Very little has been written on seizure management in palliative care (PC). Given this situation, and considering the forthcoming setting up of the Palliative Care Unit at our neurorehabilitation centre, the Clínica San Vicente, we decided to establish a series of guidelines on the use of antiepileptic drugs (AEDs) for handling seizures in PC.
MethodsWe conducted a literature search in PubMed to identify articles, recent manuals, and clinical practice guidelines on seizure management in PC published by the most relevant scientific societies.
ResultsClinical practice guidelines are essential to identify patients eligible for PC, manage seizures adequately, and avoid unnecessary distress to these patients and their families. Given the profile of these patients, we recommend choosing AEDs with a low interaction potential and which can be administered by the parenteral route, preferably intravenously. Diazepam and midazolam appear to be the most suitable AEDs during the acute phase whereas levetiracetam, valproic acid, and lacosamide are recommended for refractory cases and long-term treatment.
ConclusionsThese guidelines provide general recommendations that must be adapted to each particular clinical case. Nevertheless, we will require further well-designed randomised controlled clinical trials including large samples of patients eligible for PC to draft a consensus document recommending adequate, rational, and effective use of AEDs, based on a high level of evidence, in this highly complex area of medical care.
Dada la escasez de directrices abordando este tema y con motivo de la futura creación de la Unidad de Cuidados Paliativos (CP) en nuestro centro de neurorrehabilitación, los miembros del equipo médico de la Clínica San Vicente hemos decidido proponer una serie de sugerencias sobre el empleo de fármacos antiepilépticos (FAEs) en el manejo de las crisis epilépticas (CEs) en CP.
MétodosBúsqueda de artículos en PubMed, últimos libros y recomendaciones de las guías de práctica clínica y sociedades científicas publicadas más relevantes, referentes al manejo de las CEs en CP.
ResultadosLa confección de este tipo de guías, además de identificar pacientes candidatos a recibir CP, es fundamental para garantizar un buen control sintomático de las CEs y evitar el sufrimiento innecesario de estos enfermos y sus familiares. Dadas las características de estos pacientes, se recomienda usar FAEs con presentación vía parenteral (preferiblemente intravenosa) y un perfil bajo de interacciones. Diazepam y/o midazolam serían los más idóneos para la fase aguda, y levetiracetam, ácido valproico y/o lacosamida para casos refractarios y/o como tratamiento crónico.
ConclusionesEstas recomendaciones deben considerarse una guía de abordaje integral, debiendo adaptarse a la idiosincrasia de cada caso clínico en particular. Sin embargo, se necesitan ensayos clínicos controlados, aleatorizados, bien diseñados, que incluyan muestras amplias de pacientes subsidiarios de CP, para redactar un documento de consenso que permita recomendar con un mayor nivel de evidencia y de forma generalizada, la utilización adecuada, racional y efectiva de FAEs en este ámbito médico-asistencial de elevada complejidad.
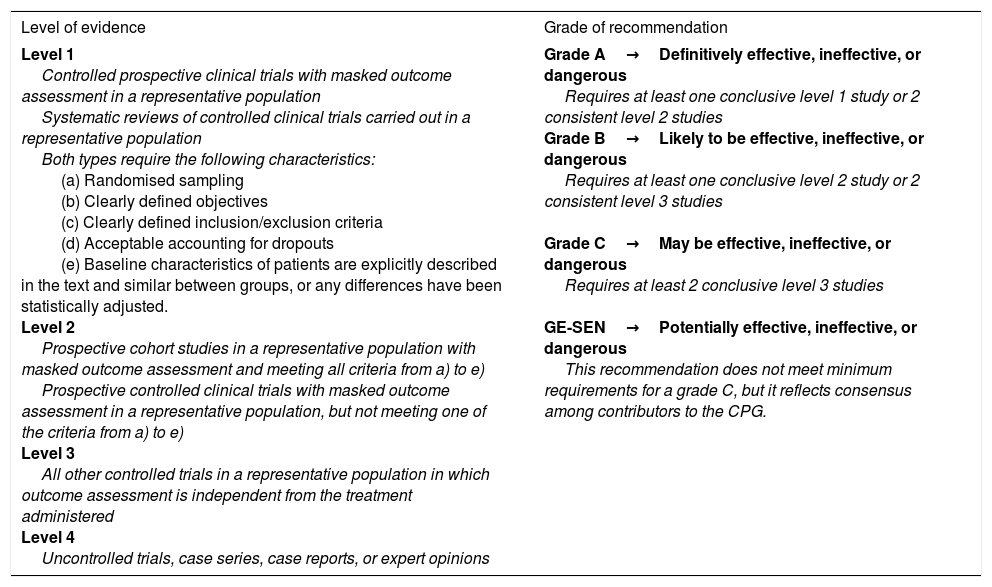
Population ageing and the increasing prevalence of cancer and chronic degenerative diseases constitute a significant challenge for health services. At the end of their lives, many of these patients are severely sick and require care involving all levels of the health service. Between 50% and 60% of people who die in Spain are estimated to have experienced a process of deterioration in the final year of life. Patients in this period of life are thought to account for 8% to 22% of hospital admissions.1–3 There is also a societal demand for quality, cost-effective, person-centred care enabling patients to live and die with dignity. The above underscores the need to reconsider the aims of today's medicine, which so far has excessively emphasised curative care. In his 2000 article in the prestigious New England Journal of Medicine, Callaghan4 argued for the recognition of a peaceful death as an objective of equal value and importance as prolonging life and fighting disease. The neurological deficits observed in patients with brain tumours may result from the primary effects of cancer, systemic complications, or from adverse reactions to oncological treatment. In the first stages of the neoplastic process, neurorehabilitation generally aims to restore patients’ cognitive function following cancer treatment, whereas at later stages it focuses on maintaining their autonomy and quality of life. Neurorehabilitation has been shown to be beneficial, especially in the acute phase of oncological disease, with functional gains comparable to those achieved by other models designed for such other neurological diseases as stroke or traumatic brain injury. Despite this evidence, neurorehabilitation is underused in treating these patients.5 The overall view of palliative care (PC) is that such treatment may be beneficial for patients with irreversible, progressive, non-cancer diseases with a terminal phase, such as advanced-stage chronic obstructive pulmonary disease; heart, liver, or kidney failure; and such neurological diseases as stroke, dementia, Parkinson's disease, multiple sclerosis, and amyotrophic lateral sclerosis.1–8 However, the present guidelines focus on oncological patients, who merit special consideration on account of the increasing prevalence of cancer and the interactions between antiepileptic treatment (especially with classic antiepileptic drugs [AED]) and antineoplastic drugs.1–10 To that end, we performed a systematic literature review of the most important articles, books, and clinical practice guidelines (CPG) published in the last 16 years (taking the PC CPG from the Spanish National Health System's National Plan as the reference document up to 2016, the year of publication of the Spanish Society of Neurology's latest official CPG for epilepsy, as well as the systematic review by Sauro et al.10 on the current situation of epilepsy guidelines) in order to produce an appropriate model for the management of epileptic seizures in PC. Scientific evidence is classified according to the revised recommendations of the European Federation of Neurological Societies, published in 2004 (Table 1).11
Classification of level of evidence for therapeutic actions.
| Level of evidence | Grade of recommendation |
|---|---|
| Level 1 Controlled prospective clinical trials with masked outcome assessment in a representative population Systematic reviews of controlled clinical trials carried out in a representative population Both types require the following characteristics: (a) Randomised sampling (b) Clearly defined objectives (c) Clearly defined inclusion/exclusion criteria (d) Acceptable accounting for dropouts (e) Baseline characteristics of patients are explicitly described in the text and similar between groups, or any differences have been statistically adjusted. Level 2 Prospective cohort studies in a representative population with masked outcome assessment and meeting all criteria from a) to e) Prospective controlled clinical trials with masked outcome assessment in a representative population, but not meeting one of the criteria from a) to e) Level 3 All other controlled trials in a representative population in which outcome assessment is independent from the treatment administered Level 4 Uncontrolled trials, case series, case reports, or expert opinions | Grade A→Definitively effective, ineffective, or dangerous Requires at least one conclusive level 1 study or 2 consistent level 2 studies Grade B→Likely to be effective, ineffective, or dangerous Requires at least one conclusive level 2 study or 2 consistent level 3 studies Grade C→May be effective, ineffective, or dangerous Requires at least 2 conclusive level 3 studies GE-SEN→Potentially effective, ineffective, or dangerous This recommendation does not meet minimum requirements for a grade C, but it reflects consensus among contributors to the CPG. |
CPG: clinical practice guidelines; GE-SEN: Epilepsy Study Group of the Spanish Society of Neurology.
Taken with permission from Mercadé Cerdá et al.,51 2016.
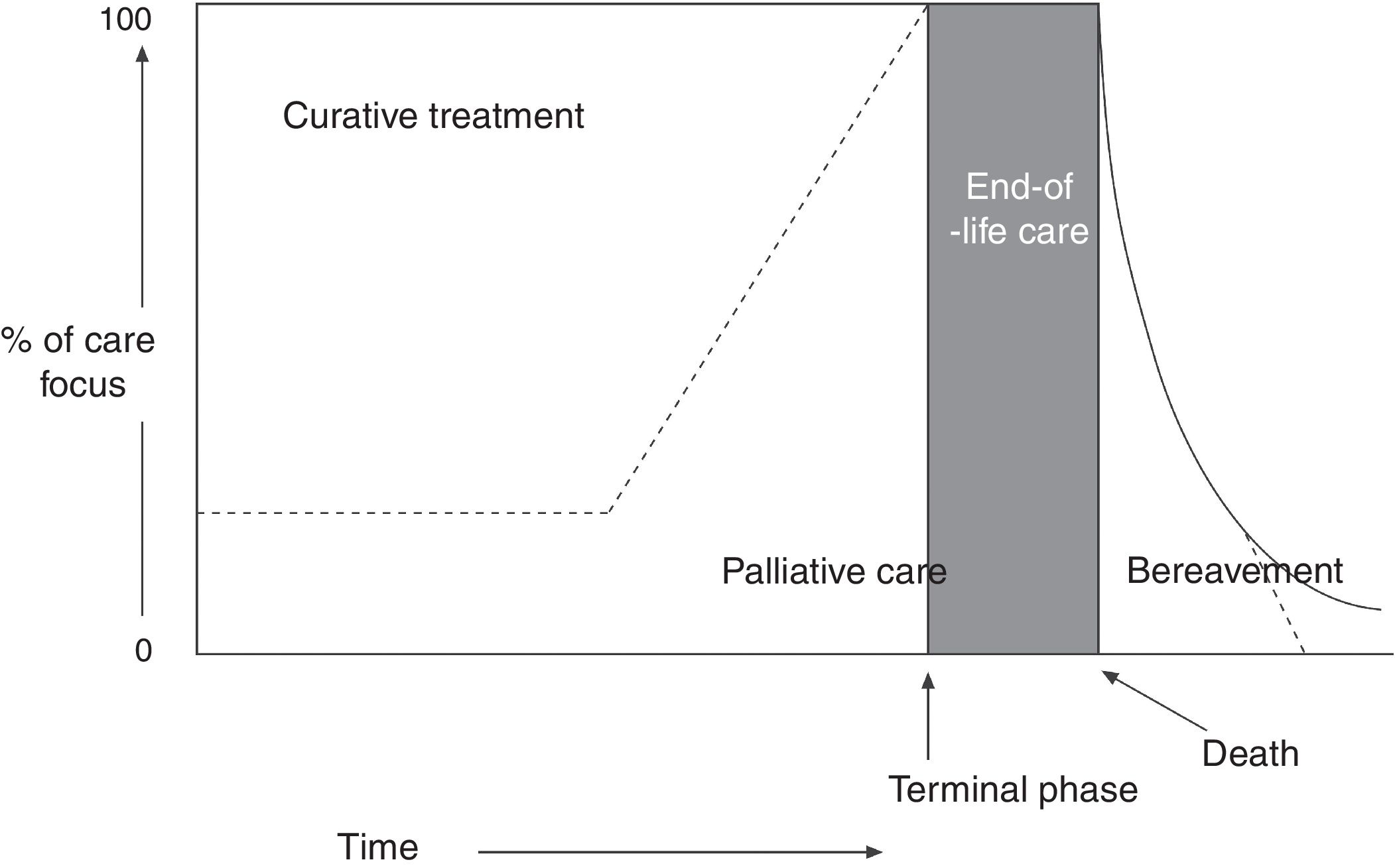
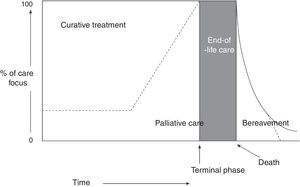
The World Health Organization (WHO) defines PC as “an approach that improves the quality of life of patients and their families facing the problem associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial, and spiritual.” Furthermore, healthcare professionals should build relationships with patients and their families in order to respond to their needs. According to the WHO definition,1,2 PC: (a) provides relief from pain and other distressing symptoms; (b) affirms life and regards dying as a normal process; (c) intends neither to hasten or postpone death; (d) integrates the psychological and spiritual aspects of patient care; (e) offers a support system to help patients live as actively as possible until death; (f) offers a support system to help the family cope during the patient's illness and in their own bereavement; (g) will enhance quality of life; (h) is applicable early in the course of illness, in conjunction with other therapies that are intended to prolong life, such as chemotherapy or radiation therapy (Fig. 1); and (i) includes those investigations needed to better understand and manage distressing clinical complications.
Conceptual, chronological representation of palliative care. PC is implemented alongside curative care following diagnosis of a life-threatening disease. Similarly, even at the final stages of disease, when care is predominantly palliative, there may continue to be a place for curative care. At the final stage of life, curative care ends, and palliative care makes way for terminal care. Finally, the family's bereavement may require specialised care over a prolonged period. Source: Working Group for Clinical Practice Guidelines in Palliative Care.1 Figure adapted with permission from Koekkoek et al.,2 2016.
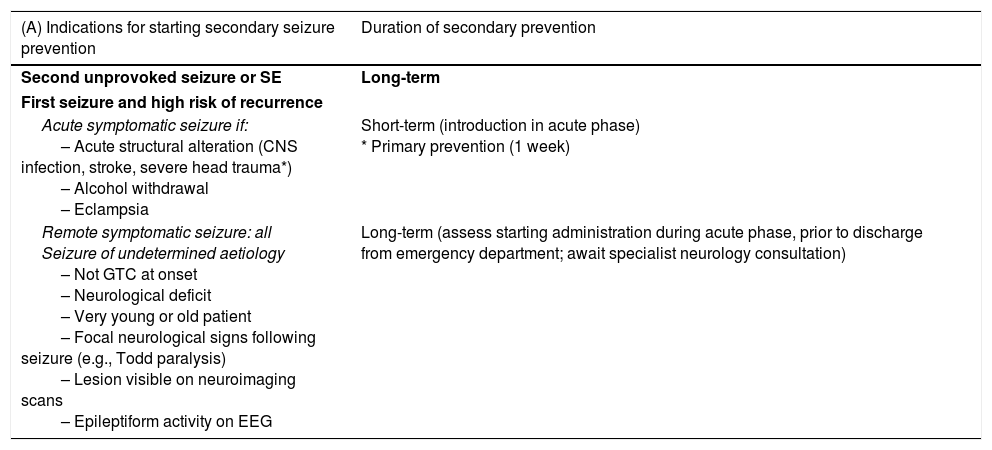
A. Terminal illness: a situation of incurable, progressive, advanced-stage disease with no reasonable expectation of response to a specific treatment; which gives rise to such issues as severe, changing, multifactorial symptoms with a great emotional impact on patients, their family members, and care teams; which has a limited vital prognosis; which is associated with significant demand for care; and in which the fundamental objective consists in promoting well-being and quality of life for patients and their families through control of symptoms, emotional support, and communication (based on the Spanish National Palliative Care Strategy).8
B. Palliative patient: a patient with an advanced, progressive, incurable disease for which specific treatment has been optimised as far as possible, who has multiple problems and/or severe symptoms that do not improve despite proper treatment, and whose vital prognosis is limited. They may be oncological or non-oncological patients (Table 2).6,8
Criteria for terminal illness in cancer patients and other patients.6,8
| Cancer patient (the focus of these guidelines) | Non-cancer patient |
|---|---|
| – Presence of an advanced, progressive, incurable oncological disease with a confirmed histological diagnosis, after provision of standard effective treatment. Histological diagnosis is not required in exceptional circumstances in which, given the patient's clinical situation, it is not considered appropriate to perform a comprehensive examination of the tumour; potentially treatable tumours must be excluded. – Low or zero possibility of response to the active, targeted treatment. In certain scenarios, specific resources must be used due to their positive impact on quality of life (oral chemotherapy, radiotherapy, hormone therapy, bisphosphonates, third- or fourth-line drugs, etc.). – Intense, multiple, multicausal, and changing problems or symptoms – Process of dying has an emotional impact on the patient, family, and care team – Specialist opinion that vital prognosis is limited to the last months of life, except in complex clinical situations in which PC is recommended due to an anticipated improvement in quality of life | – Presence of an advanced, progressive disease with no response to medical or surgical treatment – Specific treatment for the underlying disease has been optimised as far as possible. Where a specific treatment exists, it generally must be maintained until the final stage of disease. Replacing specific treatment with palliative care is justified only in the final stage of life. – Intense, multiple, multicausal, and changing problems or symptoms, despite specific treatment, causing repeated emergency department visits, hospital admissions, etc. over a period of 6 months – The explicit or implicit presence of death has an emotional impact on the patient, family, and care team, leading to numerous requests for health care visits to the home, care homes, etc. – Limited vital prognosis: patients closer to the end of life are eligible for specific care. For the majority of diseases, palliative care candidates are identified according to diagnosis and prognosis. |
| Criteria for terminal illness for specific non-oncological conditions | |
| – CHF: CHF symptoms when at rest, despite treatment (including at least one ACE inhibitor and one diuretic); CHF grade IV with LVEF≤20%. Refractory arrhythmia, history of syncope, and/or severe dyspnoea – Chronic respiratory failure and chronic obstructive pulmonary disease: very severe chronic obstructive pulmonary disease (stage IV) with a LVEF<30% or a LVEF < 50% combined with cor pulmonale or right cardiac failure, hypoxaemia at rest with domiciliary oxygen therapy (PO2≤55mm Hg or O2 saturation≤88%), hypercapnia (PCO2>45mmHg), weight loss of ≥ 10% over the previous 6 months, and or tachycardia at rest of ≥ 100bpm Liver failure: patient not eligible for liver transplant; ascites with no response to fluid restriction or diuretics; bacterial peritonitis; hepatorenal syndrome; hepatic encephalopathy with no response to protein restriction, lactulose, or neomycin; recurrent bleeding due to oesophageal varices despite liver transplant; progressive weight loss; and/or malnutrition – Kidney failure: patient potentially eligible for dialysis but refuses this treatment or kidney transplant; life expectancy less than 6 months; oliguria (< 400mL/24h); uraemic pericarditis; and/or hepatorenal syndrome – Advanced dementia: very severe cognitive impairment with patient unable to produce meaningful verbal communication, recognise carers, etc.; medical complications arising in the last year, including aspiration pneumonia, urinary tract infections, sepsis, recurrent fever following antibiotherapy, and/or dysphagia – Amyotrophic lateral sclerosis: treatment is always palliative, as the condition causes motor neuron death without affecting sensory neurons, eye muscles, sphincter control, or cognitive function (these patients always meet criteria for PC, regardless of severity). |
ACE: angiotensin-converting enzyme; CHF: chronic heart failure; LVEF: left ventricle ejection fraction; PC: palliative care.
Epilepsy and cancer.1–104 Epilepsy affects 0.5% to 1% of the population, with peak incidence during childhood and old age.26 Seizures are common in PC, especially in patients with brain tumours; up to 88% of patients with glioma (the most frequent type of primary brain tumour) present seizures at some point of tumour progression.5,78–89,100 Seizure origin appears to be multifactorial, with significant involvement of the healthy peritumoural neuronal tissue.78,90 Seizure control is essential in the management of these patients, and constitutes a frequent reason for consultation. The impact of seizures on the health system justifies a diagnostic and therapeutic approach centred around appropriate, effective protocols to be implemented in the shortest possible time. It should be taken into account at all times that not all convulsive seizures are epileptic, and not all epileptic seizures are convulsive.55 Up to 4% of oncological patients have seizures due to other causes (Table 3).57–64
Aetiology.1–106 Central nervous system (CNS) tumours are the most common cause of epilepsy in the 41-60-year age group,26 making seizures a common, severe complication of cancer. The most frequent cause is intracranial tumour,52 which is common in cases of dysembryoplastic neuroepithelial tumour (100%); ganglioglioma (80%-90%); primary glial tumours, particularly low-grade glioma (75%),92,93 which promote epileptogenesis through astrocytic glutamate release78,90; meningioma (22%-60%); glioblastoma multiforme (29%-49%); brain metastases (20%-35%); leptomeningeal tumours (10%-15%); and brain primary CNS lymphoma (10%). Seizures are the first semiological manifestation of the tumour in 45% of cases, and are more common in patients with multiple lesions and/or melanoma in histological studies.65,81,82,100,101 Tumour growth initially generates focal signs; in more than 80% of cases, they are detected subsequently to the diagnosis of primary tumour (metachronous metastasis). They are less frequently diagnosed as the first manifestation of the disease (synchronous metastasis).65 As mentioned above, seizures can be caused by multiple factors (Table 2)1: (a) Primary brain tumours and brain metastases, particularly from lung cancer, breast cancer, or melanoma (multiple lesions are most frequent in the latter case), with brain metastases being less common in prostate, oropharyngeal, or skin cancer; (b) chemotherapy, especially at high doses and in the event of liver/kidney failure; (c) metabolic disorders, caused either by the tumour itself (hypercalcaemia in lung, prostate, or breast cancer and multiple myeloma) or by drugs (cyclophosphamide-induced hyponatraemia, bisphosphonate-induced hypocalcaemia, cisplatin-induced hypomagnesaemia, etc.); (d) drugs (Table 3); (e) paraneoplastic syndromes; (f) cerebrovascular diseases (stroke, venous sinus thrombosis, etc.; seizures were recorded in 8% of a series of 96 patients with stroke and cancer at the Memorial Sloan Kettering Cancer Center in New York76); (g) CNS infections (mainly herpesvirus infection); (h) acquired immunodeficiency syndrome (AIDS) (cryptococcosis, neurotoxoplasmosis, acute fulminant encephalopathy, etc.)105,106; and (i) cranial radiotherapy (Table 3).
Aetiology of seizures in patients with cancer.
| Related to CNS involvement |
| — Primary brain tumour |
| — Brain metastases |
| — Cerebrovascular diseases |
| — Reversible posterior leukoencephalopathy syndrome |
| — Meningoencephalitis |
| — Leptomeningeal metastases, etc. |
| Treatment-related |
| — Chemotherapy: cytarabine, methotrexate, cisplatin, bevacizumab, etoposide, interferon alfa, ifosfamide, cyclophosphamide, L-asparaginase, vincristine, interleukin-2, nitrosoureas (carmustine, lomustine), anthracyclines (doxorubicin), etc. |
| — Toxic/metabolic: kidney failure, liver failure, tumour lysis syndrome, thrombotic thrombocytopenic purpura, hydroelectrolytic disorders, hypoglycaemia, hypoxia/pulmonary embolism, etc. |
| — Other drugs: pethidine, neuroleptics, bisphosphonates, ondansetron, imipenem, etc. |
| — Cranial radiotherapy: radiation-induced acute encephalopathy, radiation-induced temporal lobe necrosis, etc. |
CNS: central nervous system.
Adapted with permission from Corredera García and Becerra Cuñat,57 2012.
Clinical manifestation.1–78 Patients may display different types of seizure and even status epilepticus (SE). Seizure type depends on the speed of tumour growth and the degree of circumscription; temporal, frontal, and parietal tumours most frequently cause focal seizures.52 SE is rare in patients with brain tumours, but is associated with a mortality rate of 6%-35%.78
2010 and 2015 definitions and classifications of the International League Against Epilepsy7–51,107–116A. Convulsion: involuntary movement, generally sustained (tonic) or interrupted (clonic), resulting from an alteration in brain function caused by an abnormal, asynchronous, self-limited discharge of CNS neurons.
B. Epileptic seizure: the clinical manifestation of these discharges; focal (partial) seizures involve one hemisphere, whereas generalised seizures involve both. The clinical expression of any epileptic seizure may include impaired consciousness and/or motor, sensory, autonomic, or psychic manifestations perceived by the patient or in many cases by external bystanders. These episodes are usually stereotyped, paroxysmal, brief, and transient or self-limited.32,44
C. Status epilepticus: the second most common neurological emergency after stroke.38 SE is of great relevance as it is associated with high rates of morbidity and mortality; prognosis is established in terms of survival.23 SE is “a condition characterised by an epileptic seizure that is sufficiently prolonged or repeated at sufficiently brief intervals so as to produce an unvarying and enduring epileptic condition.”32,44 There are 2 subtypes: (1) convulsive (CSE): epileptic activity characterised by (a) ≥ 5minutes continuous tonic–clonic seizure, ≥ 10minutes focal seizure with altered level of consciousness, or ≥ 10-15minutes absence seizure; (b) ≥ 2 seizures without recovery between seizures; or (c) seizures in clusters (≥ 3 convulsive seizures within 24h)32–44,51,111–116; and (2) nonconvulsive (NCSE): seizure with a continuous abnormal electroencephalography (EEG) trace and no recognisable or predominant motor activity. The typical clinical manifestation of this type of SE is impaired consciousness.
D. Refractory SE (RSE): SE persisting despite treatment with 2 indicated AEDs (first- and second-line) at the correct dose, or epileptic activity lasting ≥ 30minutes SE is refractory in 31%-43% of patients, of whom it is necessary in almost half to induce coma in order to control seizures; RSE is associated with a mortality rate of up to 39%.107
E. Super-refractory SE: epileptic activity lasting ≥ 24hours despite AED treatment.
F. Epilepsy: a chronic condition characterised by predisposition to recurrent epileptic seizures (≥ 2 seizures or 1 seizure if a structural lesion is identified via complementary testing, neuroimaging, and/or EEG), with a cognitive and/or psychosocial impact.
G. Refractory epilepsy: a situation in which seizure freedom is not achieved after trying at least 2 appropriate AEDs, in monotherapy or in combination, administered correctly, and not withdrawn due to intolerance. Seizure freedom is defined as freedom from seizures for a minimum of 3 times the longest preintervention interseizure interval in the 12 months prior to treatment, or 12 months, whichever period is longest.14,15 Epilepsy is refractory in 12%-50% of patients with brain tumours, especially low-grade tumours; multidrug resistance genes are thought to be involved.82
For practical and treatment purposes, seizures should be classified by their clinical and aetiological characteristics27–74: (a) 2010 classification of the International League Against Epilepsy (Table 4)16,17,27–30,48,49,101; (b) aetiological classification of seizures (Table 5)27,31; (c) 2015 classification of SE.26–44 Theoretically, there are as many types of SE as there are types of seizure. In clinical practice, we refer to SE and NCSE (Tables 6 and 7).
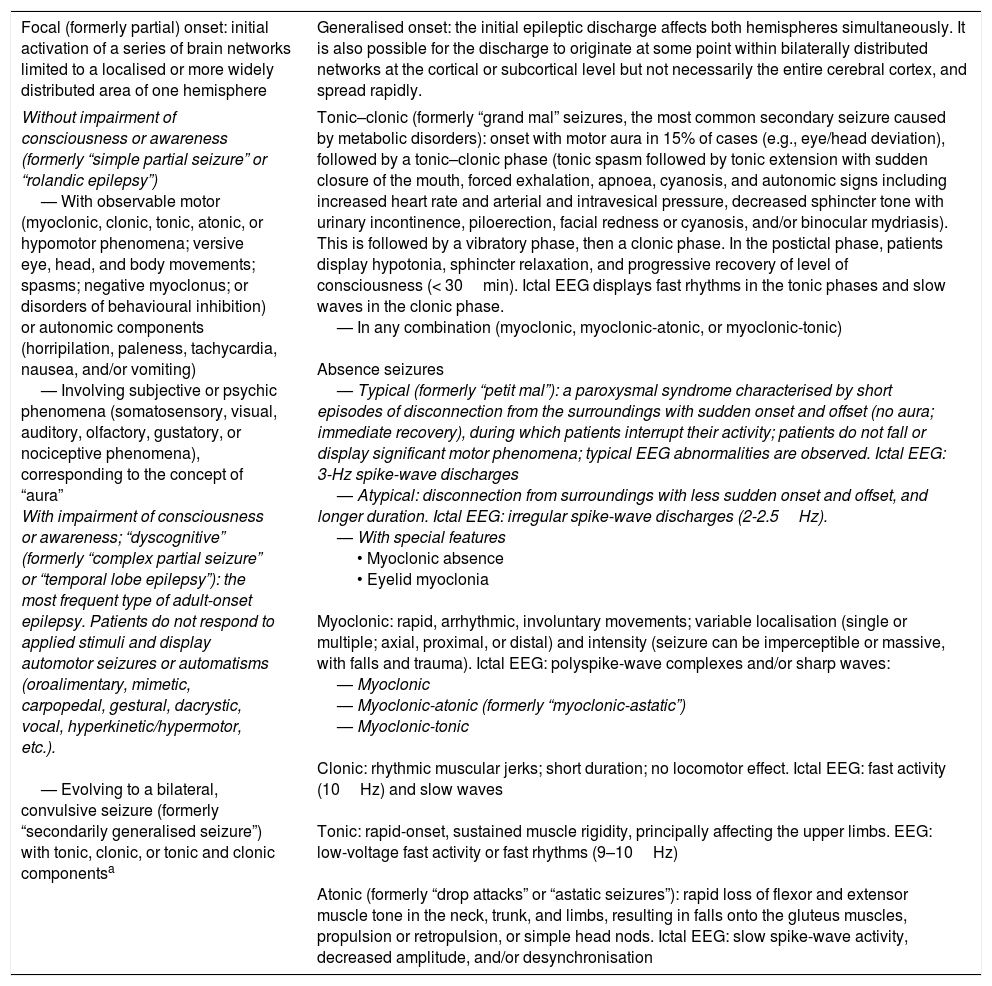
Classification of seizure types according to the 2010 International League Against Epilepsy criteria.16–18,27–30,50,51,101
| Focal (formerly partial) onset: initial activation of a series of brain networks limited to a localised or more widely distributed area of one hemisphere | Generalised onset: the initial epileptic discharge affects both hemispheres simultaneously. It is also possible for the discharge to originate at some point within bilaterally distributed networks at the cortical or subcortical level but not necessarily the entire cerebral cortex, and spread rapidly. |
|---|---|
| Without impairment of consciousness or awareness (formerly “simple partial seizure” or “rolandic epilepsy”) — With observable motor (myoclonic, clonic, tonic, atonic, or hypomotor phenomena; versive eye, head, and body movements; spasms; negative myoclonus; or disorders of behavioural inhibition) or autonomic components (horripilation, paleness, tachycardia, nausea, and/or vomiting) — Involving subjective or psychic phenomena (somatosensory, visual, auditory, olfactory, gustatory, or nociceptive phenomena), corresponding to the concept of “aura” With impairment of consciousness or awareness; “dyscognitive” (formerly “complex partial seizure” or “temporal lobe epilepsy”): the most frequent type of adult-onset epilepsy. Patients do not respond to applied stimuli and display automotor seizures or automatisms (oroalimentary, mimetic, carpopedal, gestural, dacrystic, vocal, hyperkinetic/hypermotor, etc.). — Evolving to a bilateral, convulsive seizure (formerly “secondarily generalised seizure”) with tonic, clonic, or tonic and clonic componentsa | Tonic–clonic (formerly “grand mal” seizures, the most common secondary seizure caused by metabolic disorders): onset with motor aura in 15% of cases (e.g., eye/head deviation), followed by a tonic–clonic phase (tonic spasm followed by tonic extension with sudden closure of the mouth, forced exhalation, apnoea, cyanosis, and autonomic signs including increased heart rate and arterial and intravesical pressure, decreased sphincter tone with urinary incontinence, piloerection, facial redness or cyanosis, and/or binocular mydriasis). This is followed by a vibratory phase, then a clonic phase. In the postictal phase, patients display hypotonia, sphincter relaxation, and progressive recovery of level of consciousness (< 30min). Ictal EEG displays fast rhythms in the tonic phases and slow waves in the clonic phase. — In any combination (myoclonic, myoclonic-atonic, or myoclonic-tonic) Absence seizures — Typical (formerly “petit mal”): a paroxysmal syndrome characterised by short episodes of disconnection from the surroundings with sudden onset and offset (no aura; immediate recovery), during which patients interrupt their activity; patients do not fall or display significant motor phenomena; typical EEG abnormalities are observed. Ictal EEG: 3-Hz spike-wave discharges — Atypical: disconnection from surroundings with less sudden onset and offset, and longer duration. Ictal EEG: irregular spike-wave discharges (2-2.5Hz). — With special features • Myoclonic absence • Eyelid myoclonia Myoclonic: rapid, arrhythmic, involuntary movements; variable localisation (single or multiple; axial, proximal, or distal) and intensity (seizure can be imperceptible or massive, with falls and trauma). Ictal EEG: polyspike-wave complexes and/or sharp waves: — Myoclonic — Myoclonic-atonic (formerly “myoclonic-astatic”) — Myoclonic-tonic Clonic: rhythmic muscular jerks; short duration; no locomotor effect. Ictal EEG: fast activity (10Hz) and slow waves Tonic: rapid-onset, sustained muscle rigidity, principally affecting the upper limbs. EEG: low-voltage fast activity or fast rhythms (9–10Hz) Atonic (formerly “drop attacks” or “astatic seizures”): rapid loss of flexor and extensor muscle tone in the neck, trunk, and limbs, resulting in falls onto the gluteus muscles, propulsion or retropulsion, or simple head nods. Ictal EEG: slow spike-wave activity, decreased amplitude, and/or desynchronisation |
Another section will address seizures that are unclassifiable due to incomplete or inadequate data, or which do not coincide with the described classification. These include neonatal seizures (e.g., rhythmic eye, chewing, or swimming movements), according to the terminology of the 1981 ILAE classification, and epileptic spasms according to the 2010 document. In the 2010 classification, Berg et al.28 stress the importance of precisely describing seizures, addressing motor, cognitive, autonomic, and/or sensory/subjective manifestations. When these occur sequentially, the order of the different manifestations should also be recorded. Focal motor seizures are the predominant type of seizure observed in patients with brain metastases.90
EEG: electroencephalography.
According to the 2016 ILAE operational classification, there may be a progression to “bilateral tonic–clonic” seizure.30
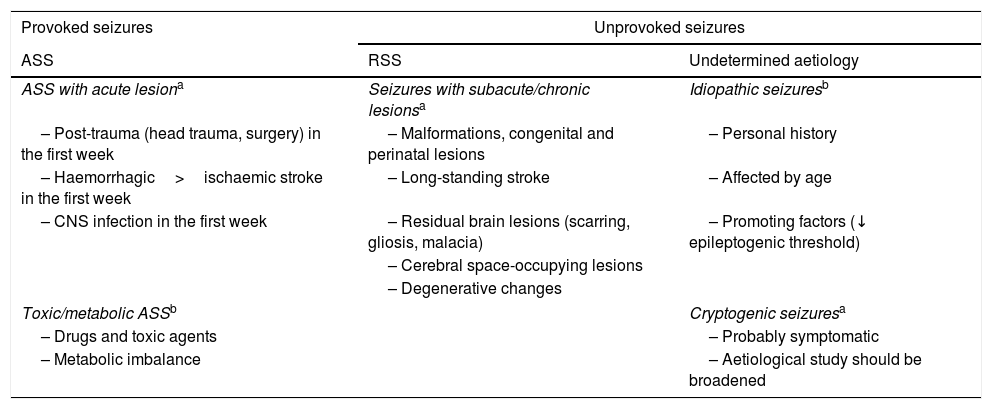
American College of Emergency Physicians aetiological classification of seizures.22,27,31
| Provoked seizures | Unprovoked seizures | |
|---|---|---|
| ASS | RSS | Undetermined aetiology |
| ASS with acute lesiona | Seizures with subacute/chronic lesionsa | Idiopathic seizuresb |
| – Post-trauma (head trauma, surgery) in the first week | – Malformations, congenital and perinatal lesions | – Personal history |
| – Haemorrhagic>ischaemic stroke in the first week | – Long-standing stroke | – Affected by age |
| – CNS infection in the first week | – Residual brain lesions (scarring, gliosis, malacia) | – Promoting factors (↓ epileptogenic threshold) |
| – Cerebral space-occupying lesions | ||
| – Degenerative changes | ||
| Toxic/metabolic ASSb | Cryptogenic seizuresa | |
| – Drugs and toxic agents | – Probably symptomatic | |
| – Metabolic imbalance | – Aetiological study should be broadened | |
ASS: acute symptomatic seizure; CNS: central nervous system; RSS: remote symptomatic seizure.
Adapted with permission from Fernández Alonso et al.,27 2013.
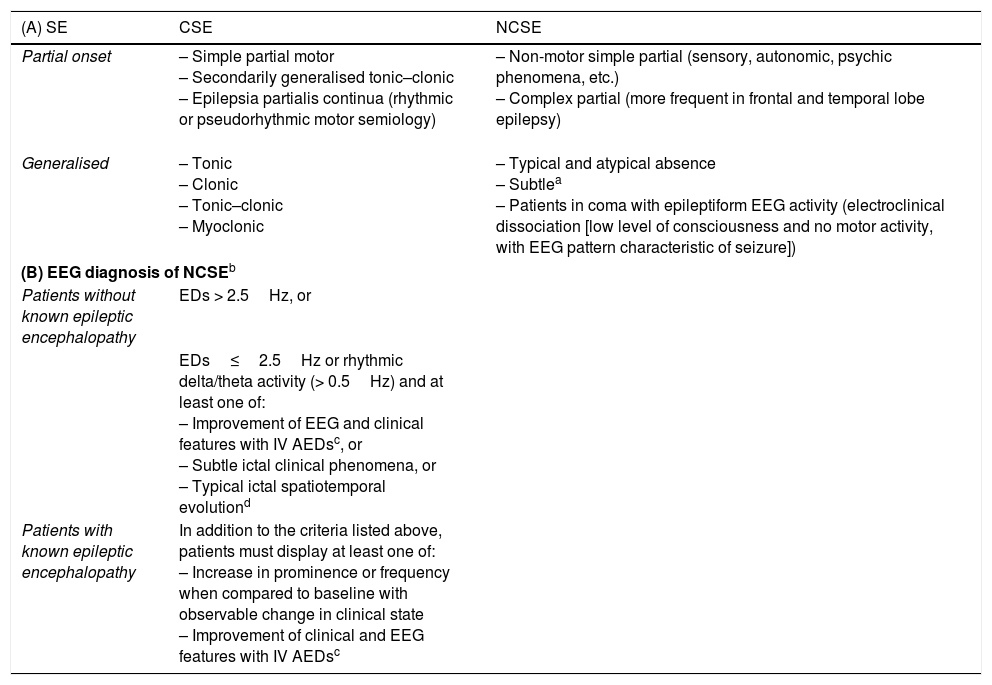
(A) Classical classification of status epilepticus.19,27–37,51 (B) Salzburg consensus criteria for nonconvulsive status epilepticus.
| (A) SE | CSE | NCSE |
|---|---|---|
| Partial onset | – Simple partial motor – Secondarily generalised tonic–clonic – Epilepsia partialis continua (rhythmic or pseudorhythmic motor semiology) | – Non-motor simple partial (sensory, autonomic, psychic phenomena, etc.) – Complex partial (more frequent in frontal and temporal lobe epilepsy) |
| Generalised | – Tonic – Clonic – Tonic–clonic – Myoclonic | – Typical and atypical absence – Subtlea – Patients in coma with epileptiform EEG activity (electroclinical dissociation [low level of consciousness and no motor activity, with EEG pattern characteristic of seizure]) |
| (B) EEG diagnosis of NCSEb | ||
| Patients without known epileptic encephalopathy | EDs > 2.5Hz, or | |
| EDs≤2.5Hz or rhythmic delta/theta activity (> 0.5Hz) and at least one of: – Improvement of EEG and clinical features with IV AEDsc, or – Subtle ictal clinical phenomena, or – Typical ictal spatiotemporal evolutiond | ||
| Patients with known epileptic encephalopathy | In addition to the criteria listed above, patients must display at least one of: – Increase in prominence or frequency when compared to baseline with observable change in clinical state – Improvement of clinical and EEG features with IV AEDsc | |
CSE: convulsive status epilepticus; ED: epileptiform discharge; EEG: electroencephalography; IV AED: intravenous antiepileptic drug; NCSE: nonconvulsive SE; SE: status epilepticus.
aSubtle NCSE: subtle motor activity following the ictal epileptiform activity after offset of evident motor activity following untreated or undertreated generalised CSE. Characterised by minor motor signs (facial and/or distal clonic movements, nystagmus, eye deviation, etc.). These seizures usually manifest in severely ill patients in ICUs, with a severely reduced level of consciousness (generally in coma) and focal brain injury; mortality can reach 65%. It is therefore essential to open these patient's eyes in order to detect the condition.39,40
Adapted with permission from Fernández Alonso et al.,27 2013.
Diagnosis of NCSE should be based on the combination of clinical and EEG findings. Clinical signs lasting ≥ 10min indicate possible NCSE.
NCSE should be suspected if EEG improvements are not associated with clinical improvements, or fluctuate with no defined progression.
Initial increase (increase in voltage and change in frequency), progression of EEG pattern (change in frequency [> 1Hz] or localisation), or decrementing termination (voltage or frequency).
Taken with permission from Trinka and Kälviäinen,44 2016.
2015 International League Against Epilepsy classification of status epilepticus.32–44
| Axis I: semiology | Axis II: aetiology |
|---|---|
| With prominent motor symptoms | |
| A.1 Convulsive SE (CSE, synonym: tonic–clonic SE) | Known |
| A.1.a Generalised convulsive | – Acute (e.g. stroke, intoxication, malaria, encephalitis, etc.) |
| A.1.b Focal onset evolving into bilateral convulsive SE | – Remote (e.g., post-traumatic, postencephalitic, poststroke, etc.) |
| A.1.c Unknown whether focal or generalised | – Progressive (e.g., brain tumour, Lafora's disease and other PMEs, dementias) |
| – SE in defined electroclinical syndromes | |
| Unknown (i.e., cryptogenic) | |
| A.2 Myoclonic SE (prominent epileptic myoclonic jerks) | Axis III: electroencephalographic correlates |
| A.2.a With coma | (1) Location: generalised (including bilateral synchronous patterns), lateralised, bilateral independent, or multifocal |
| A.2.b Without coma | (2) Name of the pattern: periodic discharges, rhythmic delta activity, or spike–slow wave/sharp wave–slow wave complexes, plus subtypes, etc.a |
| (3) Morphology: sharpness, number of phases (e.g., triphasic morphology), absolute and relative amplitude, polarity | |
| (4) Time-related features: prevalence, frequency, duration, daily pattern duration and index, onset (sudden vs gradual), and dynamics (evolving, fluctuating, or static) | |
| (5) Modulation: stimulus-induced vs spontaneous | |
| (6) Effect of intervention (medication) on EEG | |
| A.3 Focal motor | |
| A.3.a Repeated focal motor seizures (Jacksonian) | |
| A.3.b EPC | |
| A.3.c Adversive SE | |
| A.3.d Oculoclonic SE | |
| A.3.e Ictal paresis (e.g., focal inhibitory SE) | |
| A.4 Tonic SE | |
| A.5 Hyperkinetic SE | |
| Without prominent motor symptoms (i.e., NCSE) | |
| B.1 NCSE with coma (including so-called “subtle” SE) | |
| B.2 NCSE without coma | Axis IV: age |
| B.2.a Generalised | (1) Neonatal (0 to 30 days) |
| B.2.a.a Typical absence SE | (2) Infancy (1 month to 2 years) |
| B.2.a.b Atypical absence SE | (3) Childhood (2 to 12 years) |
| B.2.a.c Myoclonic absence SE | (4) Adolescence and adulthood (12 to 59 years) |
| B.2.b Focal | (5) Elderly (≥ 60 years) |
| B.2.b.a Without impairment of consciousness (aura continua, with autonomic, sensory, visual, olfactory, gustatory, emotional/psychic/experiential, or auditory symptoms) | |
| B.2.b.b Aphasic SE | |
| B.2.b.c With impaired consciousness | |
| B.2.c Unknown whether focal or generalised | |
| B.2.c.a Autonomic SE | |
SE is a condition resulting either from the failure of the mechanisms responsible for termination of seizure or from the initiation of mechanisms responsible for prolonged seizures (after time point t1). Depending on the type and duration of the seizure, the long-term consequences of SE (after time point t2) may include neuronal death, neuronal damage, and the alteration of neural networks. This conceptual definition involves 2 time dimensions. The first is the duration of the seizure and the time point (t1) after which seizure can be considered “abnormally prolonged,” and at which point AEDs should be administered, established as 5min for generalised tonic–clonic SE, 10min for focal SE (with or without impaired level of consciousness), and 10-15min for absence SE. Time point (t2), from which point continued epileptic activity may entail a risk of long-term consequences, is established at 30min for generalised tonic–clonic SE.32,44
NB: although generalised convulsive or nonconvulsive SE with coma corresponds almost perfectly to the semiological classification of SE, focal SE is notoriously variable and appears to be better described in the new ILAE classification by Trinka's32 study group, who provide more clinically relevant subdivisions and different mortality rates. This enhanced knowledge enables the development of more precise prognostic scales than the existing clinical tools; these should be taken into account in future epidemiological studies into SE.42
AED: antiepileptic drug; EEG: electroencephalography; EPC: epilepsia partialis continua; ILAE: International League Against Epilepsy; NCSE: nonconvulsive SE; PME: progressive myoclonic epilepsy; SE: status epilepticus.
Diagnosis.1–119 Evidence on the diagnosis and treatment of seizures in patients receiving PC is very scarce; it is therefore necessary to extrapolate from the general population and from patients with cancer.1 The first step in diagnosing seizures is recognising them as such; they must therefore be distinguished from other types of episodic involuntary muscle contraction (e.g., opioid-induced myoclonus), hyperkinesis (e.g., due to haloperidol or prokinetics), and disorders of consciousness related to increased intracranial pressure, which is observed in 85%-94% of patients.1,102 A thorough account of the episode is therefore essential. This step is practically simultaneous with therapeutic decision-making. Postictal aetiological diagnosis requires: (a) detailed medical history and physical examination to rule out other non-epileptic conditions (syncope, transient ischaemic attack, psychogenic non-epileptic seizure, transient global amnesia, etc)20–22; if epileptic seizure is diagnosed, potential trigger factors should be ruled out: non-adherence and/or changes to AED treatment (the most significant cause in patients with known epilepsy), changes in the sleep–wake cycle, infections, systemic diseases, drugs, ingestion of toxic agents, stress, strobe lighting, menstruation, etc; and (b) laboratory testing (complete blood count and biochemical tests for glycaemia, liver and kidney function including Na, K, Ca, Mg, lactate, etc.; plasma AED levels; urine toxicology; arterial blood gas), EEG, and neuroimaging studies (emergency contrast-enhanced CT scan and preferably a brain MRI scan, given its greater diagnostic sensitivity). It is important to be familiar with the indications for: (1) emergency head CT scan: adult patients with no known epilepsy (always) and epileptic patients with severe head trauma (Glasgow Coma Scale score≤8), history of stroke or transient ischaemic attack, unknown focal neurological signs, suspected CNS infection, cancer, immunodeficiency (e.g., due to HIV infection), anticoagulant treatment, suspected subarachnoid haemorrhage, or SE with no obvious cause; (2) emergency EEG: prolonged confusional state, coma of unknown origin (NCSE accounts for up to 8% of patients treated in intensive care units [ICU] and 37% of hospitalised patients with coma and no clinical manifestation of epileptic activity),22,41,117 delayed recovery of level of consciousness after SE, brief episodes of loss of consciousness of unknown origin (rule out absence seizures), acute seizures following trauma (which increase the risk of post-traumatic epilepsy if an irritative zone is observed in the acute-phase [1st week] EEG),118 and/or herpes simplex encephalitis (observation of periodic, lateralised discharges assists in diagnosis; however, these discharges are also observed in patients with stroke, tumours, postanoxic encephalopathy, etc.)119; in cases of high clinical suspicion in which the first EEG showed normal results, it is recommended to perform a sequence including a second EEG, a sleep-deprived EEG, a sleep EEG, and a long-term video-EEG26; and (3) lumbar puncture: in the absence of radiological lesions or metabolic causes, lumbar puncture should be performed to rule out infection and/or meningeal carcinomatosis. Diagnostic tests should be selected according to the patient's condition and the preferences of the patient and his/her family.
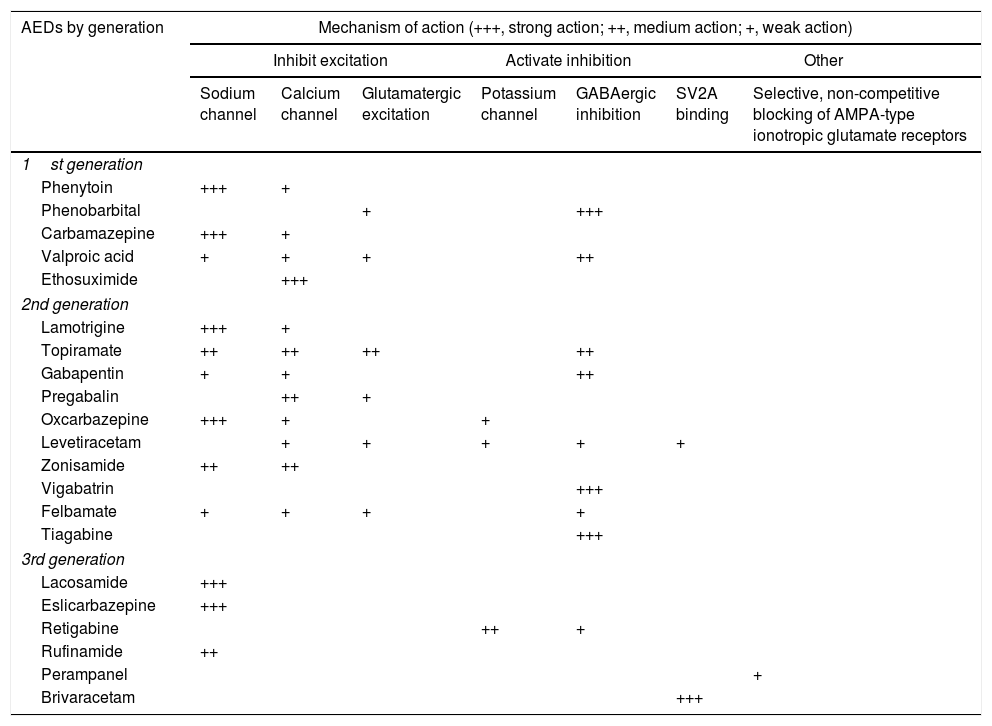
Treatment1–160Antiepileptic drugs.27,31AEDs are currently the main treatment for epilepsy and seizures. Treatment is symptomatic, as no antiepileptogenic drugs are yet available. Table 8 summarises the main mechanisms of action of AEDs, in order to better explain the indications and possible combinations of these drugs:
Antiepileptic drugs and their mechanisms of action.27,49,90,154–158
| AEDs by generation | Mechanism of action (+++, strong action; ++, medium action; +, weak action) | ||||||
|---|---|---|---|---|---|---|---|
| Inhibit excitation | Activate inhibition | Other | |||||
| Sodium channel | Calcium channel | Glutamatergic excitation | Potassium channel | GABAergic inhibition | SV2A binding | Selective, non-competitive blocking of AMPA-type ionotropic glutamate receptors | |
| 1st generation | |||||||
| Phenytoin | +++ | + | |||||
| Phenobarbital | + | +++ | |||||
| Carbamazepine | +++ | + | |||||
| Valproic acid | + | + | + | ++ | |||
| Ethosuximide | +++ | ||||||
| 2nd generation | |||||||
| Lamotrigine | +++ | + | |||||
| Topiramate | ++ | ++ | ++ | ++ | |||
| Gabapentin | + | + | ++ | ||||
| Pregabalin | ++ | + | |||||
| Oxcarbazepine | +++ | + | + | ||||
| Levetiracetam | + | + | + | + | + | ||
| Zonisamide | ++ | ++ | |||||
| Vigabatrin | +++ | ||||||
| Felbamate | + | + | + | + | |||
| Tiagabine | +++ | ||||||
| 3rd generation | |||||||
| Lacosamide | +++ | ||||||
| Eslicarbazepine | +++ | ||||||
| Retigabine | ++ | + | |||||
| Rufinamide | ++ | ||||||
| Perampanel | + | ||||||
| Brivaracetam | +++ | ||||||
AED: antiepileptic drug; AMPA: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; SV2A: synaptic vesicle glycoprotein 2A (involved in neurotransmitter vesicle fusion and exocytosis).
Adapted with permission from Fernández Alonso et al.,27 2013.
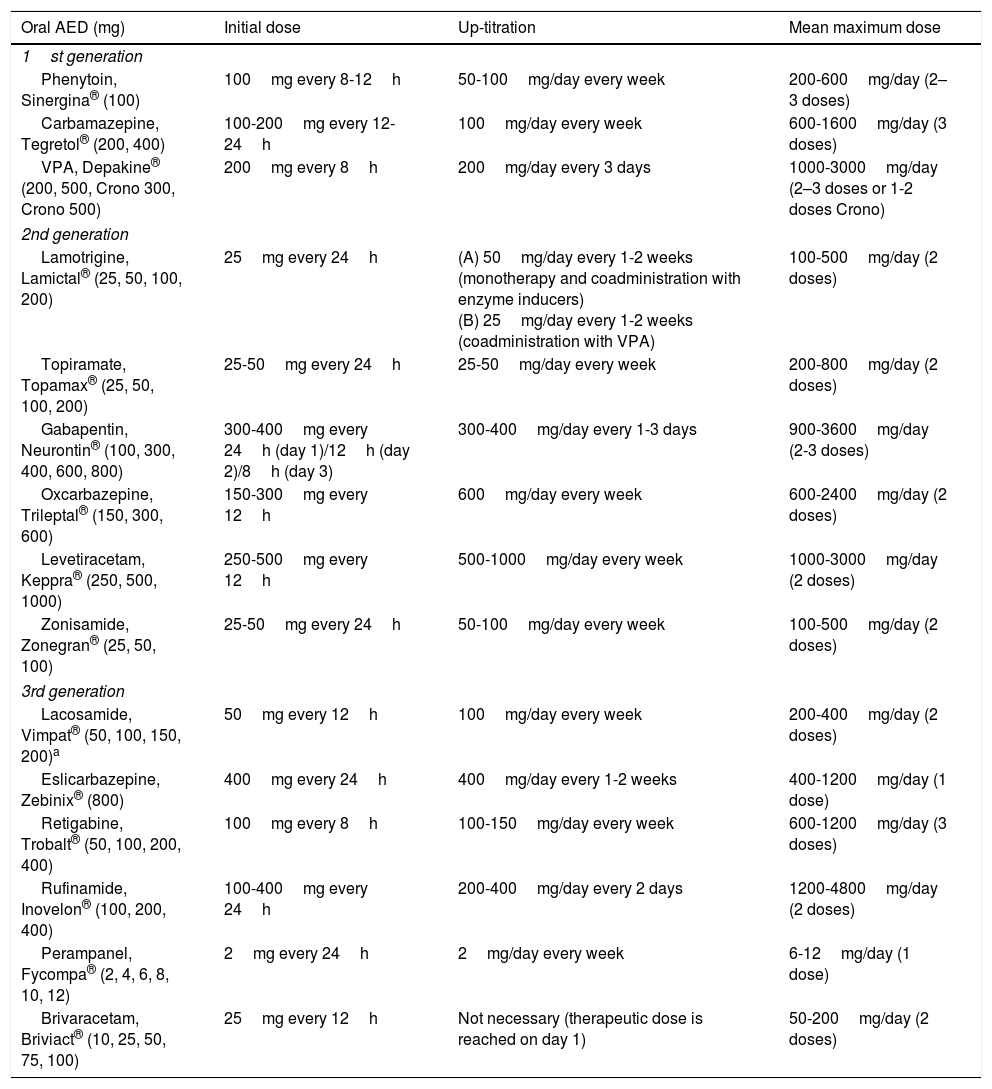
The criteria for selecting an “ideal AED” in emergency departments are: (a) good pharmacological profile: completely and rapidly absorbed via oral route, linear kinetics, low plasma protein binding, extrahepatic metabolism, absence of active metabolites and interactions, renal clearance, and long half-life. In accordance with these criteria, the AEDs with the best pharmacokinetic profiles are levetiracetam (LEV), lacosamide (LCM), gabapentin (GBP), and pregabalin (PGB). Drugs with an intermediate pharmacokinetic profile are eslicarbazepine (ESL), lamotrigine (LTG), oxcarbazepine (OXC), retigabine (RTG), topiramate (TPM), zonisamide (ZNS), and rufinamide (RFN). Finally, the AEDs with the worst kinetics are phenytoin (PHT), carbamazepine (CBZ), valproic acid (VPA), felbamate (FBM), primidone (PRM), and tiagabine (TGB). (b) Parenteral administration, comfortable oral conversion, and possibility of sequential therapy: AEDs must be suitable for parenteral administration (preferably intravenously [IV]). Eighty-five percent of terminally ill patients display dysphagia,85,89 including dysphagia caused by decreased level of consciousness due to the progression of the tumour in the brain and/or adverse drug reactions.85,89,102,103 Parenteral administration enables therapeutic levels to be reached quickly, both in patients with SE and for acute-phase preventive treatment. Where possible, it is desirable to comfortably continue administering the same drug orally (1:1 conversion) and safely at therapeutic doses. Of the AEDs that can be administered intravenously, LEV and LCM meet this criterion. VPA is the next drug of choice, preferable to PHT and anaesthetics. (c) Broad spectrum of action: ideally, the AED selected will be able to control both focal and generalised seizures; because there are often no witnesses to seizures, they are often poorly defined, with unclear medical history. There must also be no risk of exacerbating any specific type of seizure; CBZ and PHT, for example, exacerbate myoclonic and absence seizures (Table 9). LEV, LTG, VPA, TPM, and ZNS meet this criterion. In patients with clearly focal onset seizures, LEV, LTG, or OXC are recommended as the treatment of first choice, and ZNS, CBZ, TPM, GBP, LCM, or ESL as alternatives. In patients with generalised seizures, VPA is preferred for tonic–clonic, myoclonic, or absence seizures, LEV for tonic–clonic or myoclonic seizures, and LTG for tonic–clonic or absence (but not myoclonic) seizures. Table 8 shows the mechanisms of action of the different AEDs; Table 9 shows their efficacy according to seizure and epilepsy type. (d) Safety is a fundamental consideration. AEDs must be well tolerated, with no significant adverse reactions or drug–drug interactions, including with other AEDS, and must be suitable for use in specific clinical contexts (old age; women of childbearing age; heart, liver, or kidney comorbidities, etc). Based on current evidence from case series, retrospective studies, and expert opinions, monotherapy with LEV, LTG, OXC, TPM, VPA, and GPB and combined therapy with LCM, perampanel, and brivaracetam (BRV) are recommended in these situations, with exceptions. PHT and phenobarbital (PB) should be avoided, and CBZ and VPA should not be administered to patients with liver disease or polymedicated individuals. GBP, CBZ and derivatives, TPM, LEV, BRV, and LCM should be administered with caution (adjusted doses) in patients with kidney failure (Tables 10 and 11).27,45–49,59–63,74,80,89,96,100,152–159
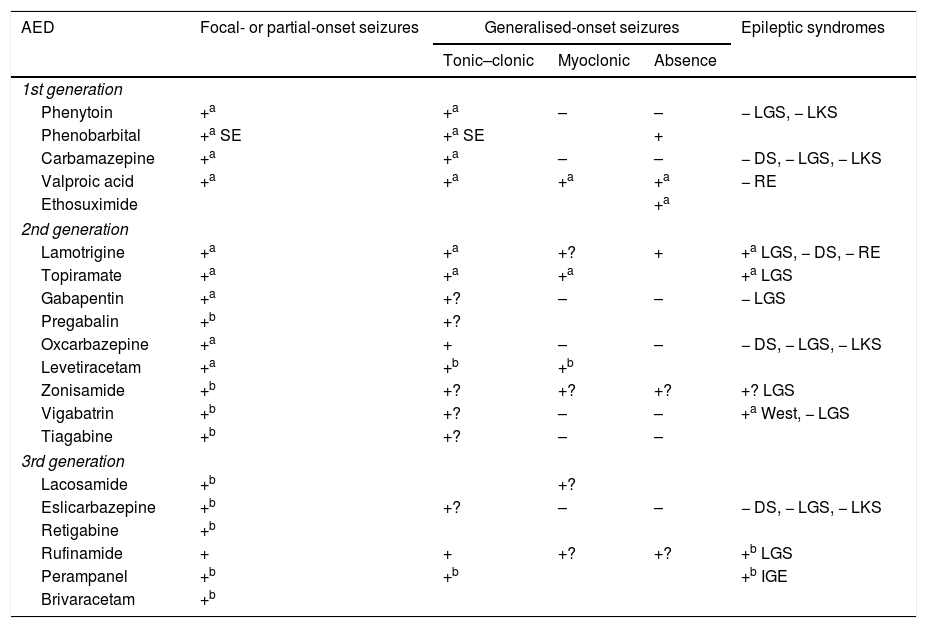
Efficacy and indications for antiepileptic drugs.27,49,97,154–158
| AED | Focal- or partial-onset seizures | Generalised-onset seizures | Epileptic syndromes | ||
|---|---|---|---|---|---|
| Tonic–clonic | Myoclonic | Absence | |||
| 1st generation | |||||
| Phenytoin | +a | +a | – | – | − LGS, − LKS |
| Phenobarbital | +a SE | +a SE | + | ||
| Carbamazepine | +a | +a | – | – | − DS, − LGS, − LKS |
| Valproic acid | +a | +a | +a | +a | − RE |
| Ethosuximide | +a | ||||
| 2nd generation | |||||
| Lamotrigine | +a | +a | +? | + | +a LGS, − DS, − RE |
| Topiramate | +a | +a | +a | +a LGS | |
| Gabapentin | +a | +? | – | – | − LGS |
| Pregabalin | +b | +? | |||
| Oxcarbazepine | +a | + | – | – | − DS, − LGS, − LKS |
| Levetiracetam | +a | +b | +b | ||
| Zonisamide | +b | +? | +? | +? | +? LGS |
| Vigabatrin | +b | +? | – | – | +a West, − LGS |
| Tiagabine | +b | +? | – | – | |
| 3rd generation | |||||
| Lacosamide | +b | +? | |||
| Eslicarbazepine | +b | +? | – | – | − DS, − LGS, − LKS |
| Retigabine | +b | ||||
| Rufinamide | + | + | +? | +? | +b LGS |
| Perampanel | +b | +b | +b IGE | ||
| Brivaracetam | +b | ||||
AED: antieplieptic drug; DS: Dravet syndrome; IGE: idiopathic generalised epilepsy; LGS: Lennox–Gastaut syndrome; LKS: Landau-Kleffner syndrome; RE: rolandic epilepsy; SE: status epilepticus only; +: effective; +?: uncertain effectiveness; –: harmful.
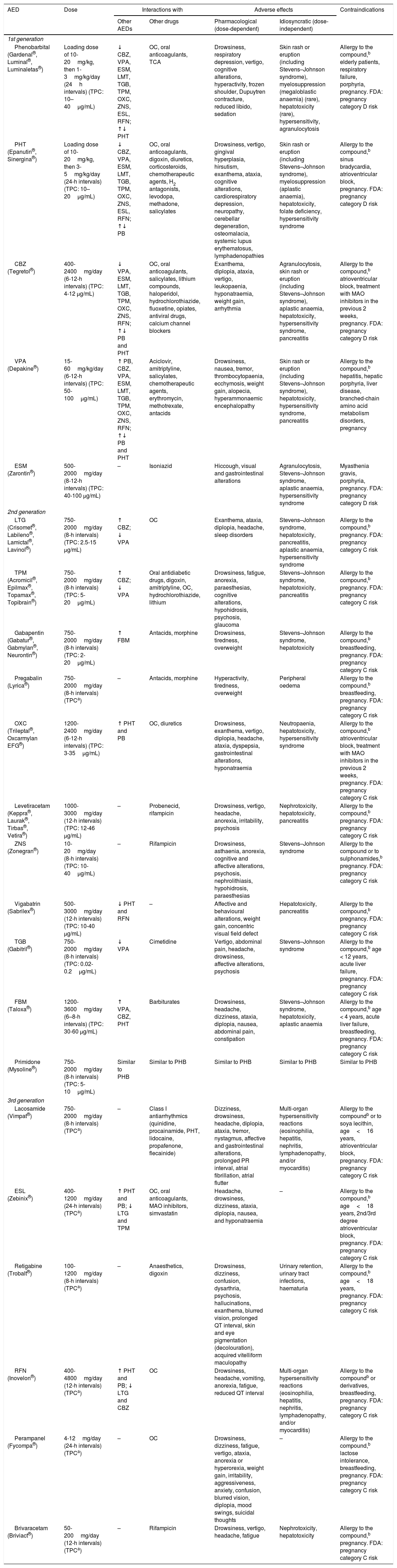
Antiepileptic drugs used in patients (particularly cancer patients) receiving palliative care: interactions, secondary effects, and contraindications.27,45,46,49,60–64,75,81,90,97,101,154–159
| AED | Dose | Interactions with | Adverse effects | Contraindications | ||
|---|---|---|---|---|---|---|
| Other AEDs | Other drugs | Pharmacological (dose-dependent) | Idiosyncratic (dose-independent) | |||
| 1st generation | ||||||
| Phenobarbital (Gardenal®, Luminal®, Luminaletas®) | Loading dose of 10-20mg/kg, then 1-3mg/kg/day (24h intervals) (TPC: 10–40μg/mL) | ↓ CBZ, VPA, ESM, LMT, TGB, TPM, OXC, ZNS, ESL, RFN; ↑↓ PHT | OC, oral anticoagulants, TCA | Drowsiness, respiratory depression, vertigo, cognitive alterations, hyperactivity, frozen shoulder, Dupuytren contracture, reduced libido, sedation | Skin rash or eruption (including Stevens–Johnson syndrome), myelosuppression (megaloblastic anaemia) (rare), hepatotoxicity (rare), hypersensitivity, agranulocytosis | Allergy to the compound,b elderly patients, respiratory failure, porphyria, pregnancy. FDA: pregnancy category D risk |
| PHT (Epanutin®, Sinergina®) | Loading dose of 10-20mg/kg, then 3-5mg/kg/day (24-h intervals) (TPC: 10–20μg/mL) | ↓ CBZ, VPA, ESM, LMT, TGB, TPM, OXC, ZNS, ESL, RFN; ↑↓ PB | OC, oral anticoagulants, digoxin, diuretics, corticosteroids, chemotherapeutic agents, H2 antagonists, levodopa, methadone, salicylates | Drowsiness, vertigo, gingival hyperplasia, hirsutism, exanthema, ataxia, cognitive alterations, cardiorespiratory depression, neuropathy, cerebellar degeneration, osteomalacia, systemic lupus erythematosus, lymphadenopathies | Skin rash or eruption (including Stevens–Johnson syndrome), myelosuppression (aplastic anaemia), hepatotoxicity, folate deficiency, hypersensitivity syndrome | Allergy to the compound,b sinus bradycardia, atrioventricular block, pregnancy. FDA: pregnancy category D risk |
| CBZ (Tegretol®) | 400-2400mg/day (6-12-h intervals) (TPC: 4-12 μg/mL) | ↓ VPA, ESM, LMT, TGB, TPM, OXC, ZNS, RFN; ↑↓ PB and PHT | OC, oral anticoagulants, salicylates, lithium compounds, haloperidol, hydrochlorothiazide, fluoxetine, opiates, antiviral drugs, calcium channel blockers | Exanthema, diplopia, ataxia, vertigo, leukopaenia, hyponatraemia, weight gain, arrhythmia | Agranulocytosis, skin rash or eruption (including Stevens–Johnson syndrome), aplastic anaemia, hepatotoxicity, hypersensitivity syndrome, pancreatitis | Allergy to the compound,b atrioventricular block, treatment with MAO inhibitors in the previous 2 weeks, pregnancy. FDA: pregnancy category D risk |
| VPA (Depakine®) | 15-60mg/kg/day (6-12-h intervals) (TPC: 50-100μg/mL) | ↑ PB, CBZ, VPA, ESM, LMT, TGB, TPM, OXC, ZNS, RFN; ↑↓ PB and PHT | Aciclovir, amitriptyline, salicylates, chemotherapeutic agents, erythromycin, methotrexate, antacids | Drowsiness, nausea, tremor, thrombocytopaenia, ecchymosis, weight gain, alopecia, hyperammonaemic encephalopathy | Skin rash or eruption (including Stevens–Johnson syndrome), hepatotoxicity, hypersensitivity syndrome, pancreatitis | Allergy to the compound,b hepatitis, hepatic porphyria, liver disease, branched-chain amino acid metabolism disorders, pregnancy |
| ESM (Zarontin®) | 500-2000mg/day (8-12-h intervals) (TPC: 40-100 μg/mL) | – | Isoniazid | Hiccough, visual and gastrointestinal alterations | Agranulocytosis, Stevens–Johnson syndrome, aplastic anaemia, hypersensitivity syndrome | Myasthenia gravis, porphyria, pregnancy. FDA: pregnancy category D risk |
| 2nd generation | ||||||
| LTG (Crisomet®, Labileno®, Lamictal®, Lavinol®) | 750-2000mg/day (8-h intervals) (TPC: 2.5-15 μg/mL) | ↑ CBZ; ↓ VPA | OC | Exanthema, ataxia, diplopia, headache, sleep disorders | Stevens–Johnson syndrome, hepatotoxicity, pancreatitis, aplastic anaemia, hypersensitivity syndrome | Allergy to the compound,b pregnancy. FDA: pregnancy category C risk |
| TPM (Acromicil®, Epilmax®, Topamax®, Topibrain®) | 750-2000mg/day (8-h intervals) (TPC: 5-20μg/mL) | ↑ CBZ; ↓ VPA | Oral antidiabetic drugs, digoxin, amitriptyline, OC, hydrochlorothiazide, lithium | Drowsiness, fatigue, anorexia, paraesthesias, cognitive alterations, hypohidrosis, psychosis, glaucoma | Stevens–Johnson syndrome, hepatotoxicity, pancreatitis | Allergy to the compound,b pregnancy. FDA: pregnancy category C risk |
| Gabapentin (Gabatur®, Gabmylan®, Neurontin®) | 750-2000mg/day (8-h intervals) (TPC: 2-20μg/mL) | ↑ FBM | Antacids, morphine | Drowsiness, tiredness, overweight | Stevens–Johnson syndrome, hepatotoxicity | Allergy to the compound,b breastfeeding, pregnancy. FDA: pregnancy category C risk |
| Pregabalin (Lyrica®) | 750-2000mg/day (8-h intervals) (TPCa) | – | Antacids, morphine | Hyperactivity, tiredness, overweight | Peripheral oedema | Allergy to the compound,b breastfeeding, pregnancy. FDA: pregnancy category C risk |
| OXC (Trileptal®, Oxcarmylan EFG®) | 1200-2400mg/day (6-12-h intervals) (TPC: 3-35μg/mL) | ↑ PHT and PB | OC, diuretics | Drowsiness, exanthema, vertigo, diplopia, headache, ataxia, dyspepsia, gastrointestinal alterations, hyponatraemia | Neutropaenia, hepatotoxicity, hypersensitivity syndrome | Allergy to the compound,b atrioventricular block, treatment with MAO inhibitors in the previous 2 weeks, pregnancy. FDA: pregnancy category C risk |
| Levetiracetam (Keppra®, Laurak®, Tirbas®, Vetira®) | 1000-3000mg/day (12-h intervals) (TPC: 12-46 μg/mL) | – | Probenecid, rifampicin | Drowsiness, vertigo, headache, anorexia, irritability, psychosis | Nephrotoxicity, hepatotoxicity, pancreatitis | Allergy to the compound,b pregnancy. FDA: pregnancy category C risk |
| ZNS (Zonegran®) | 10-20mg/day (8-h intervals) (TPC: 10-40μg/mL) | – | Rifampicin | Drowsiness, asthaenia, anorexia, cognitive and affective alterations, psychosis, nephrolithiasis, hypohidrosis, paraesthesias | Stevens–Johnson syndrome | Allergy to the compound or to sulphonamides,b pregnancy. FDA: pregnancy category C risk |
| Vigabatrin (Sabrilex®) | 500-3000mg/day (12-h intervals) (TPC: 10-40 μg/mL) | ↓ PHT and RFN | – | Affective and behavioural alterations, weight gain, concentric visual field defect | Hepatotoxicity, pancreatitis | Allergy to the compound,b pregnancy. FDA: pregnancy category C risk |
| TGB (Gabitril®) | 750-2000mg/day (8-h intervals) (TPC: 0.02-0.2μg/mL) | ↓ VPA | Cimetidine | Vertigo, abdominal pain, headache, drowsiness, affective alterations, psychosis | Stevens–Johnson syndrome | Allergy to the compound,b age < 12 years, acute liver failure, pregnancy. FDA: pregnancy category C risk |
| FBM (Taloxa®) | 1200-3600mg/day (6–8-h intervals) (TPC: 30-60 μg/mL) | ↑ VPA, CBZ, PHT | Barbiturates | Drowsiness, headache, dizziness, ataxia, diplopia, nausea, abdominal pain, constipation | Stevens–Johnson syndrome, hepatotoxicity, aplastic anaemia | Allergy to the compound,b age < 4 years, acute liver failure, breastfeeding, pregnancy. FDA: pregnancy category C risk |
| Primidone (Mysoline®) | 750-2000mg/day (8-h intervals) (TPC: 5-10μg/mL) | Similar to PHB | Similar to PHB | Similar to PHB | Similar to PHB | Similar to PHB |
| 3rd generation | ||||||
| Lacosamide (Vimpat®) | 750-2000mg/day (8-h intervals) (TPCa) | – | Class I antiarrhythmics (quinidine, procainamide, PHT, lidocaine, propafenone, flecainide) | Dizziness, drowsiness, headache, diplopia, ataxia, tremor, nystagmus, affective and gastrointestinal alterations, prolonged PR interval, atrial fibrillation, atrial flutter | Multi-organ hypersensitivity reactions (eosinophilia, hepatitis, nephritis, lymphadenopathy, and/or myocarditis) | Allergy to the compoundb or to soya lecithin, age<16 years, atrioventricular block, pregnancy. FDA: pregnancy category C risk |
| ESL (Zebinix®) | 400-1200mg/day (24-h intervals) (TPCa) | ↑ PHT and PB; ↓ LTG and TPM | OC, oral anticoagulants, MAO inhibitors, simvastatin | Headache, drowsiness, dizziness, ataxia, diplopia, nausea, and hyponatraemia | – | Allergy to the compound,b age<18 years, 2nd/3rd degree atrioventricular block, pregnancy. FDA: pregnancy category C risk |
| Retigabine (Trobalt®) | 100-1200mg/day (8-h intervals) (TPCa) | – | Anaesthetics, digoxin | Drowsiness, dizziness, confusion, dysarthria, psychosis, hallucinations, exanthema, blurred vision, prolonged QT interval, skin and eye pigmentation (decolouration), acquired vitelliform maculopathy | Urinary retention, urinary tract infections, haematuria | Allergy to the compound,b age<18 years, pregnancy. FDA: pregnancy category C risk |
| RFN (Inovelon®) | 400-4800mg/day (12-h intervals) (TPCa) | ↑ PHT and PB; ↓ LTG and CBZ | OC | Drowsiness, headache, vomiting, anorexia, fatigue, reduced QT interval | Multi-organ hypersensitivity reactions (eosinophilia, hepatitis, nephritis, lymphadenopathy, and/or myocarditis) | Allergy to the compoundb or derivatives, breastfeeding, pregnancy. FDA: pregnancy category C risk |
| Perampanel (Fycompa®) | 4-12mg/day (24-h intervals) (TPCa) | – | OC | Drowsiness, dizziness, fatigue, vertigo, ataxia, anorexia or hyperorexia, weight gain, irritability, aggressiveness, anxiety, confusion, blurred vision, diplopia, mood swings, suicidal thoughts | – | Allergy to the compound,b lactose intolerance, breastfeeding, pregnancy. FDA: pregnancy category C risk |
| Brivaracetam (Briviact®) | 50-200mg/day (12-h intervals) (TPCa) | – | Rifampicin | Drowsiness, vertigo, headache, fatigue | Nephrotoxicity, hepatotoxicity | Allergy to the compound,b pregnancy. FDA: pregnancy category C risk |
AED: antiepileptic drug; CBZ: carbamazepine; ESL: eslicarbazepine; ESM: ethosuximide; FBM: felbamate; FDA: United States Food and Drug Administration; LTG: lamotrigine; MAO: monoamine oxidase; OC: oral contraception; OXC: oxcarbazepine; PB: phenobarbital; PHT: phenytoin; RFN: rufinamide; TCA: tricyclic antidepressant; TGB: tiagabine; TPC: therapeutic plasma concentration; TPM: topimarate; VPA: valproic acid; ZNS: zonisamide.
Compound: active ingredient and/or excipients contained in the specific preparation.
Adapted with permission from Fernández Alonso et al.,27 2013.
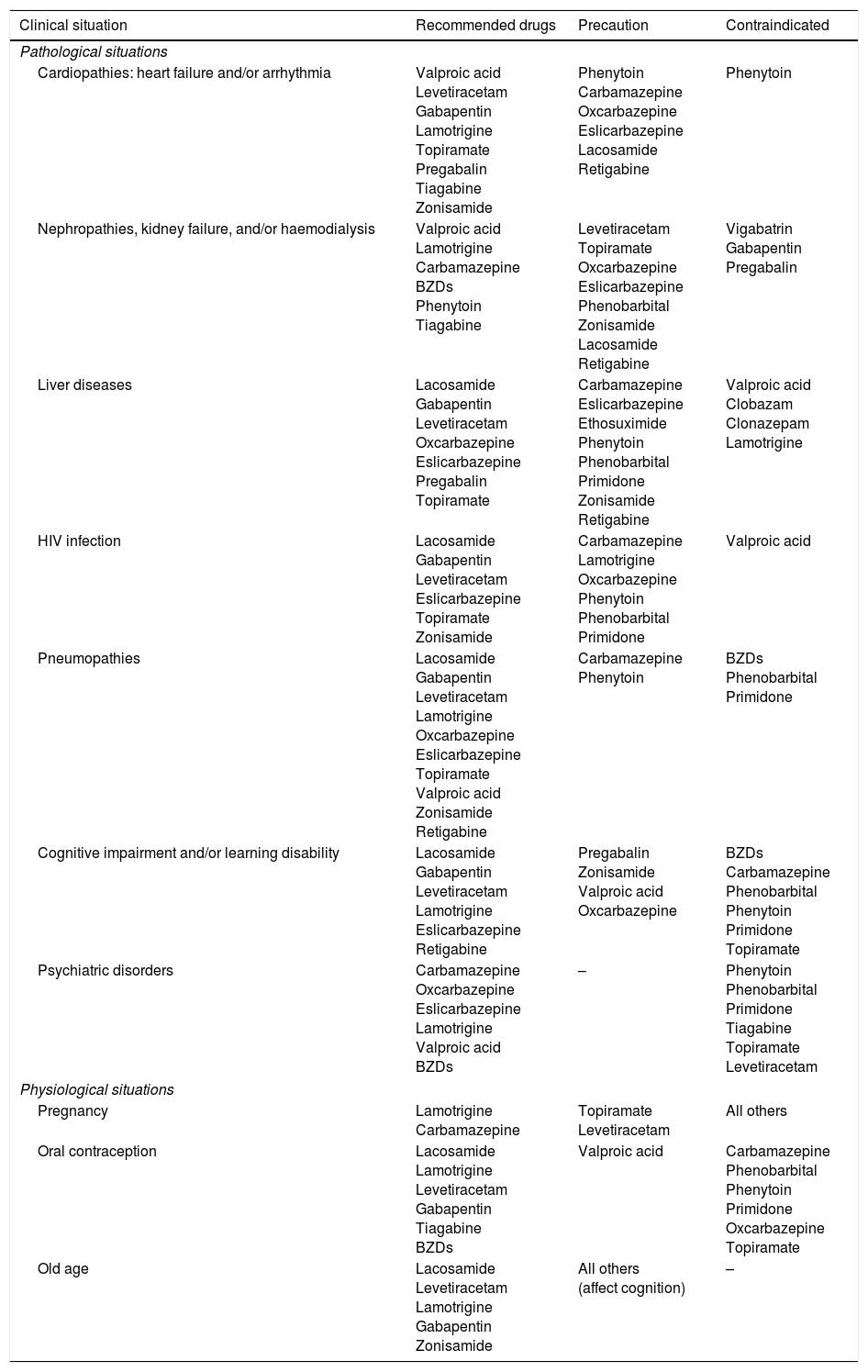
AEDs in specific situations.
| Clinical situation | Recommended drugs | Precaution | Contraindicated |
|---|---|---|---|
| Pathological situations | |||
| Cardiopathies: heart failure and/or arrhythmia | Valproic acid Levetiracetam Gabapentin Lamotrigine Topiramate Pregabalin Tiagabine Zonisamide | Phenytoin Carbamazepine Oxcarbazepine Eslicarbazepine Lacosamide Retigabine | Phenytoin |
| Nephropathies, kidney failure, and/or haemodialysis | Valproic acid Lamotrigine Carbamazepine BZDs Phenytoin Tiagabine | Levetiracetam Topiramate Oxcarbazepine Eslicarbazepine Phenobarbital Zonisamide Lacosamide Retigabine | Vigabatrin Gabapentin Pregabalin |
| Liver diseases | Lacosamide Gabapentin Levetiracetam Oxcarbazepine Eslicarbazepine Pregabalin Topiramate | Carbamazepine Eslicarbazepine Ethosuximide Phenytoin Phenobarbital Primidone Zonisamide Retigabine | Valproic acid Clobazam Clonazepam Lamotrigine |
| HIV infection | Lacosamide Gabapentin Levetiracetam Eslicarbazepine Topiramate Zonisamide | Carbamazepine Lamotrigine Oxcarbazepine Phenytoin Phenobarbital Primidone | Valproic acid |
| Pneumopathies | Lacosamide Gabapentin Levetiracetam Lamotrigine Oxcarbazepine Eslicarbazepine Topiramate Valproic acid Zonisamide Retigabine | Carbamazepine Phenytoin | BZDs Phenobarbital Primidone |
| Cognitive impairment and/or learning disability | Lacosamide Gabapentin Levetiracetam Lamotrigine Eslicarbazepine Retigabine | Pregabalin Zonisamide Valproic acid Oxcarbazepine | BZDs Carbamazepine Phenobarbital Phenytoin Primidone Topiramate |
| Psychiatric disorders | Carbamazepine Oxcarbazepine Eslicarbazepine Lamotrigine Valproic acid BZDs | – | Phenytoin Phenobarbital Primidone Tiagabine Topiramate Levetiracetam |
| Physiological situations | |||
| Pregnancy | Lamotrigine Carbamazepine | Topiramate Levetiracetam | All others |
| Oral contraception | Lacosamide Lamotrigine Levetiracetam Gabapentin Tiagabine BZDs | Valproic acid | Carbamazepine Phenobarbital Phenytoin Primidone Oxcarbazepine Topiramate |
| Old age | Lacosamide Levetiracetam Lamotrigine Gabapentin Zonisamide | All others (affect cognition) | – |
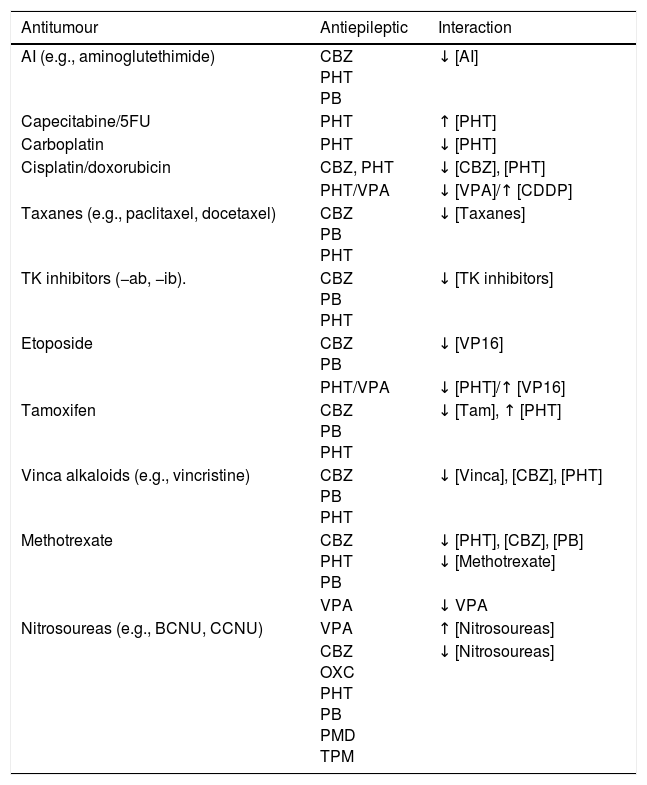
Use of AEDs in PC.22–159 Epileptic seizures are common in patients with brain tumours; seizure control is an important objective in the management of these patients. Patients with brain tumours are more likely to develop refractory epilepsy. The main issues of AED use in these patients are the following54: (a) drug–drug interactions57,58: there are numerous CYP450-mediated drug–drug interactions between AEDs and chemotherapeutic agents. Within the group of AEDs, some drugs have high potential to provoke interactions (CBZ, PHT, PB, PRM, VPA, and FBM affect other drugs and are themselves affected) while others have medium or low potential (LTG, OXC, TGB, TPM, ESM, clonazepam [CNZ], clobazam [CLB], and ZNS do not affect other drugs but are themselves affected; LCM, VGB, GBP, LEV, and PGB do not affect other drugs and are not themselves affected); the latter group are ideal for use in PC (Table 12).57–64 Finally, seizures may occur at onset of radio- or chemotharapy (e.g., with carmustine wafer, intra-arterial cisplatin, etc.) due to neural irritation of the surrounding brain tissue.90 (b) Haematologic toxicity: neutropaenia and thrombocytopaenia associated with classic AEDs are rare (0.9-1.2 cases per 104 prescriptions), although coadministration with cytostatic drugs is associated with greater toxic effects.27–65,67,68,94 (c) CNS toxicity: it is essential to thoroughly assess the adverse effects of AEDs on the CNS, as they are more frequent in patients with brain tumours than in those without; the sedation, cognitive alterations, changes in personality, and occasionally the focal neurological signs that these drugs can cause may be misinterpreted as tumour progression and/or cause the patient's overall condition to worsen. (d) Hypersensitivity syndrome: radiotherapy is associated with greater incidence of this toxic effect.51–74 These patients may present malnutrition with hypoproteinaemia, which can result in a higher than expected fraction unbound in plasma for classic AEDs, promoting toxic effects.57
Interactions between the most common antitumour and antiepileptic drugs.55,57,58,74
| Antitumour | Antiepileptic | Interaction |
|---|---|---|
| AI (e.g., aminoglutethimide) | CBZ PHT PB | ↓ [AI] |
| Capecitabine/5FU | PHT | ↑ [PHT] |
| Carboplatin | PHT | ↓ [PHT] |
| Cisplatin/doxorubicin | CBZ, PHT | ↓ [CBZ], [PHT] |
| PHT/VPA | ↓ [VPA]/↑ [CDDP] | |
| Taxanes (e.g., paclitaxel, docetaxel) | CBZ PB PHT | ↓ [Taxanes] |
| TK inhibitors (−ab, −ib). | CBZ PB PHT | ↓ [TK inhibitors] |
| Etoposide | CBZ PB | ↓ [VP16] |
| PHT/VPA | ↓ [PHT]/↑ [VP16] | |
| Tamoxifen | CBZ PB PHT | ↓ [Tam], ↑ [PHT] |
| Vinca alkaloids (e.g., vincristine) | CBZ PB PHT | ↓ [Vinca], [CBZ], [PHT] |
| Methotrexate | CBZ PHT PB | ↓ [PHT], [CBZ], [PB] ↓ [Methotrexate] |
| VPA | ↓ VPA | |
| Nitrosoureas (e.g., BCNU, CCNU) | VPA | ↑ [Nitrosoureas] |
| CBZ OXC PHT PB PMD TPM | ↓ [Nitrosoureas] |
AI: aromatase inhibitors; BCNU: carmustine; CBZ: carbamazepine; CCNU: lomustine; CDDP: cisplatin; PB: phenobarbital; PHT: phenytoin; PMD: primidone; TK: tyrosine kinase; Vinca: vinca alkaloids; VPA: valproic acid; VP16: etoposide; 5FU: 5-fluorouracil.
Patients with brain tumours are at much greater risk of adverse reactions to AEDs than other epileptic patients. AEDs are associated with such severe adverse reactions as Stevens–Johnson syndrome, especially during dose up-titration (first 4-8 weeks); these effects have been described for CBZ, PHT, PB, VPA, LMT, ESM, TPM, GBP, ZNS, TGB, and FBM. Stevens–Johnson syndrome has also been reported in patients receiving cranial radiotherapy simultaneously with PHT, CBZ, and/or PB. Given the increased risk of cutaneous adverse reactions, AEDs are not recommended for patients receiving whole-brain radiotherapy. Patients with brain tumours and receiving AEDs and radiotherapy more frequently present cognitive adverse reactions. Patients receiving both treatments simultaneously have shown 6 times greater impairment in neuropsychological tests (attention deficit, psychomotor delay, and/or alterations in executive function) than patients receiving radiotherapy only, in both medium- and long-term assessments. Other frequent adverse reactions include increased incidence of headache (PHT, LEV, ZNS), myelosuppression (CBZ, LMT), cognitive and behavioural alterations (TPM, LEV, PHT, PB, CBZ, LMT), poor coordination (PHT), and increased risk of shoulder-hand syndrome (hemiplegic patients receiving PB). To summarise, patients with brain tumours receiving chemotherapy, radiotherapy, or corticosteroids should not be treated with classic AEDs due to possible interactions and/or idiosyncratic adverse reactions (level of evidence: 4).51
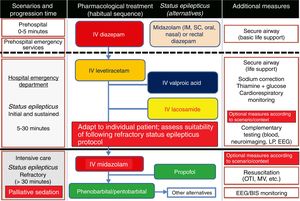
Therapeutic approach.27,32,66–70 Acute treatment of epileptic seizures in patients receiving PC should be symptomatic and aetiological. It is essential to resolve situations entailing vital risk (obstructed airway, intracranial hypertension, etc.) and which may reduce quality of life (incoercible vomiting, refractory pain, etc). The following considerations must be observed when treating these patients, especially if they are terminally ill: (1) symptom control will be the main aim of emergency treatment. When necessary, and in situations in which it is indicated, administration of opioids and/or sedatives may improve the well-being of patients and/or their families. (2) Maintaining constant, effective communication with the patient and/or family can be difficult. However, physicians should aim to ascertain the family's understanding of the disease and communicate as clearly as possible both with them and with the patient to facilitate subsequent joint decision-making. (3) Supporting the family: it is important to ensure that family members are informed and understand the patient's situation; families often refuse to accept the reality of the disease. Patients may also need other levels of care, such as psychiatric, social, neuropsychological, religious, and spiritual support.
General life support measures.27,31,52Life support measures are taken from the onset of the seizure, and aim to stabilise the patient, prevent potential traumatic lesions, and control or prevent complications during and immediately after the seizure. The adapted protocol of the Advanced Trauma Life Support programme should be followed. The ABCDE mnemonic (airway, breathing/ventilation, circulation, disability, exposure and environment) comprises: (a) keeping the airway clear; (b) ensuring proper ventilation/oxygenation; (c) ensuring good haemodynamic control: monitoring vital signs, inserting a peripheral venous catheter (preferably 2, one for extracting blood for analysis and another for administering serum therapy and drug treatment), and (where possible) correcting the primary cause (metabolic disorder, infection, etc.); (d) assessing level of consciousness, pupils (anisocoria>1mm is considered abnormal and may signal uncal herniation of the temporal lobe), and motor function; and (e) controlling exposure to prevent hypothermia.66
Measures to improve patient well-being.In PC, palliative sedation refers to the administration of drugs to decrease patients’ level of consciousness in order to partially or completely reduce their perception of symptoms and/or signs causing unnecessary suffering due to their high severity or poor treatment response (refractory symptoms). Palliative sedation may be continuous or intermittent. In terminally ill patients, we refer to terminal sedation, which is administered continuously. The refractory symptoms most frequently leading to palliative sedation are delirium, agitation, dyspnoea, pain, anxiety, and first or recurrent acute haemorrhage. Drug families of choice are benzodiazepines (BZDs: midazolam [MDZ] and diazepam [DZP]), opioids (morphine), neuroleptics (NLP), sedatives (chlorpromazine and levomepromazine), antipsychotics (haloperidol), barbiturates (PB), and anaesthetics (propofol). Specific drugs are selected according to symptoms, with delirium and agitation being treated with NLPs (first choice) or BZDs (second choice), especially MDZ; and dyspnoea, anxiety, and haemorrhage being treated with MDZ (first choice) or NLPs (second choice). Specific doses and administration guidelines are beyond the scope of this article; we recommend consulting articles, protocols, and CPGs on the subject.1,7,8,12,52,53
Treatment of peritumoural brain oedema and intracranial hypertension.1–159 Treatment of recent-onset seizure aims to minimise the possibility of additional lesions. To that end, the patient's family should be trained to respond to a seizure.1 Basic treatment of epileptic seizures is largely similar in palliative patients to in any other patient. AEDs should be selected according to seizure type, adverse reactions, and potential drug–drug interactions (with chemotherapeutic drugs, corticosteroids, etc.). If corticosteroids are administered, it may be necessary to monitor blood levels of many AEDs (especially dexamethasone [DXT] coadministered with PHT, as they reduce one another's levels via induction of the hepatic CYP450 enzyme system). Prophylactic corticosteroids are not indicated for seizures secondary to primary or metastatic brain tumours or brain radiation necrosis. This is also the case if there are no symptoms or signs of mild-to-moderate intracranial hypertension (headache, vomiting) or severe hypertension (intense vomiting and/or headache, altered level of consciousness, rapidly progressing neurological deficits) with stable neurological deficits. Cancer patients receiving AED treatment and displaying signs or symptoms of intracranial hypertension should be treated with corticosteroids (preferably DXT due to its reduced likelihood of causing salt retention and inhibition of leucocyte migration, and the resulting lower risk of superinfection, compared to other corticosteroids)59 at 12 to 24mg/24hours for the shortest time possible (a 10-mg bolus may be administered intravenously as a loading dose, followed by a dose of 12mg for signs and/or symptoms of mild-to-moderate intracranial hypertension, and 24mg for severe intracranial hypertension, at intervals of 4, 6, or 8hours, increasing the dose if there is no improvement within 48hours and gradually reducing it by 4mg every 48hours if improvement is observed; the treatment may be withdrawn if no clinical response is observed within 48hours of the 24-mg dose being administered), together with manitol for the first 48hours (1mg/kg per 6-8hours, maintaining plasma osmolality at 310-320mOsm/kg). The patient's bed should be elevated > 30°, water intake should be restricted to < 1 to 1.5L/day, and diuretic drugs should be administered (furosemide, 1 IV ampoule each 6-8h).52,59,65
Pharmacological treatment of acute symptomatic seizures.27,31,52,90,91(a) Patients with brain tumours should not receive AEDs if they have not presented seizures (grade of recommendation: A).51 (b) AED treatment should be started if the patient displays ≥ 2 unprovoked seizures, or one seizure with high likelihood of recurrence (e.g., focal seizure with a structural aetiology detected by neuroimaging or EEG) or in cases where the patient and/or family are very concerned about the seizure. (c) Initial treatment should be monotherapy at low doses. (d) If seizures persist, the dose should be increased until seizure control is achieved or the maximum tolerable dose is reached. (e) In patients with poor seizure control, a different AED should be prescribed in addition to or in place of the first. (f) Blood AED levels should be used only as a guide; higher than normal AED levels should not prevent doses being increased if seizure control is poor. It is important to perform periodic monitoring to assess suboptimal levels. (g) AEDs should be selected according to the type of epilepsy and the adverse reactions associated with each drug. Second-generation AEDs with extrahepatic metabolism are recommended in patients with acute symptomatic seizures caused by brain tumours during radiotherapy, chemotherapy, or corticotherapy (recommendation of the Epilepsy Study Group of the Spanish Society of Neurology). (h) In patients with myoclonus (which all opioids may cause), it is necessary to investigate the cause; if it is treatable, it should be corrected or opioids rotated. If no cause is identified and/or the patient is terminal, BZDs should be administered (see first-line antiepileptic drugs section).
Recommended antiepileptic drug treatment schedule for oncological patients.. Prophylactic AED administration is not recommended for patients with brain tumours as it does not reduce seizure incidence or increase seizure-free time, and increases the likelihood of adverse reactions. Prophylactic AED treatment is acceptable prior to surgery, but should be progressively withdrawn a week thereafter (level of evidence: 1).54,70–73,81 The ideal drug will not interact with CYP450 isoenzymes and will show weak protein binding. New-generation AEDs have these qualities, although limited experience has been reported and many of these drugs are not without problems. LEV (20mg/kg/day in 2 doses) is recommended as the first line of treatment and VPA (15mg/kg/day in 3 doses) as the second-line AED.70 There are a number of preliminary considerations to be taken into account for each drug.27–101,120–133 OXC is a weak enzyme inducer. LMT often provokes skin toxicity, and dose up-titration is very slow. TPM may cause language problems, paraesthesia, and/or focal neurological signs; besides being a weak hepatic enzyme inducer, it can cause cachexia and a degree of metabolic acidosis. GBP is a weak AED requiring high doses, with a risk of CNS toxicity; the same is true of PGB, which also has a long up-titration period. Extensive evidence has been published on the use of VPA, a CYP450 inhibitor; the drug is highly effective in controlling seizures and presents significantly lower haematologic toxicity than would be expected, given its mechanism of action. VPA-induced hyperammonaemic encephalopathy is very rare. In addition to the increased haematologic toxicity associated with adjuvant treatment with temozolomide in patients with glioblastoma, longer survival times have been reported in comparison with patients not taking VPA,132 as the drug has a degree of antineoplastic activity, attributed to inhibition of histone deacetylase activity and reduced protein kinase C activation. It is not recommended in patients receiving nitrosoureas (carmustine, lomustine); caution should also be exercised when coadministering with irinotecan. LEV has an optimal pharmacokinetic profile and is an effective alternative for treating seizures secondary to brain tumours. In vitro studies have also found that it inhibits expression of O(6)-methylguanine-DNA methyltransferase (a DNA repair enzyme with an important role in tumour ‘cells’ resistance to alkylating agents and the cytostatic drug temozolomide).133 Both VPA and LEV perform adequately in treating refractory epilepsy: LEV is not a substrate of P-glycoprotein (also known as multidrug resistance protein 1), and VPA inhibits its expression. A further advantage of both drugs is that they can be administered intravenously. In patients with refractory primary or metastatic brain tumours, coadministration of these drugs is recommended over sequential administration in monotherapy (achieving 81.5% responders, a 55.6% reduction in seizure frequency, 59% seizure-free patients, and an adequate safety profile).57,123 Dose adjustment of such other new drugs as ZNS and LCM is a slow process; little evidence is available on their use to treat tumour-related epilepsy. ESL and RTG dose is adjusted quickly, although no evidence has been reported on their use in oncological patients.54
Withdrawal of antiepileptic treatment in oncological patients following seizure remission.Most authors recommend continuing AED treatment in adult patients with brain tumours and a history of seizures, given the risk of recurrence.57
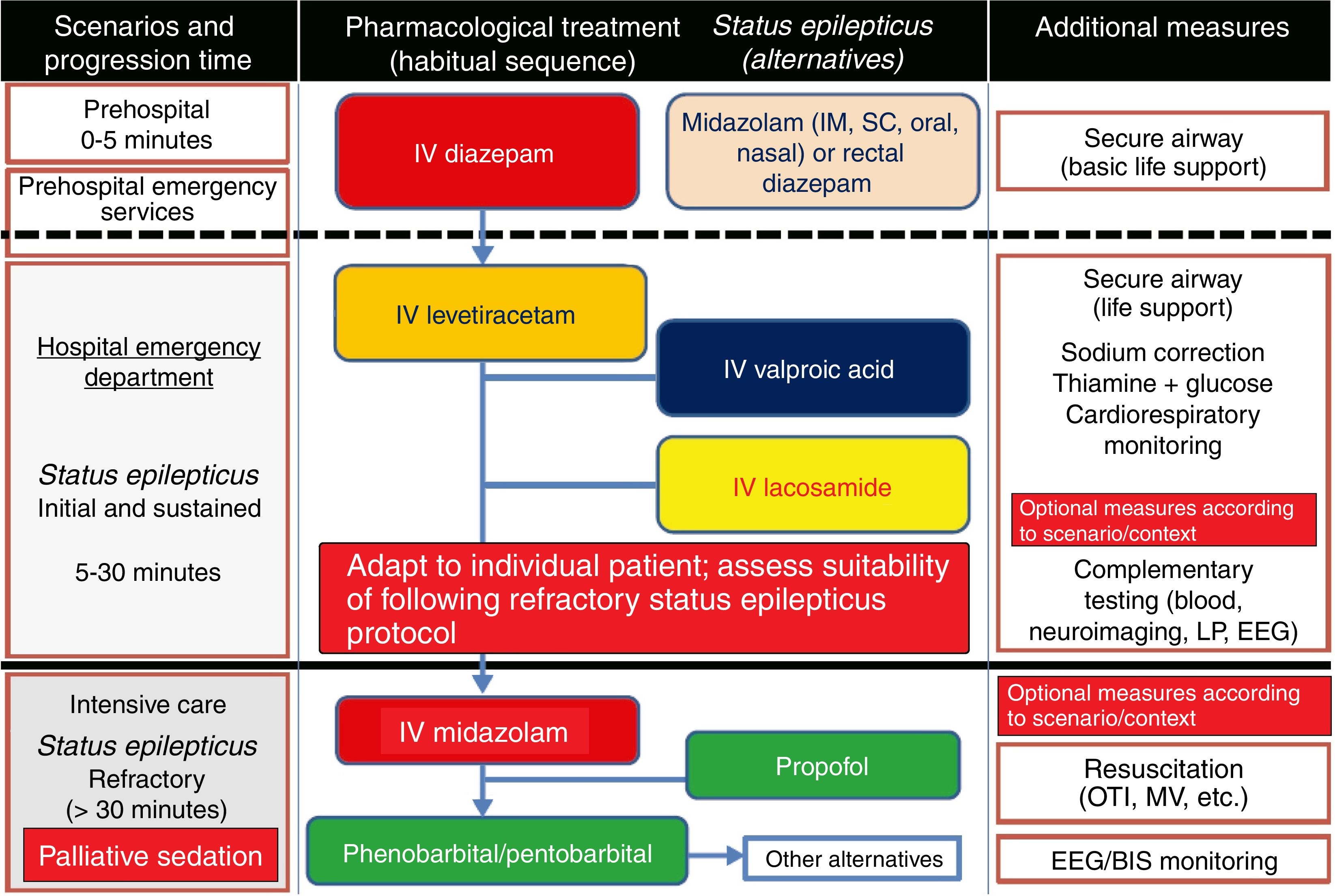
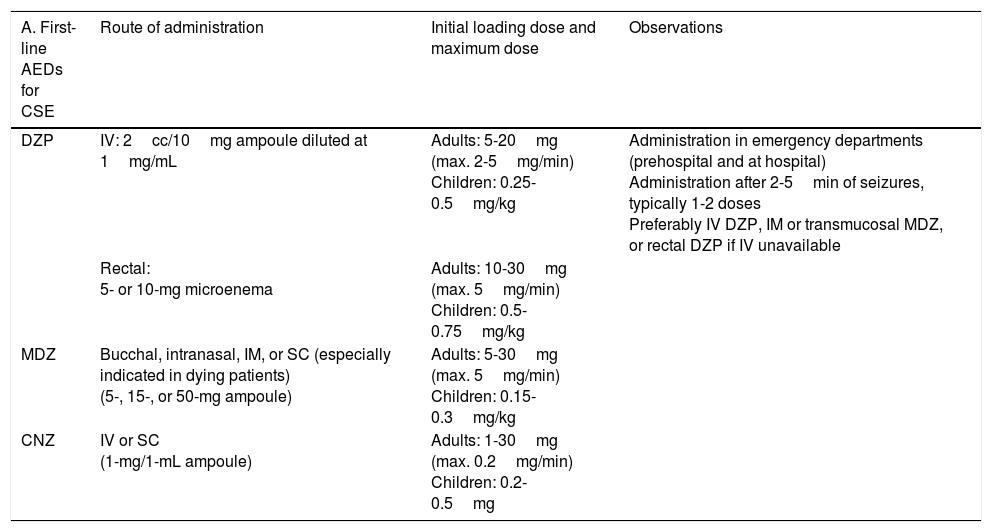
Treatment of convulsive status epilepticus.12,27–40,107–114 Treatment beginning within 60minutes of SE onset is highly successful (80%); a delay of over 2hours is associated with a 40%-50% success rate.12,34 Depending upon the clinical context, IV DZP may be the first-line treatment. DZP (0.15-0.25mg/kg) reaches the brain within seconds, although its antiepileptic effects are short-lasting; a second dose (maximum 20mg) must therefore be administered 20-30minutes later. DZP may be administered rectally in doses of 5mg for children and 10mg for adults. Intramuscular administration should be avoided due to the possibility of incorrect absorption. Another option is IV lorazepam (LZP) in a slow bolus (0.1-0.15mg/kg over 1-2min), which may be repeated after 5minutes (this preparation is not available in Spain). A further option is MDZ, which can be administered subcutaneously. It is water-soluble, with onset of action of 3minutes, 5minutes, 10-15minutes, and 15minutes for IV, intramuscular, subcutaneous, and oral administration, respectively. For refractory cases, the initial dose is 0.2mg/kg, followed by infusion at 0.05-0.5mg/kg/hour. It is best to start at a dose of 1-2mg in elderly patients. Rectal DZP and subcutaneous MDZ are particularly useful for treating seizures in dying patients. Patients with refractory seizures require emergency referral to the nearest reference hospital (given they are not terminally ill and/or the patient/family agrees to the referral) (see algorithm 1, Fig. 2).12,27–40 Prognosis depends mainly on level of consciousness at SE onset; SE type, aetiology, and duration; and the patient's age. Stupor or coma at baseline predict poor neurological recovery; cerebral anoxia has the highest mortality rate (nearly 100%); generalised CSE or NCSE have poorer prognosis; and duration≥60minutes before onset of AED treatment, age≥65, and absence of history of seizures are all associated with higher mortality rates.110–113
Algorithm 1. Management of convulsive status epilepticus in patients receiving palliative care, particularly cancer patients. Other alternatives to anaesthetics: see Third-line antiepileptic drugs (induced coma) section. AED: antiepileptic drug; BIS: bispectral index; CSE: convulsive status epilepticus; EEG: electroencephalography; IM: intramuscular; IV: intravenous; LP: lumbar puncture; MV: mechanical ventilation; OTI: orotracheal intubation; PC; palliative care; SC: subcutaneous. Adapted with permission from the algorithm published by Fernández Alonso27 in 2013 and modified for the context of palliative care based on the protocol of the Virginia Commonwealth University's Thomas Palliative Care Unit (Richmond, Virginia, USA).139
- -
Intravenous benzodiazepines: LRZ and DZP are the drugs of choice, given the high level of evidence (1) and grade of recommendation for use in emergency departments.51 DZP has a faster onset of action than LRZ (1-3 vs 5min), although its effect lasts a shorter time (10-30min vs 12-24h) as LRZ is less liposoluble and is not rapidly distributed into peripheral tissues, as is DZP, making it more effective.44 No IV preparation is available in Spain. In addition to DZP, MDZ and CNZ are available in IV preparations. MDZ has a faster onset of action (1min) and greater potency, but its half-life is very short; it therefore requires continuous perfusion, for which reason it is usually used only for controlling RSE. CNZ has a slower onset of action (3-10min) and longer half-life (12h), and is more often used for maintenance therapy.17–40,44
- -
Alternative routes of administration for benzodiazepines: MDZ is the drug of choice for intramuscular administration, showing similar efficacy to IV LZP as the initial prehospital treatment (level of evidence: 2).51 In a randomised study of prehospital patients, Silbergleit et al.138 found that MDZ (10mg intramuscular) was at least as effective as LRZ (4mg IV) in adults, particularly when venous access was not immediately achieved. However, it should be noted that metabolism by CYP450 3A4 enzymes may increase the likelihood of drug–drug interactions with DZP or LRZ.37 Transmucosal administration (oral or nasal) has been shown to be effective in recent years, with a certain preference for this route. Transmucosal administration with oral solutions is accepted for use in paediatric patients (3 months to < 18 years). Non-IV (oral/nasal transmucosal, intramuscular, and rectal) MDZ is as effective as IV DZP; oral transmucosal MDZ is superior to rectal DZP (level of evidence: 2).5,27–40,44,51,84,97,99,101,102,139–143 (a) Rectal DZP is the alternative to non-IV MDZ. Extensive evidence has been published on its use in both children (5-mg tube) and adults (10-mg tube). (b) Subcutaneous CNZ is a reasonable alternative in such settings as PC. (c) LRZ via enteral route (oral, nasogastric tube, or percutaneous endoscopic gastrostomy) may be a good option in PC.
- -
Alternatives to benzodiazepines: in cases of respiratory insufficiency or high risk of respiratory compromise due to sedation, or cases in which orotracheal intubation is not recommended, alternative treatment may be started with VPA (see dosage in section on SE) or IV lidocaine (bolus of 2mg/kg in children and 100-200mg in adults) (Table 13).27–40
Table 13.A. BZDs recommended for first-line treatment for CSE.19,27–39,96,138,139 B. AEDs recommended for second-line treatment for CSE, administration schedule, and practical recommendations.19,23,27–40,136,137 C. AEDs recommended for third-line treatment for CSE, administration schedule, and practical recommendations.22,23,27–40,134,135 D. Treatment of NCSE.22,23,27,31,40–46,102
A. First-line
AEDs for CSERoute of administration Initial loading dose and maximum dose Observations DZP IV: 2cc/10mg ampoule diluted at 1mg/mL Adults: 5-20mg (max. 2-5mg/min)
Children: 0.25-0.5mg/kgAdministration in emergency departments (prehospital and at hospital)
Administration after 2-5min of seizures, typically 1-2 doses
Preferably IV DZP, IM or transmucosal MDZ, or rectal DZP if IV unavailableRectal:
5- or 10-mg microenemaAdults: 10-30mg (max. 5mg/min)
Children: 0.5-0.75mg/kgMDZ Bucchal, intranasal, IM, or SC (especially indicated in dying patients)
(5-, 15-, or 50-mg ampoule)Adults: 5-30mg (max. 5mg/min)
Children: 0.15-0.3mg/kgCNZ IV or SC
(1-mg/1-mL ampoule)Adults: 1-30mg (max. 0.2mg/min)
Children: 0.2-0.5mg(B) Second-line
AEDs for CSEAdministration schedule Practical recommendations Initial dose Maintenance dose PHT (100- or 250-mg ampoule) 15-20mg/kg at 20-50mg/min
E.g., 1g in 250 cc 0.9% saline over 30min1-2mg/kg/8h
(12h after initial dose)
E.g., 100mg/8h, IV administrationAEDs of choice for young adults with no comorbidities (cardiovascular) in a stable condition VPA (400-mg ampoule) 15-30mg/kg (4–6mg/kg/min)
E.g., 1.2g diluted/undiluted over 5-15min0.5-1mg/kg/h
(0.5h after initial dose)
E.g., 800mg/24hAlternative treatment if PHT is contraindicated or insufficient in patients without liver disease LEV (500-mg ampoule) 20mg/kg (250-3000mg) over 15min
or 2-5mg/kg/min in 100 cc 0.9% saline
E.g., 1g over 15min or 500mg over 5min (3×)20-30mg/kg/24h
(12h after initial dose)
max. 3000mg/day
E.g., 500-1500/12h in 100 cc 0.9% salineAlternative treatment in elderly patients
or patients with heart or liver comorbidities and/or lack of response to PHT and/or VPALCM (200-mg ampoule) 100-400mg over 3-15min
E.g., 200mg undiluted over 5min100-200mg undiluted (12h after initial dose) Optional AEDs if no response to previous AEDs (PHT, VPA, and LEV) (C) Third-line
AEDs for CSEAdministration schedule Practical recommendations Initial dose Maintenance dose Coma (non-barbiturates) MDZ (5-, 15-, or 50-mg ampoule) 0.2-0.3mg/kg bolus (2mg/min) 0.05-2mg/kg/h Preferable in haemodynamically unstable patients PPF (10- or 20-mg ampoule) 1-2-mg/kg bolus (20μg/kg/min) 5-10mg/kg/h (dangerous if > 80μg/kg/min) Infusion syndrome, contraindicated in patients aged<16, requires OTI+MV Coma (barbiturates) PB (200-mg ampoule) 5-20mg/kg over 20-30min (20-50mg/min) 2–4mg/kg/day; 0.1-5mg/kg/h (12-24h after initial dose) Alternative to non-barbiturates: greater efficacy and fewer adverse reactions
Requires OTI+MVTPT (500-mg ampoule) 2-7mg/kg; 100-200mg over 1min (1-5mg/kg/h) 50mg/2–5min until seizure control achieved (0.05-2mg/kg/h) (D) NCSE Treatment of choice Other options Typical absence BZD (IV or transmucosal; SC administration should be considered in oncological patients) VPA
LEV
Atypical absence IV DZP
VPA/LEV, IV or oralLTG
TPMPartial complex DZP+PHT
PBCLB
LEV (treatment of choice in oncological patients)Subtle NCSE (patient in coma) PB MDZ (treatment of choice in oncological patients)
TPT
PPFAED: antiepileptic drug; BZD: benzodiazepine; CLB: clobazam; CNZ: clonazepam; DZP: diazepam; IM: intramuscular; IV: intravenous; LCM: lacosamide; LEV: levetiracetam; LTG: lamotrigine; MDZ: midazolam; MV: mechanical ventilation; OTI: orotracheal intubation; PB: phenobarbital; PHT: phenytoin; PPF: propofol; SC: subcutaneous; TPM: topiramate; TPT: thiopental; VPA: valproic acid.
A, B, C: adapted with permission from Fernández Alonso et al.,27 2013.
D: adapted with permission from Mercadé-Cerdá et al.,23 2013.
The most recent guidelines recommend administering AEDs as early as possible; the latest trends follow this approach. Delayed onset of IV AEDs and/or administration at low doses is associated with poorer treatment response and prognosis. Seizures not resolving in the predetermined time with the measures described above are classified as SE. In these patients, treatment should be started with intravenous PHT, VPA, LEV, or LCM; no study with level of evidence 1 has shown any of these drugs to be superior. Therefore, the AED should be selected by exclusion according to comorbidities, tolerability, and potential drug–drug interactions; IV AEDs of choice in oncological patients are LEV, VPA, and LCM.44 The following second-line AEDs are available (Table 13)27–40,44:
- -
PHT27–40,94–97: given the extensive published evidence and the US Food and Drug Administration recommendation, PHT is the second-line AED of choice for CSE in the majority of clinical guidelines. It is more effective in treating focal than generalised SE, and is not recommended for myoclonic or absence SE. PHT does not suppress respiratory function or impair consciousness. The drug's greatest limitation is its cardiovascular toxicity (arterial hypertension, arrhythmias, etc); great caution should be exercised when prescribing it to elderly patients and/or those with heart disease. Due to its alkalinity (pH: 12)34 it can also cause phlebitis; it should be administered via a different route than BZDs and should not be administered in sugar solutions. An additional disadvantage is that at the highest rate of administration, the necessary dose takes 30minutes to be administered, which is too long to verify effectiveness and administer a second drug, where necessary, before considering anaesthesia as the third line of treatment. Due to potential interactions with antineoplastic drugs and the possible negative effects on lymphocyte function,94,95 LEV is now recommended over PHT, with VPA and/or LCM to be added should that treatment be ineffective.27–40
- -
VPA: despite the absence of any study with level of evidence 1 on its use (only level 2B), many countries have approved use of the drug based on the cumulative evidence, published in over 300 studies,34 that it is as effective as PHT. Most guidelines recommend adding VPA if PHT is unsuccessful or contraindicated for patients with CSE. In a randomised, non-blinded study including 68 patients with CSE, high-dose VPA (30mg/kg over 15min) was more effective than PHT (18mg/kg at 50mg/min). In patients who were unresponsive to the first AED, VPA was more effective than PHT (79% vs 25% responders, respectively).144 VPA has a series of advantages over PHT, including faster onset of action, better tolerability, mild sedation, absence of cardiotoxicity, and a broad spectrum of action. No adjustment needs to be made in patients with kidney failure; due to the high level of plasma protein binding, metabolism of the drug is not affected by dialysis. The most common adverse effects include hypotension, dizziness, and thrombocytopaenia. In clinical practice, VPA has become established as an alternative to PHT in elderly patients and those with heart disease. The drug's main limitation is its hepatic metabolism. It is contraindicated in patients with mitochondrial diseases, liver diseases, bleeding disorders, porphyria, immunosuppression, and HIV infection. Its use is not recommended in women of childbearing age as it can cause polycystic ovary syndrome and teratogenic effects.27–40
- -
LEV is one of the newest alternatives to PHT and VPA when these drugs are contraindicated or ineffective. It became available for IV administration in 2007, so published experience is more limited. The main evidence is from case series of patients with CSE or NCSE treated with LEV as the first second-line AED, or after PHT or VPA. Effective dose is 500-3000mg/day, with seizure control achieved 12-96hours after administration. Up to 1500mg/minute is tolerable. A safe and effective rate of administration is 500mg every 5minutes for 15minutes. No sufficiently well-designed study compares LEV to PHT and VPA. In a 2011 study, Alvarez et al.145 performed an analysis adjusted for SE aetiology and severity and found that PHT was more effective than LEV and less effective than VPA. Various studies have reported similar effectiveness for LEV and VPA at 30minutes for treating CSE and absence NCSE.146–148 The drug's main advantages are its safety, absence of severe adverse reactions, few drug–drug interactions, linear pharmacokinetics, and ease of administration. Due to its metabolism, dose should be reduced by half in patients with creatinine clearance below 30mL/minute. LEV is a good option as the first AED in elderly, oncological, or polymedicated patients, or patients with cardiovascular or liver comorbidities, and constitutes a good alternative if PHT or VPA are ineffective.27 LEV has also been observed to have an antiemetic effect, although the specific mechanism of action involved is unclear.125 Finally, a 2014 literature review and a recent case report have observed a good balance between efficacy and safety for subcutaneous administration of LEV as an alternative route of administration in PC.149,150
- -
LCM, available in Spain since 2011, is the newest AED to be used in SE, and was initially developed for IV administration. It is a new treatment option for situations where SE is unresponsive to the drugs discussed above, particularly for focal CSE, although good results have also been reported for generalised CSE. The level of evidence and grade of recommendation are limited (3D). Nineteen articles (10 clinical cases and 9 case series) have been published, including 136 patients (50% CSE: 31% focal and 19% generalised CSE) aged between 8 and 90, treated with LCM after lack of response to other AEDs; LCM was successful in nearly 60% of patients, particularly when combined with VPA and/or LEV. Efficacy appears to improve with earlier administration of the drug. Dosage typically ranges between 100-400mg over 3-15minutes. A dose of 200-300mg over 15minutes showed similar efficacy to a 400-mg dose, but greater safety. The 400-mg dose, in turn, was safer than a 600-mg dose (not indicated in the summary of product characteristics), with fewer adverse reactions. LCM has a good safety profile, with no severe adverse reactions, with the exception of one case of complete heart block associated with class I antiarrhythmics (sodium channel blockers). The most frequent adverse reactions are mild, dose-dependent neurological alterations. Like LEV, LCM has a profile similar to that considered ideal in emergency departments, with optimal potential for treating SE.27–40
- -
PB: barbiturates were the first drugs to be used regularly to treat SE; from the early 20th century until the arrival of BZDs in the 1960s, they were first-line AEDs. PB continues to be the second most frequently used AED after BZDs in developing countries, with comparable efficacy to PHT. In Spain, it is indicated as an alternative to PHT in SE. It continues to be a good option for neonatal seizures. The main limitation is the drug's poor safety profile: it is associated with a risk of haemodynamic instability and a need for mechanical ventilation, as well as cognitive and behavioural alterations. With the introduction of the new IV AEDs, its use as a second-line AED has fallen; it is now used as a third-line drug to induce coma (Table 12).27–40
According to the available evidence on established CSE, intravenous DZP+PHT, PB, and LZP are equally effective for controlling CSE at 20minutes after perfusion onset and during the first hour (level of evidence: 1).51 Intravenous PHT and VPA, and VPA and LEV are equally effective for controlling CSE at 30minutes after perfusion onset and in patients with adverse reactions (level of evidence: 2).51 Intravenous LCM has been shown to be effective in various non-prospective, non-controlled studies and case series for different types of CSE (level of evidence: 4).51 Most CPGs recommend LZP (4mg IV) or DZP (10mg IV), followed by PHT (18mg/kg IV) or PB (20mg/kg IV) (level of evidence: 4).51 Treatment with VPA, LEV, or LCM is indicated in cases of RSE or when PHT is contraindicated, as an alternative to IV PB (level of evidence: 3).51 LEV plus LCM is not indicated for treating SE.51 According to grade of recommendation, BZDs should be used for the initial pharmacological treatment of any prolonged seizure or SE episode (grade of recommendation: A). Intravenous PHT and/or PB should be used if SE is not controlled with BZDs (grade of recommendation: A). SE should be treated with IV VPA and/or LEV if PHT is contraindicated (grade of recommendation: B). LEV and LCM may be used to treat SE if PHT is contraindicated, as an alternative to IV PB, or in RSE (grade of recommendation: C). In PC of oncological patients, second-line treatments (to be administered for no longer than 30minutes after CSE onset) should be, in order of choice: LEV, LEV+VPA, LEV+LCM, VPA+LCM, and LEV+VPA+LCM.27–40 PMP via nasogastric tube has been tested in several small case series (the largest including 12 patients), which are too heterogeneous to allow reliable conclusions to be drawn regarding its efficacy in treating CSE.44 BRV is available in an IV preparation; although epilepsy experts consider it to be a potential adjuvant AED for treatment of SE, no studies have yet been published on its use in humans.44
Third-line antiepileptic drugs (induced coma).27–40,139,140If after 30minutes SE does not respond to treatment with second-line AEDs, anaesthetics should be considered (provided that the patient does not meet criteria for terminal illness and the patient and/or family consents). One limitation of these drugs is their systemic effects (arterial hypotension, myocardial depression, and hepatotoxicity). Anaesthetics should be administered in ICUs; patients should be monitored via ECG and EEG and receive life support. If orotracheal intubation is required, muscle relaxants with short half-lives, such as vecuronium, are preferred as they do not interfere with subsequent neurological assessment. Selection of drugs used to induce medical coma for RSE should be based on the experience and/or protocols of the ICU. ICU admission is ideally avoided in cancer patients receiving PC.102 There are 2 means of inducing coma: barbiturates and non-barbiturates. No sufficiently high-quality studies have shown that either type of drug is superior to the other (level of evidence: 4).27–40,44,51
- -
Coma induced with non-barbiturates (MDZ/propofol): these drugs are preferred over barbiturates in haemodynamically unstable patients due to their greater safety and faster onset of action. MDZ and propofol are the most commonly used drugs. MDZ has the better safety profile; it should be noted that adverse reactions and tachyphylaxis are more common if constant infusion is used.39,139 While propofol is safe at low doses, there is a risk of potentially fatal propofol infusion syndrome (severe metabolic acidosis, rhabdomyolysis, kidney failure, arterial hypotension, apnoea, bradycardia, etc). Vital signs must be monitored for this reason; the drug is contraindicated in children.17–40,139–143
- -
Coma induced with barbiturates (PB/thiopental): barbiturates are only used as a rescue drug when non-barbiturates fail, and should be avoided in haemodynamically unstable patients due to the associated adverse reactions (especially hypotension and sedation). Efficacy is comparable to that of PHT. No clear differences have been observed between PB and thiopental (which is converted to its active metabolite pentobarbital) with the exception of the latter's cardiovascular toxicity (Table 13).151
- -
Alternatives to anaesthetics: inhaled anaesthetics (isoflurane, desflurane); IV lidocaine; IV ketamine; IV magnesium sulphate (especially in women with eclampsia); AEDs (enteral TPM; oral CBZ or CLB); immunotherapy (corticosteroids and/or immunosuppressors); and non-drug treatments (ketogenic diet, therapeutic hypothermia, or vagus nerve stimulation), among other treatments.27–40,44,47,81,83,89
No guidelines or expert consensus documents on treatment of NCSE are currently available due to a lack of studies and efficacy data. The recommendations are the same as those for CSE, but with a lower level of evidence (recommendation of the Epilepsy Study Group of the Spanish Society of Neurology).51 Aggressive approaches are not recommended for patients who are not in deep coma, as the consequences are less severe. It is therefore recommended to start treatment with BZDs and monitor the response. Should epileptic activity continue, second-line AEDs should be administered: VPA and LEV are preferred for absence SE and LEV and PHT are recommended for partial complex SE. Due to its efficacy, safety, and broad spectrum of action, LCM seems to be a good option for refractory cases (Table 13).27,40,41
Summary of treatment for convulsive status epilepticus (Fig. 2)1–151Preventive antiepileptic treatment after the first seizure27- -
Initial AEDs for preventive treatment: the first course of action is to decide whether secondary preventive treatment in the acute phase should be applied in the short term, to prevent early recurrence, or maintained in the long term (difficult to determine during the acute phase). The AED treatment strategy must ensure greater benefits than risks to the patient and must be agreed with the patient and family, taking into account their preferences.
- -
Risks and benefits related to the initial AED: the introduction of AED treatment should aim to minimise the risk of recurrences, as additional seizures are associated with increased risk of accidents, work restrictions, social stigma, and even sudden death, as well as repeated emergency department visits. Patients presenting SE or a second unprovoked seizure have a greater risk of recurrence (70% in the first year). Patients only experiencing one stroke are at less risk (40% at 2 years). AED treatment after a first seizure reduces the risk of recurrence to a mean of 34%, especially in the short term (< 2 years). However, no clear differences have been observed in the long term (> 5 years); nor does vital prognosis significantly improve (level of evidence: 1).27,51 A series of risk factors have been described for seizure recurrence: seizure type (focal onset, symptomatic aetiology), number of seizures (≥ 2), abnormalities detected in neurological examination, epileptiform activity on EEG, and structural alteration on neuroimaging scans. The Multicentre Trial for Early Epilepsy and Single Seizures established a prognostic index for seizure recurrence, based on these factors: a) one point if the patient experienced 2 or 3 seizures prior to consultation, and 2 points for 4 or more seizures; and b) one additional point if the patient displays neurological disorder, learning disorder, or developmental delay, and a further point for EEG abnormalities (epileptiform activity or slow waves). Risk is classified as low (0 points), medium (1 point), or high (2-4 points).152
- -
Indications for preventive treatment (Table 14)27: treatment is generally recommended following SE, ≥ 2 unprovoked seizures, or one seizure (provoked or not) accompanied by EEG alterations, some potentially epileptogenic neurological disorder (acute structural alteration and/or previous disease), or great psychosocial demand on the part of the patient. AED treatment should be started in patients at medium or high risk (≥ 1 point). In any case, patients should be referred to a neurology consultation (epilepsy unit) in order to complete the aetiological study and to start or continue long-term antiepileptic treatment.
Table 14.A. Summary of the main indications for AEDs for secondary prevention. B. AEDs as preventive treatment.
(A) Indications for starting secondary seizure prevention Duration of secondary prevention Second unprovoked seizure or SE Long-term First seizure and high risk of recurrence Acute symptomatic seizure if:
– Acute structural alteration (CNS infection, stroke, severe head trauma*)
– Alcohol withdrawal
– EclampsiaShort-term (introduction in acute phase)
* Primary prevention (1 week)Remote symptomatic seizure: all
Seizure of undetermined aetiology
– Not GTC at onset
– Neurological deficit
– Very young or old patient
– Focal neurological signs following seizure (e.g., Todd paralysis)
– Lesion visible on neuroimaging scans
– Epileptiform activity on EEGLong-term (assess starting administration during acute phase, prior to discharge from emergency department; await specialist neurology consultation) (B) AEDs as preventive treatment Observations Recommendations during acute phase (order of preference)
1. LEV
2. VPA
3. PHT
4. CBZ (oral)IV administration recommended during acute phase After acute phase, secondary prevention with
LEV
Alternatives
LTG
VPA
OXCUpon discharge from the emergency department, treatment should continue with oral administration, if possible. AED: antiepileptic drug; CBZ: carbamazepine; CNS: central nervous system; EEG: electroencephalography; GTC: generalised tonic clonic; IV: intravenous; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHT: phenytoin; SE: status epilepticus; VPA: valproic acid.
Adapted with permission from Fernández Alonso et al.,27 2013.
- -
Selection of AEDs for preventive treatment (Table 14)27: drug choice should be based on seizure type (classification), patient characteristics (age, comorbidities, and other treatments), and drug characteristics (efficacy, safety, availability, and ease of use).
Decompensation may occur in various clinical scenarios; safety is the primary concern in all cases. Patients should be referred to hospital if they present specific risk factors: suspicion of a new cerebral lesion (different seizure characteristics, abnormal neurological examination, and/or underlying disease such as cancer, AIDS, etc), prolonged or recurrent seizures (SE or cluster seizures [≥ 3 seizures in 24h), and/or suspected systemic and/or traumatic complications. Pragmatically, we can distinguish between decompensation related to seizure recurrence (efficacy) and decompensation associated with adverse drug reactions (safety) (Fig. 3).
- -
Recurrent seizures: (a) similar to typical seizures: patients with no change in seizure type or frequency. A conservative approach should be taken with these patients, with normal follow-up (complementary testing is unnecessary), maintaining or restarting the habitual treatment, and stressing the importance of avoiding triggers and ensuring treatment adherence. (b) Increased seizure frequency: it is important to consider proper treatment adherence and whether AED levels are within the therapeutic range. Tests should be performed to determine levels of PHT (10-20μg/mL), PB (10-40μg/mL), CBZ (4-12μg/mL), and VPA (50-100μg/mL). If the trigger factor is clearly treatment withdrawal or non-adherence and seizures were controlled when the patient was using the drugs, treatment should be restarted. In cases where adherence is thought to be good and AEDs are detected at subtherapeutic levels, drug–drug interactions and/or intercurrent disease should be suspected. In these cases, we should increase the AED dose or consider changing to another drug without these interactions. If AED levels do fall within the therapeutic range, we can increase the dose and/or add other AEDs. If the maximum dose for the drug in question is prescribed and levels of the drug are unknown, we may add a new AED or await the next neurology consultation.
- -
Adverse reactions to AEDs27,45: (a) harmacological adverse reactions (dose-dependent)p: most effects involve the CNS (drowsiness, ataxia, dysarthria, diplopia, blurred vision, etc.). This is managed by reducing the dose and the speed of up-titration. Monitoring AED levels is useful if they are below the therapeutic range; in this way we can identify a parallel between clinical improvement and normalisation of AED levels. (B) Idiosyncratic adverse reactions (dose-independent): mild adverse reactions (particularly skin rashes) are relatively frequent, although more severe reactions can affect the skin (Stevens–Johnson syndrome), bone marrow (agranulocytosis), or liver (toxic hepatitis). Hypersensitivity reactions (fever, exanthema, adenopathy, oedema, etc) can be very severe, and appear more frequently in association with CBZ, PHT, PB, LTG, OXC, and ZNS. In patients with exanthema, the AED should be changed for one with lower risk, such as LEV, GBP, or TPM. Cognitive adverse effects are frequent in elderly patients, especially in association with classic AEDs. VPA is contraindicated in women of childbearing age due to its effects on the reproductive organs and foetus. Hyponatraemia is an issue frequently associated with CBZ and derivatives (OXC and ESL) and often leads to changes or withdrawal of AEDs.
Algorithm 2. Treatment adjustment for decompensation. AED: antiepileptic drug. Adapted with permission from Fernández Alonso et al.27
Regarding rational polytherapy for refractory epilepsy (which is frequent in oncological patients), most authors recommend trying the new AEDs if seizure control is not achieved and the drugs are indicated. LCM is a good option in these situations as it features a novel mechanism of action and a near-ideal clinical profile. Current evidence is limited, as it comes from non-randomised, open-label trials, case series, post hoc analyses, and expert opinions. The following are considered “potentially useful combinations”: non–sodium channel blocking AEDs (e.g., VPA or LEV)+LCM; LCM or VPA+LEV, TPM, ZNS, or LCM; CBZ, OXC, ESL, or PHT+LEV, LCM, ZNS, or RTG; and VPA+ESM. Caution is necessary when administering CBZ, OXC, ESL, or PHT+LTG; VPA+PHT; or CBZ or PHT+TPM or TGB. The following combinations are not recommended: CBZ+PHT; and OXC+ESL, PRM, TGB, or VGB. Finally, changing brand-name for generic AEDs is not recommended in patients who are seizure-free or have refractory epilepsy.27,153Table 15 lists the most commonly used brand-name oral AEDs.
Most frequently administered brand-name AEDs for oral administration (where possible) and dosing information.
| Oral AED (mg) | Initial dose | Up-titration | Mean maximum dose |
|---|---|---|---|
| 1st generation | |||
| Phenytoin, Sinergina® (100) | 100mg every 8-12h | 50-100mg/day every week | 200-600mg/day (2–3 doses) |
| Carbamazepine, Tegretol® (200, 400) | 100-200mg every 12-24h | 100mg/day every week | 600-1600mg/day (3 doses) |
| VPA, Depakine® (200, 500, Crono 300, Crono 500) | 200mg every 8h | 200mg/day every 3 days | 1000-3000mg/day (2–3 doses or 1-2 doses Crono) |
| 2nd generation | |||
| Lamotrigine, Lamictal® (25, 50, 100, 200) | 25mg every 24h | (A) 50mg/day every 1-2 weeks (monotherapy and coadministration with enzyme inducers) (B) 25mg/day every 1-2 weeks (coadministration with VPA) | 100-500mg/day (2 doses) |
| Topiramate, Topamax® (25, 50, 100, 200) | 25-50mg every 24h | 25-50mg/day every week | 200-800mg/day (2 doses) |
| Gabapentin, Neurontin® (100, 300, 400, 600, 800) | 300-400mg every 24h (day 1)/12h (day 2)/8h (day 3) | 300-400mg/day every 1-3 days | 900-3600mg/day (2-3 doses) |
| Oxcarbazepine, Trileptal® (150, 300, 600) | 150-300mg every 12h | 600mg/day every week | 600-2400mg/day (2 doses) |
| Levetiracetam, Keppra® (250, 500, 1000) | 250-500mg every 12h | 500-1000mg/day every week | 1000-3000mg/day (2 doses) |
| Zonisamide, Zonegran® (25, 50, 100) | 25-50mg every 24h | 50-100mg/day every week | 100-500mg/day (2 doses) |
| 3rd generation | |||
| Lacosamide, Vimpat® (50, 100, 150, 200)a | 50mg every 12h | 100mg/day every week | 200-400mg/day (2 doses) |
| Eslicarbazepine, Zebinix® (800) | 400mg every 24h | 400mg/day every 1-2 weeks | 400-1200mg/day (1 dose) |
| Retigabine, Trobalt® (50, 100, 200, 400) | 100mg every 8h | 100-150mg/day every week | 600-1200mg/day (3 doses) |
| Rufinamide, Inovelon® (100, 200, 400) | 100-400mg every 24h | 200-400mg/day every 2 days | 1200-4800mg/day (2 doses) |
| Perampanel, Fycompa® (2, 4, 6, 8, 10, 12) | 2mg every 24h | 2mg/day every week | 6-12mg/day (1 dose) |
| Brivaracetam, Briviact® (10, 25, 50, 75, 100) | 25mg every 12h | Not necessary (therapeutic dose is reached on day 1) | 50-200mg/day (2 doses) |
VPA: valproic acid.27,45,46,154–158
Loading dose: 200mg over 15min (oral or intravenous). Maintenance dose: 200mg/day (2 doses), 12h after the loading dose.
Adapted with permission from Fernández Alonso et al.,27 2013.
Given the relative frequency of seizures in PC and the creation of the new PC unit being opened at our neurorehabilitation centre, we deemed it necessary to produce these guidelines. In addition to informing the proper selection of candidates for PC, it is essential to achieving optimal symptomatic control of seizures and preventing distress, suffering, and pain for these patients and their families. This enables us to provide a standard of well-being and comfort in the final stage of life. Based on the findings of a thorough literature search and the needs of these patients, we recommend using AEDs via parenteral route (preferably IV) and selecting drugs with few interactions. DZP and MDZ are the most appropriate drugs in the acute stage; LEV, VPA, and/or LCM should be used for long-term treatment or for epilepsy refractory to DZP or MDZ, where more effective symptomatic treatment is needed. These guidelines should be considered a global approach and should be adapted holistically, from a multidisciplinary perspective, according to the inherent characteristics of each individual case, observing the wishes and priorities of the patient/family. No national consensus document has yet been published on this subject. There is a need for well-designed, randomised, controlled trials with large samples of patients receiving PC. This would enable such a national document to establish well-founded, generalised recommendations on appropriate, rational, and effective use of AEDs in this highly delicate, complex area of healthcare.
LimitationsWe include a table clarifying the levels of evidence and grades of recommendation for the different treatments discussed. This information is needed for all treatments, as the main issue in the preparation of these guidelines is the lack of published evidence. This will be addressed in future updates of this initial version of the CPG as new studies are published on the subject.
FundingThis study received no public or private funding.
Conflicts of interestAll authors have given their approval for the publication of the manuscript. The authors have no conflicts of interest to declare.
We wish to thank all the patients and employees of San Vicente Clinic.
Please cite this article as: León Ruiz M, Rodríguez Sarasa ML, Sanjuán Rodríguez L, Pérez Nieves MT, Ibáñez Estéllez F, Arce Arce S, et al. Guía para el manejo de las crisis epilépticas en cuidados paliativos: propuesta de un modelo actualizado de práctica clínica basado en una revisión sistemática de la literatura. Neurología. 2019;34:165–197.