre (H. U. Miguel Servet, Zaragoza), Sonia Santos Lasaosa (H. C. U. Lozano Blesa, Zaragoza), Cristina Iñiguez Martínez (H. C. U. Lozano Blesa, Zaragoza), Jose Angel Mauri Llerda (H. C. U. Lozano Blesa, Zaragoza), Pilar Larrode Pellicer (H. C. U. Lozano Blesa, Zaragoza), Sara Sánchez Valiente (C. M. especialidades Grande Covián, Zaragoza), Consuelo Rios Gómez (H. de Barbastro, Huesca), María Itziar Gastón Zubirendi (H. Virgen del Camino, Navarra), Francisco Lacruz Bescos (H. de Navarra, Navarra), Gerardo Joaquín Soriano Hernández (H. de Navarra, Navarra), José María Sánchez Álvarez (H. de Cabueñes, Asturias), Manuel Menéndez González (H. Álvarez Buylla, Asturias), Roberto Suárez Moro (H. Valle del Nalón, Asturias), Alejandro Formica Martínez (A. Ntra. Sra. del Coro, Guipúzcoa), Nerea Foncea Beti (H. de Galdakao, Vizcaya), Beatriz Tijero Merino (H. de Cruces, Vizcaya), Jesús Alberto Bergareche Yarza (H. Comarcal de Bidasoa, Guipúzcoa), Enrique Corredera García (H. Clínico Santiago, A Coruña), Jose Manuel Paz González (H. Xeral de Lugo, Lugo), Robustiano Pego Reigosa (H. Xeral de Lugo, Lugo), José Marey López (C. Saúde O Ventorrillo, A Coruña), Youssef Sayed Hachem (Clínica Rotger, Baleares), Eloy Elices Palomar (Clínica Rotger, Baleares), Esteban Taleti Depego (H. Mateu Orfila, Baleares), Bartolomé Rossiñol Miralles (C. de Neurodiagnosis, Baleares), Javier Esteve Balzola (H. Pius de Valls, Tarragona), Fernando Herrero Cerezo (H. de Mataró, Barcelona), Jordi Batlle Nadal (H. Santa Tecla, Tarragona), Jaume Burcet Darde (H. El Vendrell, Tarragona), Fernando Espada Olivan (H. Sant Jaume, Girona), Joan Bello López (H. G. de L’Hospitalet, Barcelona), Asunción Ávila Rivera (H. G. de L’Hospitalet, Barcelona), Francesc Valldeoriola Serra (H. Clínic i Provincial de Barcelona, Barcelona), Serge Jaumà Classen (H. U. de Bellvitge, Barcelona), Laura Gubieras Lillo (H. Duran i Reynals, Barcelona), Ana Jaén Peraire (CAP Numancia, Barcelona), Alain Luna Rodríguez (H. de Palamós, Girona), Mercè Martínez Corral (H. Sant Joan de Déu, Martorell, Barcelona), Matilde Calopa Garriga (H. U. de Bellvitge, Barcelona), Joan Massons Cirera (H. Sagrat Cor, L’Aliança, Barcelona), Jaume Camp de la Creu Fumado (H. Universitari de Bellvitge, Barcelona), Luis Borrás Roca (ABS Manso 4-Casanovas, Barcelona), Núria Caballol Pons (CAP La Rambla, Barcelona), Sonia Huertas Folch (H. Mutua de Terrassa, Barcelona), Sonsoles Aranceta Arilla (CAP Cerdanyola-Ripollet, Barcelona), Antonio Palasí Franco (H. G. Vall d’Hebron, Barcelona), César Castejón Gabriel (ABS Chafarinas, Barcelona), Marta Fragoso Martínez (Consorci Sanitari de Terrassa, Barcelona), Jose Antonio Arenas Sánchez (Clínica Ponent, Lleida), M. Eugenia Villar Villar (C. Especialidades Móstoles, Madrid), Pilar Sánchez Alonso (H. Puerta de Hierro, Madrid), María Ángeles del Real Francia (H. G. de Ciudad Real, Ciudad Real), Ramón Ernesto Ibáñez Alonso (H. G. de Ciudad Real, Ciudad Real), Fernando Alonso Frech (H. de Fuenlabrada, Madrid), Jacinto Duarte (H. G. de Segovia, Segovia), José Balseiro Gómez (H. de Getafe, Madrid), Samira Fanjul Arbos (H. Severo Ochoa, Madrid), Cristina Ruiz Huete (Sanatorio del Rosario, Madrid), Jerónimo Almajano Martínez (H. U. 12 de Octubre, Madrid), Antonio Yusta Izquierdo (H. G. Universitario Guadalajara, Guadalajara), Miguel Angel Conde Sendin (C. Salud Federica Montseny, Madrid), Miguel Angel Garcia Soldevila (C. Salud Virgen del Val, Madrid), Yasmina El Berdei Montero (A. Central Salamanca, Salamanca), Julián Benito León (A. Villaverde Cruce, Madrid), Hari Bhathal Guede (C. Especialidades Peña Prieta-Hermanos Sangro, Madrid), Paloma Alonso Béjar (H. del Tajo, Madrid), Yolanda Fernández Bullido (A. Moratalaz, Madrid), Ignacio López-Zuazo Aroca (H. U. de Guadalajara, Guadalajara), Wadih Bowakim Dib (H. G. U. de Alicante, Alicante), Antonio Ortiz Pascual (H. C. Cruz Roja, Madrid), Manuel Lara Lara (H. La Paz, Madrid), José Miguel Velázquez Pérez (H. Virgen de la Salud, Toledo), Pedro Enrique Jiménez Caballero (H. Virgen de la Salud, Toledo), Jesús Olivares Romero (H. Torrecardenas, Almería), Francisco Pérez Errazquin (H. Virgen de la Victoria, Málaga), María José Gómez Heredia (H. Virgen de la Victoria, Málaga), José María Ramírez Moreno (H. U. Infanta Cristina, Badajoz), Juan José Asencio Marchante (H. U. Puerto Real, Cádiz), Francisca Terriza Garcia (H. G. Santa Maria del Puerto, Cádiz), Esther Dionisia Cancho García (C. Especialidades Don Benito, Badajoz), José Carlos Estevez María (CPE VVA, Serena, Badajoz), Francisco Sánchez Caballero (H. Llerena, Badajoz), M. Carmen García de Casasola García (H. Rambla, S.L., Santa Cruz de Tenerife), Jesús Norelys Lorenzo Brito (H. Ntra. Sra. de la Candelaria, Santa Cruz de Tenerife), José Andrés Suárez Muñoz (H. G. de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria), Pablo Eguia del Rio (H. G. de Lanzarote, Las Palmas de Gran Canaria), Santiago Díaz Nicolás (H. G. de Gran Canaria Dr. Negrín, Las Palmas de Gran Canaria), Carlos Perla Muedra (H. Arnau de Vilanova, Lleida), Vicente Fernando Peset Mancebo (C. Salut Aldaia-Antic Regne, Valencia), José F. Folgado Montesinos (H. G. d’Ontinyent, Valencia), José Andrés Domínguez Morán (H. de la Ribera, Valencia), José Cerdà Fayos (H. Provincial de Castellón, Castellón), Pilar Taberner Andrés (H. U. Dr. Peset Aleixandre, Valencia), Alejandro Ponz De Tienda (H. Clínico U. de Valencia, Valencia), Silvia Martí Martínez (H. G. U. de Alicante, Alicante), José Manuel Puentes Gil (H. G. de Albacete, Albacete), Vicente Medrano Martínez (H. G. de Elda, Alicante), Eduardo Kahn Mesia (H. Santa María del Rosell, Murcia), Juan José Soria Torrecillas (H. Santa María del Rosell, Murcia), María Álvarez Sauco (H. G. U. de Elche, Alicante) and Francisco Gracia Fleta (H. G. U. de Alicante, Alicante).
Please cite this article as: Martínez-Martín P, Hernández B, Ricart J, en representación del Grupo de Trabajo del Estudio FAST. Factores determinantes del inicio de tratamiento con levodopa/carbidopa/entacapona en pacientes españoles con enfermedad de Parkinson. Neurología. 2014;29:153–160.
Members of the FAST Study Group are listed in Appendix A.