Chronic kidney disease (CKD) can cause anaemia and neurological disorders. Recombinant human erythropoietin (rHuEPO) is used to manage anaemia in CKD. However, there is little evidence on the effects of rHuEPO on behaviour and cognitive function in CKD. This study aimed to evaluate the impact of rHuEPO in sensorimotor and cognitive functions in a CKD model.
MethodsMale Wistar rats were randomly assigned to 4 groups: control and CKD, with and without rHuEPO treatment (1050IU per kg body weight, once weekly for 4 weeks). The Morris water maze, open field, and adhesive removal tests were performed simultaneously to kidney damage induction and treatment. Markers of anaemia and renal function were measured at the end of the study.
ResultsTreatment with rHuEPO reduced kidney damage and corrected anaemia in rats with CKD. We observed reduced sensorimotor dysfunction in animals with CKD and treated with rHuEPO. These rats also completed the water maze test in a shorter time than the control groups.
ConclusionsrHuEPO reduces kidney damage, corrects anaemia, and reduces sensorimotor and cognitive dysfunction in animals with CKD.
La enfermedad renal crónica (ERC) puede provocar anemia e inducir afectaciones neurológicas. La eritropoyetina humana recombinante (rHuEPO) se utiliza en el tratamiento de la anemia en la ERC. Sin embargo, existe poca evidencia de los efectos de la rHuEPO sobre la conducta y las funciones cognitivas en la ERC. El objetivo de este estudio fue evaluar el efecto del tratamiento con rHuEPO sobre las funciones sensoriomotoras y cognitivas en un modelo de ERC.
MétodosRatas macho de la cepa Wistar fueron asignadas a 4 grupos: control y ERC, con y sin tratamiento con rHuEPO (1.050 UI/kg de peso, una vez por semana durante 4 semanas). Las pruebas conductuales de laberinto acuático de Morris, campo abierto y cinta adhesiva se realizaron de manera simultánea a la inducción del daño renal y el tratamiento. Mientras que la determinación de marcadores de función renal y anemia se realizaron al término del estudio.
ResultadosEl tratamiento con rHuEPO redujo el daño en el riñón y corrigió la anemia en las ratas con ERC. En las pruebas conductuales, el tratamiento con rHuEPO redujo la disfunción sensoriomotora observada en los animales con ERC. Por otra parte, en los animales con ERC y tratamiento con rHuEPO resolvieron el laberinto en menor tiempo en comparación a los grupos control.
ConclusionesEl tratamiento con rHuEPO reduce el daño en el riñón, corrige la anemia y reduce la disfunción sensoriomotora y cognitiva en los animales con ERC.
Cognitive impairment is a frequent complication of chronic kidney disease (CKD), causing such alterations as attention deficits and difficulties with verbal communication, learning, and memory.1 According to epidemiological data, over 60% of patients with CKD are highly likely to develop cerebrovascular diseases, which promote cognitive impairment and dementia.2–4
The main pathogenic factors involved in neurological complications of CKD are high concentrations of circulating uraemic toxins,5 proinflammatory cytokines, and reactive oxygen species,2 which cause alterations in serotonergic and cholinergic neurotransmission, and endothelial inflammation and dysfunction.6 These factors can affect all levels of the nervous system, promoting such neurological alterations as uraemic encephalopathy, cognitive problems, and such neuromuscular disorders as mononeuropathy, polyneuropathy, and myopathy.7
At advanced stages, patients with kidney disease may present severe anaemia, known as renal anaemia, caused by a deficiency in renal erythropoietin production.8–10 Numerous studies have reported that anaemia negatively affects cognitive function11,12 due to insufficient cerebral oxygen supply caused by a decrease in the oxygen transport capacity.13 Chronic hypoxia causes toxicity secondary to oxidative stress; combined with the accumulation of uraemic toxins,7,14 this accelerates neuronal degeneration.12,15 In patients with CKD, anaemia is treated with erythropoiesis-stimulating agents, such as recombinant human erythropoietin (rHuEPO).10
While erythropoietin plays an essential role in erythropoiesis, it has been reported to have other functions, such as anti-inflammatory16 and anti-apoptotic activities,17 stimulating neurogenesis and neuronal differentiation in early developmental stages,18 and tissue protection in the kidneys19 and brain.20,21
Both in vitro17,22 and in vivo models have demonstrated the neuroprotective effects of this growth factor; in animal models, rHuEPO has promoted angiogenesis, neurogenesis, and the migration of neural precursor cells to the lesion site, and inhibited apoptosis by suppressing glutamate secretion and modulating intracellular calcium.23–28 Strikingly, clinical trials have reported that administration of rHuEPO reduces cognitive impairment, with a positive impact on memory and learning problems.29–31
In addition to learning and memory impairment, motor problems are reported in some animal models of CKD.32,33 However, there is little evidence on the neuroprotective effects of erythropoietin in CKD. This study aims to evaluate the effect of rHuEPO on sensorimotor activity, memory, and learning when the agent is administered simultaneously with the induction of kidney damage in a model of CKD.
MethodsSubjectsWe used 24 male Wistar rats (Envigo RMS) weighing 250 to 300g at the beginning of the study. Rats were housed in translucent polycarbonate cages in a vivarium with a temperature of 25±2°C and a 12:12hour light-dark cycle; rats had free access to food and water. Rats were handled according to the international ethical standards established in the Guide for care and use of laboratory animals and official Mexican guidelines established in regulation NOM-062-ZOO-1999. The institutional ethical approval code for the study is CICUAL-2016-019.
Experimental groups and treatmentsThe animals were randomly assigned to the following study groups: (1) control+sham (n=5); (2) control+rHuEPO (n=5); (3) CKD+sham (n=7); and (4) CKD+rHuEPO (n=7). CKD was induced with adenine, administered orogastrically at 100mg/kg/day for 28 days.34,35 Groups 1 and 3 received a sham treatment (saline solution) and groups 2 and 4 were treated with rHuEPO administered at 1050IU/kg via dorsal subcutaneous injection once weekly for 4 weeks. All behavioural tests were performed simultaneously with CKD induction and treatment.
Sample collection and determination of biochemical and haematological parametersAt the end of the treatment and behavioural testing period, animals were placed for 24hours in metabolic cages for urine collection. They were subsequently euthanised with an overdose of sodium pentobarbital (40-60mg/kg, intraperitoneal administration), and we collected total blood with ethylenediaminetetraacetic acid and serum. Finally, kidneys were dissected, measured, and weighed. Creatinine and urea levels were determined by dry chemistry using a Vitros 250 system (Ortho Clinical Diagnostics); haemoglobin, haematocrit, erythrocyte, and reticulocyte levels were measured with a KX-21N haematology analyser (Sysmex Corporation).
Open field testThe open field test enables general assessment of animals’ locomotor activity and anxiety.36 The test was performed in a white open-field arena measuring 50cm×50cm×50cm. Rats were placed in the centre of the arena and allowed to explore freely for 3minutes. Video footage was recorded for subsequent analysis. The entire arena was cleaned with 70% ethanol after each rat completed the test. The behavioural parameters evaluated were: (1) exploration time, (2) latency time, (3) number of escape attempts, and (4) instances of grooming.
Adhesive removal testThis test assesses somatosensory and motor function. Adhesive tape strips are placed on the rat's fore or hind paws and the animal's performance is assessed by measuring the time taken for it to feel and remove the tape.37,38 The test was performed 3 times per day for 3 days. Two adhesive tape strips of equal size were placed on the hind paws. Tactile response was determined by measuring the time between initial contact with the tape and removal of the tape. Rats that did not complete the task within 60seconds were considered unable to remove the tape.
Morris water maze testThe water maze test was performed with a circular pool (110cm diameter by 60cm depth) filled with water (mean temperature [SD], 23°C [2°C]), as specified by Tóthová et al.,39 with the modifications described below. The maze was divided virtually into 4 quadrants and a geometric shape was placed on the wall in each quadrant to act as an external clue for orientation. A 10cm×10cm polycarbonate platform was placed in the centre of one quadrant. In an initial training phase (4 sessions daily for 3 days), rats were allowed to swim freely for one minute (beginning in a different quadrant each time), enabling them to see the platform, 1cm above the surface of the water; in the final test, the platform was submerged 1cm below the water level. Animals that were unable to reach the platform within 60seconds were guided and placed on top of the platform for 20seconds to enable spatial orientation. Video footage was recorded for subsequent analysis; the parameters evaluated were time taken to reach the platform and distance swum.
Statistical analysisData on kidney function and anaemia were analysed using one-way ANOVA; data from the open-field, adhesive removal, and water maze tests were analysed using two-way repeated measures ANOVA with treatment and training days as factors. Statistical significance was set at P<.05, and the Tukey post hoc test was applied. Results are expressed as mean (standard deviation).
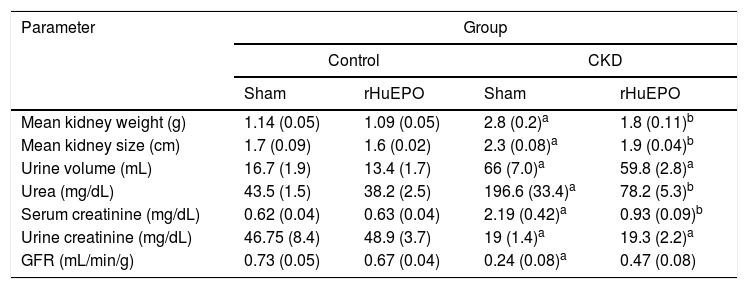
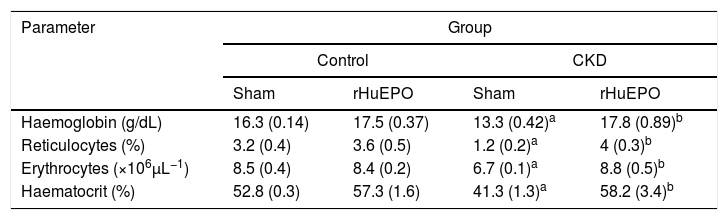
ResultsTreatment with recombinant human erythropoietin reduces kidney damage and corrects renal anaemia in rats with chronic kidney diseaseStatistical analysis found that animals with CKD had significantly lower body weight (data not shown), reduced urine creatinine levels (P<.05), and increased serum creatinine and urea levels (P<.05). These rats also had increased urine volume (P<.05) and increased kidney size and weight (39% and 152% greater, respectively) compared to control rats. This represents a 66% reduction in glomerular filtration rate (GFR), demonstrating a loss of kidney function in these animals (Table 1). Haematological findings indicated significant reductions in haemoglobin concentration (21%), haematocrit (25%), erythrocytes (21%), and reticulocytes (65%) in CKD rats vs. controls (P<.05) (Table 2).
Kidney function markers.
| Parameter | Group | |||
|---|---|---|---|---|
| Control | CKD | |||
| Sham | rHuEPO | Sham | rHuEPO | |
| Mean kidney weight (g) | 1.14 (0.05) | 1.09 (0.05) | 2.8 (0.2)a | 1.8 (0.11)b |
| Mean kidney size (cm) | 1.7 (0.09) | 1.6 (0.02) | 2.3 (0.08)a | 1.9 (0.04)b |
| Urine volume (mL) | 16.7 (1.9) | 13.4 (1.7) | 66 (7.0)a | 59.8 (2.8)a |
| Urea (mg/dL) | 43.5 (1.5) | 38.2 (2.5) | 196.6 (33.4)a | 78.2 (5.3)b |
| Serum creatinine (mg/dL) | 0.62 (0.04) | 0.63 (0.04) | 2.19 (0.42)a | 0.93 (0.09)b |
| Urine creatinine (mg/dL) | 46.75 (8.4) | 48.9 (3.7) | 19 (1.4)a | 19.3 (2.2)a |
| GFR (mL/min/g) | 0.73 (0.05) | 0.67 (0.04) | 0.24 (0.08)a | 0.47 (0.08) |
Data are expressed as mean (standard deviation).
Statistical significance was set at P<.05 (one-way ANOVA).
Haematological data.
| Parameter | Group | |||
|---|---|---|---|---|
| Control | CKD | |||
| Sham | rHuEPO | Sham | rHuEPO | |
| Haemoglobin (g/dL) | 16.3 (0.14) | 17.5 (0.37) | 13.3 (0.42)a | 17.8 (0.89)b |
| Reticulocytes (%) | 3.2 (0.4) | 3.6 (0.5) | 1.2 (0.2)a | 4 (0.3)b |
| Erythrocytes (×106μL−1) | 8.5 (0.4) | 8.4 (0.2) | 6.7 (0.1)a | 8.8 (0.5)b |
| Haematocrit (%) | 52.8 (0.3) | 57.3 (1.6) | 41.3 (1.3)a | 58.2 (3.4)b |
Data are expressed as mean (standard deviation).
Statistical significance was set at P<.05 (one-way ANOVA).
Treatment with rHuEPO significantly reduced the increase in serum creatinine and urea levels in CKD rats (P<.05). The treatment also prevented kidney hypertrophy: kidney weight and size were 1.5 times and 1.2 times smaller than in CKD rats not receiving rHuEPO (Table 1). In addition, animals receiving rHuEPO presented decreased urine volume and GFR (9.4% and 33% less, respectively), although these differences were not statistically significant (Table 1). Treatment with rHuEPO in rats with CKD also corrected renal anaemia, with haemoglobin levels, reticulocyte and erythrocyte counts, and haematocrit returning to the levels observed in the control group (Table 2).
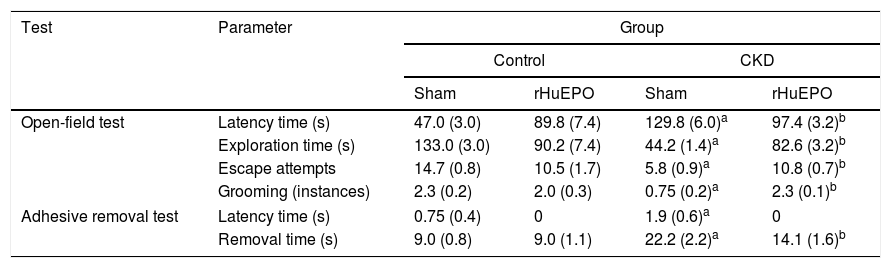
Treatment with recombinant human erythropoietin reduces sensorimotor dysfunction in rats with chronic kidney diseaseAnimals with CKD showed increased latency times in the open field and adhesive removal tests, with increased periods of inactivity compared to control animals (P<.05) (Table 3). They also showed significant reductions in exploration time, escape attempts, and instances of grooming in the open-field test (P<.05); similarly, they took longer to remove adhesive strips, demonstrating reduced tactile response compared to control rats (P<.05) (Table 3).
Tests of sensorimotor function.
| Test | Parameter | Group | |||
|---|---|---|---|---|---|
| Control | CKD | ||||
| Sham | rHuEPO | Sham | rHuEPO | ||
| Open-field test | Latency time (s) | 47.0 (3.0) | 89.8 (7.4) | 129.8 (6.0)a | 97.4 (3.2)b |
| Exploration time (s) | 133.0 (3.0) | 90.2 (7.4) | 44.2 (1.4)a | 82.6 (3.2)b | |
| Escape attempts | 14.7 (0.8) | 10.5 (1.7) | 5.8 (0.9)a | 10.8 (0.7)b | |
| Grooming (instances) | 2.3 (0.2) | 2.0 (0.3) | 0.75 (0.2)a | 2.3 (0.1)b | |
| Adhesive removal test | Latency time (s) | 0.75 (0.4) | 0 | 1.9 (0.6)a | 0 |
| Removal time (s) | 9.0 (0.8) | 9.0 (1.1) | 22.2 (2.2)a | 14.1 (1.6)b | |
Data are expressed as mean (standard deviation) for the final test session. Statistical significance was set at P<.05 (two-way ANOVA).
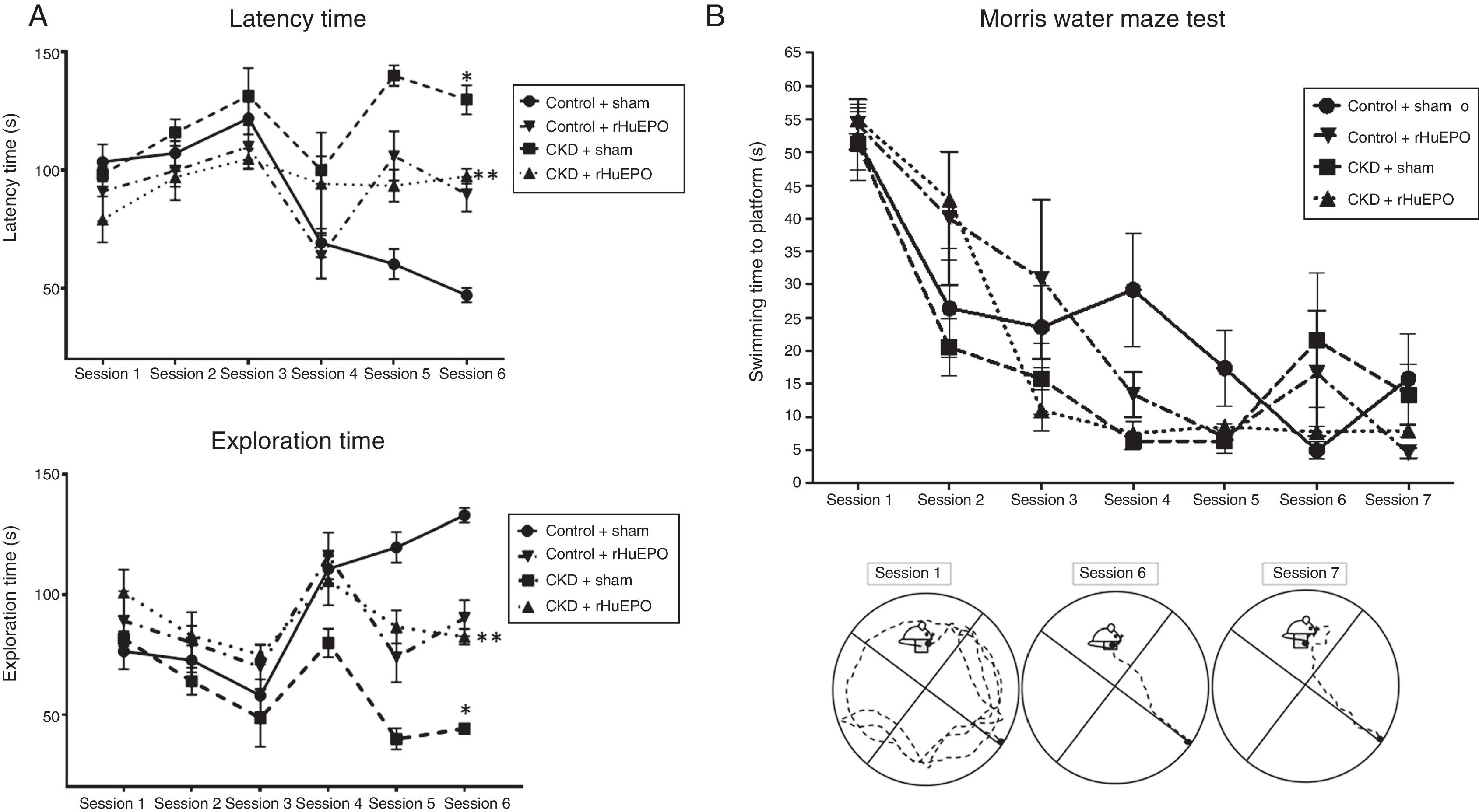
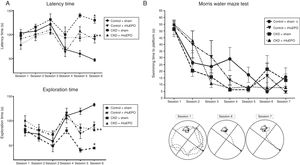
Treatment with rHuEPO was associated with significant increases in the number of escape attempts and instances of grooming in the open field test (P<.05) and reduced time taken to remove adhesive tape strips, compared to animals receiving the sham treatment (Table 3). Interestingly, rHuEPO treatment was associated with decreased latency times in both tests and increased exploration time in CKD rats (P<.05) (Fig. 1A).
Behavioural tests. (A) Open field test. The graphs show latency and exploration times for each session. (B) Morris water maze test. The graph shows swimming time to platform for each session, and a representative illustration of paths taken to the platform.
Data are expressed as means (error bars denote standard deviation). Statistical significance was set at P<.05 (one-way ANOVA). *Significant difference between CKD and control rats. **Significant difference between CKD+rHuEPO and CKD+sham rats.
ANOVA: analysis of variance; CKD: chronic kidney disease; rHuEPO: recombinant human erythropoietin.
In the water maze test, all groups showed significant reductions in the time taken to swim to the platform between training sessions 1 and 4 (P<.05). While all animals showed a significant reduction in swimming time in tests 5 to 7, a consistent reduction was observed from session 3 in CKD rats treated with rHuEPO (Fig. 1B).
DiscussionThis study aimed to evaluate the effects of treatment with rHuEPO on sensorimotor and cognitive function in a model of CKD. CKD is characterised by abnormalities in kidney structure and function.40 Our findings show that administration of adenine (used to induce CKD) increased serum creatinine and urea concentrations, reduced GFR, and caused renal anaemia; these observations are consistent with those of previous studies of this model.32,41 In rats with CKD, rHuEPO corrected anaemia and decreased kidney damage. Coldewey et al.42 report similar findings, with erythropoietin administration attenuating acute kidney dysfunction in a murine model of sepsis.
By performing behavioural tests in parallel with the induction of kidney damage, we were able to observe progressive sensorimotor dysfunction. Rats with CKD showed a gradual increase in latency times in these tests, as well as decreased ability to remove the adhesive tape and reductions in exploration times and the number of escape attempts and instances of grooming. Mazumder et al.43 and Karthick et al.33 make similar observations, reporting that inactivity times are 2-3 times greater in animals with kidney damage. Behavioural changes in rats with CKD may be attributed to increased plasma urea concentration and reduced GFR, which would cause neurotoxicity due to the accumulation of uraemic toxins, promoting neurodegeneration.2,44 They may also be related to anaemia, which contributes to reduced cerebral oxygen supply and consequently greater distribution of uraemic toxins, with an impact on brain metabolism.12,45
While some studies do not report that treatment with erythropoietin has any effect on cognitive function,46,47 several studies do observe beneficial effects on motor function, learning, and memory.25,27,33,48
Our results demonstrate that rHuEPO treatment reduces latency time and tape removal time and increases exploration time; this reflects an improvement in the sensorimotor impairment observed in rats with CKD. Similarly, other studies report that preventive treatment with a single dose of erythropoietin improves animals’ motor performance in models of traumatic brain injury28,46 and ischaemia.49 One study of an adenine-induced CKD model found that simultaneous and subsequent treatment with erythropoietin improved the behavioural alterations.33
Finally, it should be noted that slight alterations were observed in memory and learning during the induction of CKD. As explained by Tóthová et al.,39 the duration of the study period is an important factor in observing cognitive alterations in rats with kidney damage. Other studies report increases in memory and learning alterations several weeks after kidney failure is established.50,51 This is explained by the association between cognitive impairment and CKD severity due to uraemia and anaemia, which lead to a state of chronic hypoxia of the brain.52
In conclusion, despite the slight changes in cognitive function at 4 weeks, adenine-induced kidney damage was associated with progressive sensorimotor alterations. Simultaneous treatment with rHuEPO corrected anaemia and reduced uraemia and kidney damage, which may be associated with reductions in sensorimotor dysfunction in animals with CKD.
FundingThis project was supported by the Sectoral Fund for Research in Education (Ciencia Básica SEP-CONACyT; project code 243118); the technical supervisor is Dr Ana Laura Márquez.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Reza-Zaldívar EE, Sandoval-Avila S, Gutiérrez-Mercado YK, Vázquez-Méndez E, Canales-Aguirre AA, Esquivel-Solís H, et al. La eritropoyetina humana recombinante reduce la disfunción sensoriomotora y el deterioro cognitivo en ratas con enfermedad renal crónica. Neurología. 2020;35:147–154.