Adult chronic idiopathic hydrocephalus (ACIH) is a cause of dementia that can be treated by implanting a ventriculo-peritoneal shunt (VPS). We aim to study clinical and functional outcomes in patients with ACIH corrected with a VPS.
Subjects and methodsObservational cohort study of patients diagnosed with probable ACIH (Japan Neurosurgical Society guidelines) and undergoing shunt placement between 2008 and 2013 in a centre of reference for neurosurgery in Spain. Clinical improvement was classified in 4 categories (resolution, partial improvement, equivocal improvement, and no improvement); functional outcome was assessed on the modified Rankin scale (mRS).
ResultsThe study included 29 patients with a mean age of 73.9 years; 62.1% were male and 65.5% had hypertension. Clinical improvement (complete or partial) was observed in 58% after one year and in 48% by the end of the follow-up period (mean follow-up time was 37.8 months). Older age, presence of hypertension, and surgery-related complications were more prevalent in the group responding poorly to treatment. One patient died, 20.7% experienced severe complications, and 69% were dependent (mRS≥3) by the end of the follow-up period. Age at diagnosis was independently associated with poorer clinical response at one year and a higher degree of dependency by the end of follow-up.
ConclusionSymptomatic benefits offered by VPS were partial and transient; treatment was associated with a high complication rate and poor functional outcomes in the long term, especially in the oldest patients.
La hidrocefalia crónica del adulto idiopática (HCAI) es considerada una causa de demencia tratable mediante la implantación de una válvula de derivación ventrículo-peritoneal (VDVP). Nos planteamos estudiar la evolución clínica y funcional de la HCAI tratada con VDVP, así como los factores asociados con una mejor evolución a largo plazo.
Sujetos y métodosEstudio observacional de pacientes con diagnóstico de HCAI probable (según criterios de la Sociedad Japonesa de Neurocirugía) y tratados con VDVP entre 2008 y 2013 en un hospital de tercer nivel español. Se establecieron 4 grupos de respuesta clínica (normalización, mejoría parcial, mejoría dudosa y empeoramiento) y la situación funcional se evaluó mediante la escala de Rankin modificada (ERm).
ResultadosSe incluyó a 29 pacientes con una edad media de 73,9 años. El 62,1% eran hombres y el 65,5% presentaban HTA. Se observó una respuesta clínica al menos parcial en el 58 y el 48% al año y al final del seguimiento (seguimiento medio de 37,8 meses), respectivamente. La edad, la frecuencia de HTA y las complicaciones quirúrgicas fueron superiores en el grupo con mala respuesta. Un paciente falleció, el 20,7% presentó complicaciones graves y el 69% era dependiente (ERm ≥ 3) al final del seguimiento. La edad se asoció de manera independiente a peor respuesta clínica al año y una mayor dependencia al final del seguimiento.
ConclusiónEl beneficio de la VDVP fue parcial y transitorio, con una alta frecuencia de complicaciones y dependencia funcional en el seguimiento a largo plazo, especialmente en los pacientes de mayor edad.
Adult chronic idiopathic hydrocephalus (ACIH) is a clinical syndrome characterised by subacute gait disturbance, urinary incontinence, and cognitive impairment. Neuroimaging studies typically display communicating hydrocephalus associated with normal cerebrospinal fluid (CSF). Symptoms usually improve after implantation of a ventriculoperitoneal shunt (VPS).1,2 Initial symptoms, diagnosis, and treatment of ACIH, also known as idiopathic normal pressure hydrocephalus, remain controversial. The most recent diagnostic criteria are those issued by the Japanese Society of Normal Pressure Hydrocephalus in 2012; these guidelines include using the clinical response to Miller Fisher Test (lumbar tap test) or lumbar CSF drainage to diagnose probable ACIH.3,4
The exact pathogenic mechanism of the disease is unknown; the most widely accepted explanation is alteration of CSF reabsorption mechanisms due to multiple factors.2,5 The main clinical guidelines recommend VPS implantation, although no controlled studies of this treatment have been conducted to date.6 Response rates are lower when the aetiology is unknown (10%-53% vs. 60%-75% in secondary normal pressure hydrocephalus).4 These rates may be unsatisfactory considering the potential complications of surgery.7
Few studies have followed up patients for more than a year and the long-term clinical and functional response to surgery has been questioned.8 Likewise, there is no consensus on which factors predict good outcomes; assessing the risks and benefits of surgery is therefore problematic and may even pose a therapeutic dilemma.9
Our purpose was to study clinical and functional response to VPS implantation in patients with ACIH at a Spanish tertiary hospital and to analyse the factors associated with good long-term clinical and functional outcomes.
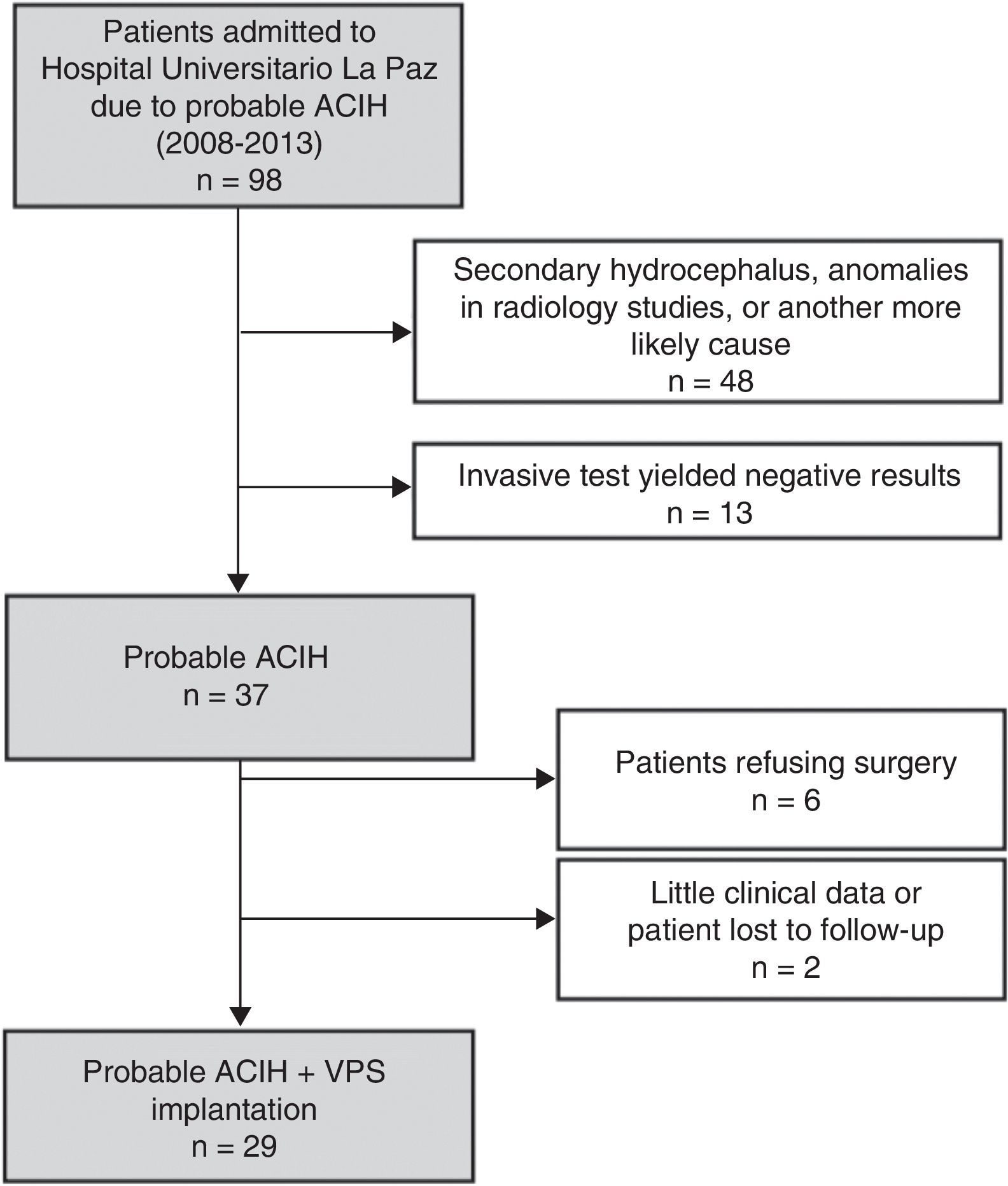
Patients and methodsWe conducted a prospective longitudinal observational study including patients with a diagnosis of probable ACIH who underwent VPS implantation between January 2008 and December 2013 and were hospitalised in a tertiary hospital providing neurosurgical care to a population of 1071666 inhabitants.
The inclusion criteria were as follows: (1) symptom onset at the age of 60 or older; (2) presence of at least 2 symptoms from the classic ACIH triad; (3) Evans index>0.3; (4) normal CSF and CSF pressure<20mm H2O; and (5) clinical improvements after lumbar puncture (30-50cc) or lumbar CSF drainage. The exclusion criteria were as follows: (1) history of subarachnoid haemorrhage, head trauma associated with loss of consciousness, congenital hydrocephalus, or aqueductal stenosis; and (2) a diagnosis of any other neurodegenerative disease able to explain the patient's symptoms.
Patients were assessed clinically during hospitalisation. We recorded the following variables: sex, age at diagnosis, vascular risk factors, drug use, normal treatment, symptom progression time, symptoms of the triad present at diagnosis, type of invasive diagnostic tests and results, and time to surgery. All participants underwent a brain MRI scan; presence of leukoaraiosis was assessed on the Fazekas scale.10 Upon patient admission, an experienced neurologist (JA) assessed gait characteristics before and after diagnostic testing. Where gait disorders were present, we analysed whether patients displayed apraxia (slow gait, short steps, wide-based stance, and marked difficulty lifting the feet off the ground). We ruled out presence of parkinsonian signs able to explain gait disturbance (vascular, drug-induced, or neurodegenerative parkinsonism, such as Parkinson's disease). Patients underwent a lumbar puncture and/or a lumbar CSF drain. We then assessed gait improvements over the next 3 days; a 30% decrease in the time and number of steps taken to cover a distance of 20metres was considered a significant improvement. During hospitalisation, those patients with memory complaints who displayed no alterations on short cognitive tests (MMSE and/or MoCA) underwent a thorough cognitive assessment (Stroop test, semantic and phonological word fluency test, parts A and B of the TMT, CERAD word list, copy and recall of the Rey-Osterrieth Complex Figure Test, and clock drawing test). Likewise, we evaluated clinical changes for each of the symptoms of the triad after surgery, between the third and the sixth months, at one year, at 3 years, and/or at the end of the follow-up period (October 2014). We classified patients in 4 groups according to clinical response for each symptom of the clinical triad based on the scale developed by Klassen et al.8: (1) worsening, when clear exacerbation was present (including death as a consequence of clinical deterioration); (2) little to no improvement, when no clear improvements were seen; (3) partial improvement, when improvement was incomplete, and (4) resolution, when patients recovered completely. To classify patients, we used data obtained from follow-up visits and a structured telephone interview with patients or their carers. Interviews were conducted by neurologists who were blind to clinical data (JMM and JPL). Partial improvement or resolution of any one of the symptoms of the triad was regarded as overall clinical improvement. A neurologist blind to clinical data (JPL) assessed functional status at the end of the follow-up period using the modified Rankin Scale (mRS).11 Patients scoring <3 on the mRS were regarded as functionally independent; in case of any discrepancies between the neurological assessment and patients’ or their carers’ impressions, we prioritised the latter.
We conducted a descriptive analysis for all variables; quantitative variables were expressed as means±SD and qualitative variables as frequencies and percentages. Inter-group differences were assessed with the t-test or the Mann–Whitney U test. Qualitative variables were analysed using the chi-square test or the Fisher exact test. We used the Kolmogorov–Smirnov test to determine goodness of fit with the normal distribution. When groups were not homogeneous for any of the baseline variables, we conducted a multivariate analysis to evaluate the association between that variable and symptom response one year after surgery (at least partial improvement of any of the triad symptoms) and/or functional independence (mRS<3) at the end of the follow-up period. For the multivariate analysis, we used a logistic regression model including variables with a P-value<.02. We compared mRS scores at time of diagnosis and at the end of the follow-up period with the Wilcoxon test for paired data. Statistical significance was set at P<.05. Data were analysed using SPSS statistical software, version 20.0 for Windows.
Our study was approved by the ethics committee at Hospital Universitario La Paz. All participants signed informed consent forms.
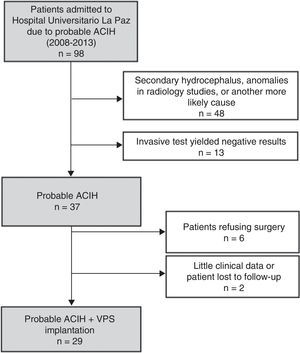
ResultsWe identified a total of 37 new cases of probable ACIH between 2008 and 2013. Fig. 1 shows the composition of the sample. Six patients declined surgery and 2 were excluded from our study due to a lack of data during follow-up (they moved to other regions). We included 29 patients with probable ACIH who underwent VPS implantation during the study period (5.4 cases per million inhabitants per year). Twenty-five of the VPS (86.2%) were programmable (21 Medtronic Strata II® and 4 Polaris Sophysa®) and 4 (13.8%) were medium-pressure (Medtronic; 110mmH2O). Men accounted for 64.5% of the sample; mean age was 3.9±7.9 years. We found a high rate of vascular risk factors: 65.5% had arterial hypertension (AHT), 37.9% diabetes mellitus, and 52.2% hypercholesterolemia. At time of diagnosis, 96.6% of the patients had gait disorders, 81.5% had cognitive impairment, and 63% had urinary incontinence. Table 1 summarises baseline characteristics in our sample. Lumbar puncture (30-50cc of CSF) was the most frequently used invasive diagnostic technique (21 patients, 72.4%). Six patients (20.7%) underwent an additional diagnostic assessment after the first evaluation yielded inconclusive results.
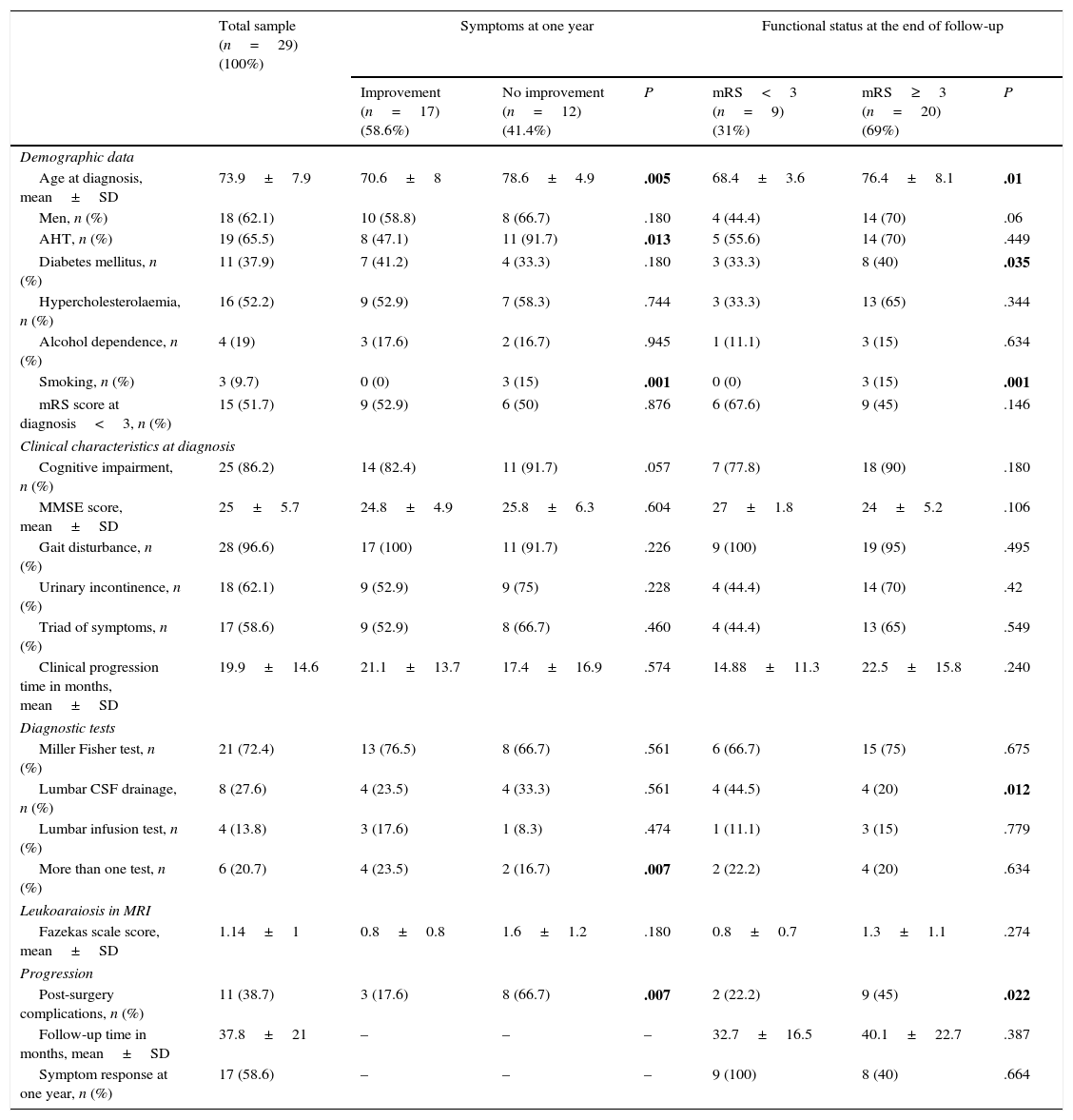
Baseline characteristics of the sample broken down by response to surgery at one year and functional status at the end of follow-up.
| Total sample (n=29) (100%) | Symptoms at one year | Functional status at the end of follow-up | |||||
|---|---|---|---|---|---|---|---|
| Improvement (n=17) (58.6%) | No improvement (n=12) (41.4%) | P | mRS<3 (n=9) (31%) | mRS≥3 (n=20) (69%) | P | ||
| Demographic data | |||||||
| Age at diagnosis, mean±SD | 73.9±7.9 | 70.6±8 | 78.6±4.9 | .005 | 68.4±3.6 | 76.4±8.1 | .01 |
| Men, n (%) | 18 (62.1) | 10 (58.8) | 8 (66.7) | .180 | 4 (44.4) | 14 (70) | .06 |
| AHT, n (%) | 19 (65.5) | 8 (47.1) | 11 (91.7) | .013 | 5 (55.6) | 14 (70) | .449 |
| Diabetes mellitus, n (%) | 11 (37.9) | 7 (41.2) | 4 (33.3) | .180 | 3 (33.3) | 8 (40) | .035 |
| Hypercholesterolaemia, n (%) | 16 (52.2) | 9 (52.9) | 7 (58.3) | .744 | 3 (33.3) | 13 (65) | .344 |
| Alcohol dependence, n (%) | 4 (19) | 3 (17.6) | 2 (16.7) | .945 | 1 (11.1) | 3 (15) | .634 |
| Smoking, n (%) | 3 (9.7) | 0 (0) | 3 (15) | .001 | 0 (0) | 3 (15) | .001 |
| mRS score at diagnosis<3, n (%) | 15 (51.7) | 9 (52.9) | 6 (50) | .876 | 6 (67.6) | 9 (45) | .146 |
| Clinical characteristics at diagnosis | |||||||
| Cognitive impairment, n (%) | 25 (86.2) | 14 (82.4) | 11 (91.7) | .057 | 7 (77.8) | 18 (90) | .180 |
| MMSE score, mean±SD | 25±5.7 | 24.8±4.9 | 25.8±6.3 | .604 | 27±1.8 | 24±5.2 | .106 |
| Gait disturbance, n (%) | 28 (96.6) | 17 (100) | 11 (91.7) | .226 | 9 (100) | 19 (95) | .495 |
| Urinary incontinence, n (%) | 18 (62.1) | 9 (52.9) | 9 (75) | .228 | 4 (44.4) | 14 (70) | .42 |
| Triad of symptoms, n (%) | 17 (58.6) | 9 (52.9) | 8 (66.7) | .460 | 4 (44.4) | 13 (65) | .549 |
| Clinical progression time in months, mean±SD | 19.9±14.6 | 21.1±13.7 | 17.4±16.9 | .574 | 14.88±11.3 | 22.5±15.8 | .240 |
| Diagnostic tests | |||||||
| Miller Fisher test, n (%) | 21 (72.4) | 13 (76.5) | 8 (66.7) | .561 | 6 (66.7) | 15 (75) | .675 |
| Lumbar CSF drainage, n (%) | 8 (27.6) | 4 (23.5) | 4 (33.3) | .561 | 4 (44.5) | 4 (20) | .012 |
| Lumbar infusion test, n (%) | 4 (13.8) | 3 (17.6) | 1 (8.3) | .474 | 1 (11.1) | 3 (15) | .779 |
| More than one test, n (%) | 6 (20.7) | 4 (23.5) | 2 (16.7) | .007 | 2 (22.2) | 4 (20) | .634 |
| Leukoaraiosis in MRI | |||||||
| Fazekas scale score, mean±SD | 1.14±1 | 0.8±0.8 | 1.6±1.2 | .180 | 0.8±0.7 | 1.3±1.1 | .274 |
| Progression | |||||||
| Post-surgery complications, n (%) | 11 (38.7) | 3 (17.6) | 8 (66.7) | .007 | 2 (22.2) | 9 (45) | .022 |
| Follow-up time in months, mean±SD | 37.8±21 | – | – | – | 32.7±16.5 | 40.1±22.7 | .387 |
| Symptom response at one year, n (%) | 17 (58.6) | – | – | – | 9 (100) | 8 (40) | .664 |
mRS, modified Rankin Scale; AHT, arterial hypertension; MMSE, Mini-Mental State Examination.
Statistically significant values are shown in bold (P<.05).
Eleven patients (37.8%) experienced post-surgical complications; these were severe in 6 cases (20.7%). Complications included surgery-related lobar haemorrhage (1 patient), sepsis during the post-operative period with secondary status epilepticus requiring admission to the intensive care unit (1), ventriculitis at one year from surgery requiring shunt replacement (1), and subdural haematoma (3). Five patients had shunt dysfunction (shunt was replaced in one case) and another patient displayed granuloma of the surgical wound. Three patients (10.3%) died during follow-up, one of them as a consequence of surgery (brain haemorrhage). The remaining 2 patients died due to infections (aspiration pneumonia at 2 and 3 years of follow-up).
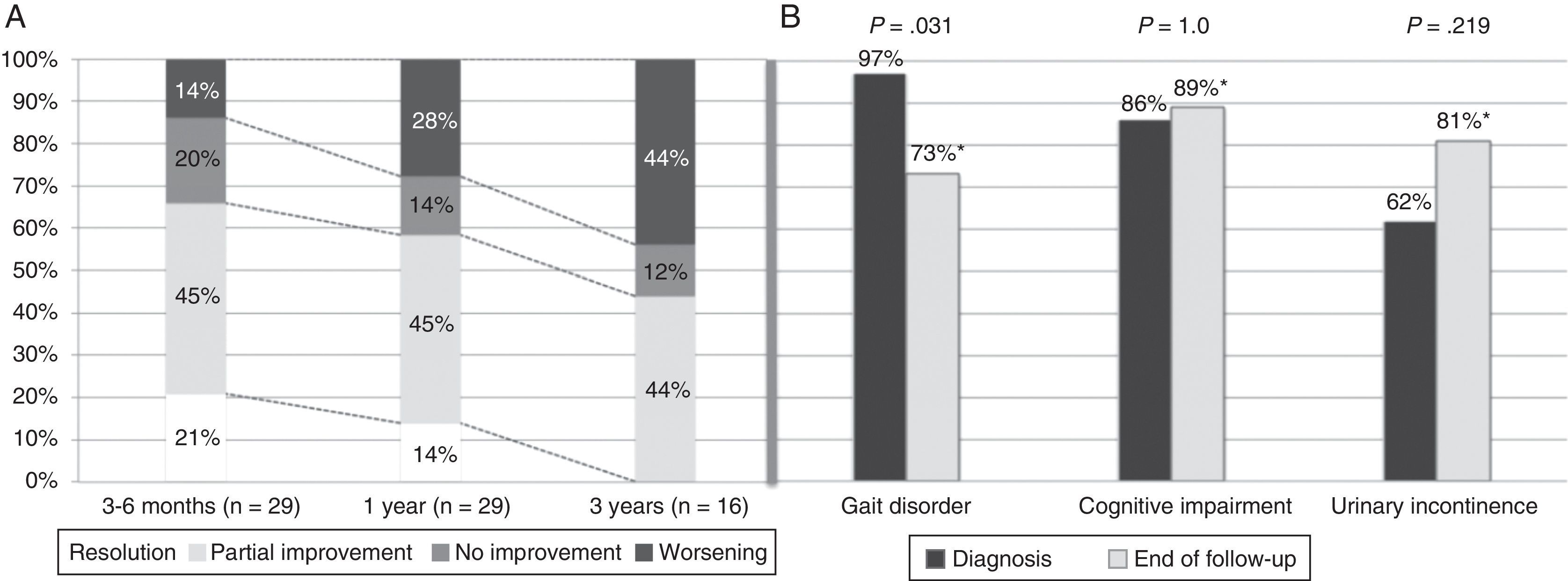
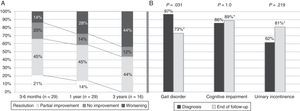
Symptoms improved at least partially in 17 patients (58.6%) at one year of follow-up and in 14 patients (48.3%) by the end of the follow-up period. Gait had improved by the end of follow-up (from 97% of patients with gait disorders at the time of diagnosis to 73% at the end of follow-up; P=.031). However, no differences were seen at the end of follow-up for the other triad symptoms after a mean follow-up period of 37.8 months (Fig. 2). At one year after diagnosis, 59% of the patients displayed some degree of improvement; symptoms resolved in 14% (Table 1). Sixteen patients (55%) were assessed at the 3-year mark; 44% displayed at least partial improvements. None of them displayed full resolution of symptoms at 3 years. Mean age, frequency of AHT, and tobacco use were higher in the group with a poor response to treatment at one year of follow-up (17 patients; 57.6%). Post-surgical complications, on the other hand, were more frequent in the group showing no response (12 patients; 41.4%). In the multivariate analysis, age at diagnosis and presence of post-surgical complications were independently associated with lack of response to VPS at one year (P=.025; OR 1.67; 95% CI, 1.066-2.623 vs. P=.030; OR 88.92; 95% CI, 1.853-180.626, respectively).
Symptom progression over follow-up period. (A) Overall symptom progression at 3 to 6 months, one year, and 3 years. (B) Percentage of patients showing each of the triad symptoms at diagnosis and at the end of the follow-up period (mean follow-up time: 37.8 months). *Percentages corresponding to the end of the follow-up period were calculated for a total of 26 patients (gait disorder 19/26, 73%; cognitive impairment 23/26, 89%; urinary incontinence 21/26, 81%).
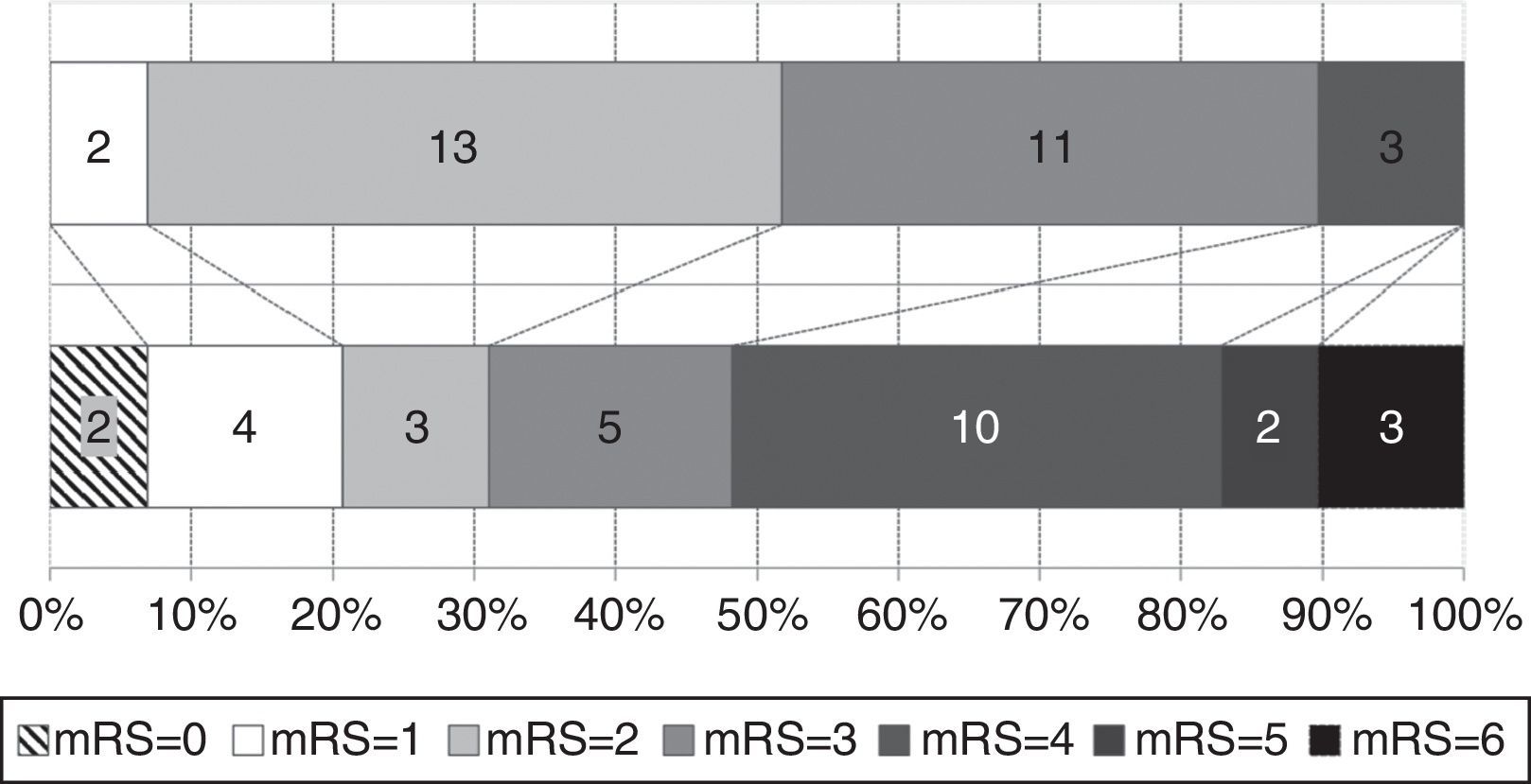
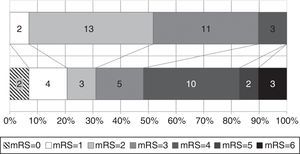
Functional status at time of diagnosis and at the end of follow-up is shown in Fig. 3. The percentage of patients scoring more than 3 on the mRS rose from 10.3% to 51.7%; functional status worsened significantly throughout the follow-up period (baseline mRS scores<final mRS scores; P=.045). Table 1 analyses patient characteristics by functional independence (mRS<3) at the end of follow-up. Eight patients (40%) who were dependent at the end of the follow-up period had achieved significant symptom improvement one year after VPS implantation; follow-up times were similar for independent and dependent patients (32.7 and 40.1 months, respectively; P=.387). Age at diagnosis and the frequency of diabetes mellitus, tobacco use, and post-surgical complications were higher in the group of dependent patients. In the multivariate analysis, however, age was the only factor independently associated with greater probability of functional dependence (P=.025; OR 1.163; 95% CI, 1.019-1.328).
At the end of follow-up, 5 patients had a diagnosis of dementia: 4 of probable mixed aetiology (probable Alzheimer disease with vascular involvement) and another case of vascular dementia.
DiscussionOur study provides data on long-term clinical and functional progression of patients with probable ACIH undergoing VPS implantation at a Spanish tertiary hospital. We observed functional worsening during a mean follow-up time of 3 years and a high rate of severe post-surgical complications. Age was independently associated with both poorer clinical outcomes at one year and greater probability of functional dependence during follow-up.
According to our data, the incidence of probable ACIH in the area covered by our tertiary hospital is 6.9 cases per million inhabitants per year. This rate is higher than that described in the Netherlands (2.2 cases per million inhabitants per year) and lower than in Norway (8.4-14.7 cases per million inhabitants per year) and Sweden (9.1 cases per million inhabitants per year).7,12,13 A recent population study conducted in a small rural area in Japan estimated the prevalence of probable ACIH at 1.4% to 2.9% of the population older than 61; diagnosis was based on neuroimaging criteria exclusively.14,15 According to a study performed at a multidisciplinary memory clinic, 3.3% of all patients visiting due to cognitive impairment had probable ACIH. Likewise, in a door-to-door survey conducted in Germany, prevalence of probable ACIH in patients older than 65 was estimated at 0.41%.16,17 These data suggest that ACIH may be underdiagnosed in our setting.18
The rate of severe complications in our study is similar to that reported in previous studies.19 In our series, 3.4% of the patients died as a consequence of surgery; post-surgery mortality rates range between 0% and 11% in the literature. Other severe complications such as subdural haematoma have been reported to range from 2% to 47% (10.3% in our series).
In our sample, the rate of clinical improvement at one year (partial improvements were seen in nearly 60% of the patients) is similar to those published in the literature (2%-100%).19 These differences may be explained by the lack of agreement between the scales used to assess clinical response in each study, the differences in follow-up times, and patient selection criteria, which makes comparison between studies difficult. Some of these studies did not adjust for drop-outs when calculating the percentage of patients showing improvements, which resulted in overly high rates.19,20
Several explanations for the lack of sustained clinical and functional response have been suggested: (a) co-presence of another neurodegenerative disease (for example, Alzheimer disease)21–23; (b) impact of surgery-related complications on functional status7; and (c) deterioration attributable to other comorbidities frequently seen in these patients.24
Overall, only the percentage of patients with gait disturbances showed a statistically significant improvement by the end of follow-up. In addition, 60% of the patients showed cognitive deterioration. These findings agree with those reported by Koivisto et al.23 in a recent study addressing initial response to VPS and long-term cognitive outcomes in patients with ACIH: according to their data, 46% and 27% of the patients had dementia and cognitive impairment, respectively, at the end of the follow-up period. In the study mentioned above, older age, male sex, longer follow-up times, and presence of memory impairment at the time of diagnosis were independently correlated with an increased risk of developing dementia.
AHT, on the other hand, was linked to poorer clinical response at one year; previous studies have observed greater rates of AHT in patients with ACIH. This has led some researchers to hypothesise that AHT may be linked to increased CSF pulsatility and could therefore play a role in ACIH pathophysiology.25,26 In fact, a recent study has shown that VPS implantation does not change CSF pulsatility, which may explain the lack of response to surgery in a subgroup of patients with AHT.4,27 According to another study, patients undergoing programmable VPS implantation (progressively decreasing CSF pressure from 20 to 4cmH2O over 6 months) achieved better outcomes than those treated with a VPS at a fixed pressure (12cmH2O).28 In our sample, however, only older age and presence of post-surgical complications were independently associated with a lower probability of response at one year after surgery.
Our study provides data on long-term functional outcomes in patients with probable ACIH. Although there is no consensus on which scales should be used to follow up on these patients, most published studies apply semi-quantitative scales that evaluate each of the triad symptoms.29 These scales can detect slight improvements with no clinical impact, which may lead to overestimations of the benefits of surgery. Functional assessment, on the other hand, provides data on the impact of symptom improvement on independence rather than detecting minor changes in gait or cognition. Although the mRS is recommended in the published clinical guidelines, only one study has used it to evaluate functional status.4,11 Some studies provide data from self-administered quality-of-life questionnaires completed after surgery; however, these studies have a marked response bias, which makes it difficult to interpret their results.19,20,29,30
In our study, mRS scores at the end of follow-up were significantly poorer than baseline scores; functional dependence rates were high after a mean of 37.8 months of follow-up. This may be explained by the presence of other diseases at the time of diagnosis or during follow-up.8,22,23 Functional dependence at the end of follow-up was not correlated with either length of follow-up or functional status at the time of surgery; age at diagnosis was the only factor independently associated with increased probability of functional dependence. Age is a risk factor for developing other neurodegenerative diseases, although numerous other comorbidities may affect prognosis in patients with ACIH.24
Our study has several limitations. Firstly, our observational study included a relatively small sample. However, we analysed clinical and functional response in a cohort of patients with ACIH and obtained statistically significant results which may be of interest for the management of these patients. Heterogeneity in the evaluation of cognitive function and other symptoms comprising the triad limited the precision of our data. Therefore, the degree of improvement or exacerbation could not be definitely established. On the other hand, while mRS scores provide objective information on patient functional status, it is impossible to determine the level of foreseeable deterioration in a cohort of elderly patients when there is no control group. We need further prospective controlled studies with a standardised neurological follow-up procedure to comprehensively evaluate the real impact of surgery and the factors associated with favourable outcomes.
Nearly 60 years have passed since ACIH was found to be a reversible cause of dementia; however, eligibility criteria for surgery in patients with ACIH have yet to be precisely established. Research on the diagnosis, treatment, and clinical progression of ACIH must aim to improve the diagnostic process (determining the role of certain comorbidities and developing biomarkers and neuroimaging techniques) and establish a consensus on how to evaluate clinical response after surgery.31,32
Symptom improvement after VPS implantation in patients with ACIH is usually partial and transient, with frequent complications and long-term functional dependence, especially in older patients.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Illán-Gala I, Pérez-Lucas J, Martín-Montes A, Máñez-Miró J, Arpa J, Ruiz-Ares G. Evolución a largo plazo de la hidrocefalia crónica del adulto idiopática tratada con válvula de derivación ventrículo-peritoneal. Neurología. 2017;32:205–212.
The results of this study were presented in poster format at the 65th Annual Meeting of the Spanish Society of Neurology (2013). Additionally, the poster was presented at the session for outstanding communications.