To assess a group of patients with parkinsonism using serial studies with FP-CIT, basically the initial false negative results.
MethodsRestrospective study of 92 patients (55 men and 37 women) who had undergone 2 different FP-CIT studies because of discrepancies between study results and clinical progression. The mean elapsed time between the studies was 26 months (SD: 6). We performed a semi-quantitative study using the patient's clinical history and the available literature to analyse discrepant cases with a normal initial study and subsequent pathological findings.
ResultsA total of 184 studies were completed for 92 patients; 11 of those 92 showed discrepancies between initial and subsequent studies. Among the 11 discrepant cases, 7 showed a normal initial study and pathological findings at a later date. Analysis of the predominant clinical features that might explain this behaviour revealed that 4 of these 7 subjects presented tremor-dominant parkinsonism. Regarding the rest, 1 presented early stage parkinsonism and was treated with antidopaminergic agents; 1 was classified as probable multisystem atrophy type C, and the third showed clinical signs of atypical parkinsonism without any causes of those signs being identified.
ConclusionsSerial FP-CIT studies are unnecessary in the large majority of cases, but they may be justifiable in certain clinical situations.
Analizar un grupo de pacientes con síndrome parkinsoniano mediante estudios seriados con FP-CIT, valorando fundamentalmente los resultados falsamente negativos iniciales.
MétodosEstudio retrospectivo en el que se incluye a 92 pacientes (55 varones y 37 mujeres), a los que se les realizó un doble estudio con FP-CIT pues existían discrepancias entre este y la evolución clínica del paciente. El tiempo medio ± desviación estándar transcurrido entre ambos estudios fue de 26 ± 6 meses. Se realizó una valoración semicuantitativa analizando mediante la historia clínica y la bibliografía disponible los casos discrepantes con estudio inicial normal y posterior patológico.
ResultadosDel total de 184 estudios realizados a 92 pacientes, 11/92 mostraron resultados discrepantes entre estudio inicial y tardío. De estos, en 7/11 el estudio inicial fue normal y el posterior patológico. Los rasgos clínicos predominantes que pudieran explicar este comportamiento mostraron que en 4/7 sujetos destacó la presentación de un cuadro parkinsoniano con predominio de la clínica tremórica; 1/7 presentó un síndrome parkinsoniano en estadio inicial en tratamiento con fármaco antidopaminérgico, 1/7 fue catalogado de probable atrofia multisistema tipo C y 1/7 presentaba un cuadro de parkinsonismo atípico, sin que encontráramos justificación para dicho comportamiento.
ConclusionesLa realización de estudios seriados con FP-CIT carece de fundamento en gran proporción de casos, aunque existen ciertas situaciones clínicas que pueden justificarlo.
Dopamine transporters (DAT) are proteins in the presynaptic terminal of dopaminergic neurons which are responsible for dopamine re-uptake. DAT density measurement, by means of SPECT or PET tomographic techniques using specific ligands, provides a direct in vivo measurement of the integrity of the striatonigral pathway. Several tracers derived from tropane and cocaine analogues, such as ioflupane or 123I-FP-CIT (N-ω-fluoropropyl-2ß-carbomethoxy-3ß-[4-iodophenyl] nortropane), have been used for these measurements. This diagnostic technique allows doctors to detect Parkinson's disease (PD) even during its premotor phase, such as in cases of olfactory deficit1 or REM sleep behaviour disorder.2 It has been determined that in cases of loss of dopaminergic neurons, DOPA decarboxylase is up-regulated3 while DAT receptors are down-regulated.4 In theory, this situation contributes to a high level of sensitivity. Results from SWEDD patients (Scan Without Evidence of Dopaminergic Deficit), a group accounting for 10% of the patient total according to data from different clinical studies on neuroprotective drugs, will decrease the diagnostic validity of the technique. Even after taking the presence of these patients into account, the test's negative predictive value does not reach optimal values.5
Annual loss of dopaminergic neurons from the nigrostriatal pathway in patients with degenerative parkinsonism (PK) has been estimated at 6% to 13%6 compared to the 0% to 2.5% change per decade measured in age-matched healthy controls. The rate of progression is not linear and loss is more rapid during initial stages than in advanced stages. For the above reasons, it seems logical to perform a new study after a reasonable period of time in order to assess the progression of a case of degenerative PK.
This study aims to analyse a group of patients with parkinsonian syndrome (PS) who underwent serial studies with FP-CIT scans. We mainly focused on those patients with an initial evaluation classified as normal (N) and a subsequent scan classified as pathological (P) (N–P sequence). By analysing each patient's clinical history and available literature, we aim to determine the most probable causes of such a pattern as a source of potential false negative results. In the same way and as a secondary aim, we also evaluated the inverse sequence (P–N).
Materials and methodsPatientsWe retrospectively studied 92 patients (55 men and 37 women) from our hospital's movement disorders unit. We performed two serial FP-CIT scans on each patient, one at baseline and the other at a later point to investigate discrepancies between initial scan results and the patient's clinical symptoms. We also included 20 patients with a clinical diagnosis of essential tremor as control subjects after a preliminary analysis.
The final clinical diagnosis was established after a minimum follow-up of 18 months for patients in our main study group (those with contradictory scan results). All patients were in a similar stage of the disease since doctors had requested a FP-CIT scan after the initial clinical evaluation in all cases. Table 1 shows the breakdown of patients according to the first provisional diagnosis.
Mean time±standard deviation elapsed between the two studies was 26±5 months. The mean age of the patients when the initial study was performed was 69.3 years (range, 36–84) with a standard deviation of 9.2 years.
MethodImages were captured 3 to 4hours after intravenous injection of 185 MBq (5mCi) of 123I-FP-CIT. Lugol solution had previously been administered to achieve thyroid blockade. Tomographies (SPECT) with ioflupane/FP-CIT were performed with a Siemens Symbia gamma camera with a dual head and a low-energy high-resolution collimator. A circular 360° orbit around the head was used to capture images with 3° azimuth stops, capturing 60 views with a duration of 35seconds per stop. Matrix size was 128×128. Tomographic reconstruction was performed using filtered back projection algorithms with no attenuation correction and applying a Hanning filter (frequency 0.7). The scanner obtained images of transaxial slices oriented on the orbitomeatal line.
Scans were studied using semi-quantitative analysis and an uptake ratio was calculated between the area with specific activity (striatal DAT binding) and the area with non-specific activity (occipital cortex) to obtain the striatal-to-occipital ratio (S/O). To this end, we drew regions of interest (ROI) on both striata (mean counts, rectangular ROI of 250 pixels) and for average uptake in the occipital lobe (rectangular ROI of 350 pixels). The 6 slices with the highest striatal activity were digitally summed (slice thickness: 3.39mm; final slice: 20.34mm). The arithmetic mean of the ratios of both hemispheres was calculated to provide an overall evaluation of the nigrostriatal pathway.
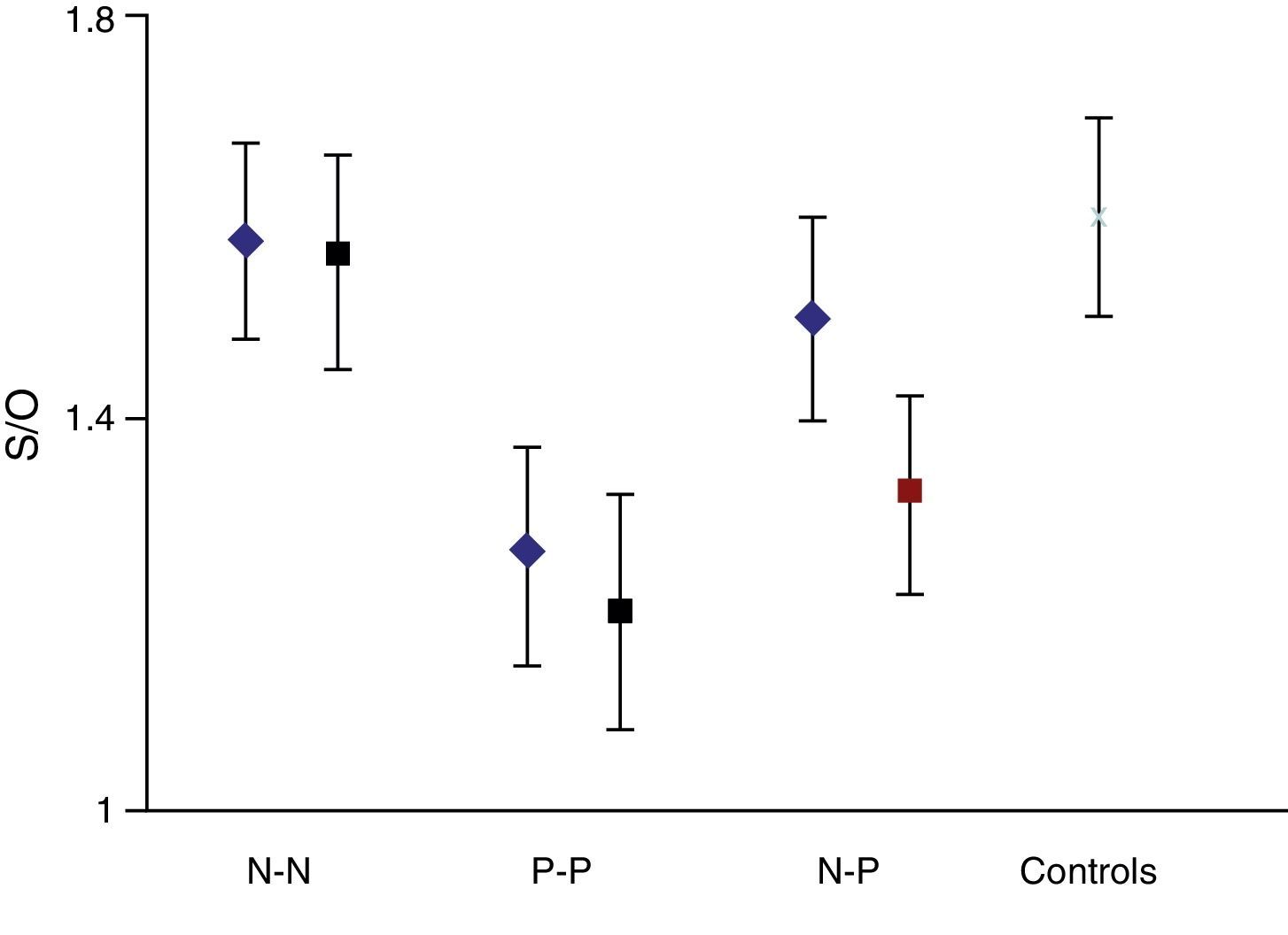
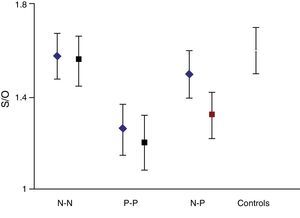
On the basis that the distribution curve of that ratio follows a normal pattern, the study was considered normal when the index fell in the range of the mean ±2 SD of the control group. It was considered pathological when the ratio fell outside the mean −2 SD. The control group had a mean S/O ratio of 1.60 with an SD of 0.10. Therefore, every S/O ratio below 1.40 was considered a pathological finding. We established 4 study groups according to FP-CIT scan results from the initial and final studies: N–N, P–P, N–P and P–N groups/sequences. These latter 2 discordant groups or sequences constitute the main focus of our study.
The discrepancy between clinical and scan results was identified when, based on the patient's clinical presentation and subsequent outcome, the neurologist in charge found signs suggesting degenerative PK after an initial FP-CIT scan study showed results in the normal range, or when a patient whose initial study was considered pathological showed no subsequent degenerative signs. In this task, the physician considered scores on outcome scales, atypical signs, response to l-DOPA or dopaminergic agonists, potential pharmacological interference, etc. In cases in which a possible interference was detected, we repeated the study once the patient had stopped taking the drug for a period equivalent to 4 times its biological half-life.7
Statistical analysisData were analysed using SPSS statistical software (version 13.0). Measures of central tendency and dispersion were used to describe quantitative variables. A parametric test (t-test) was used to compare means. In general, the level of statistical significance was P<.05 with a confidence interval of 95%. Normal distribution of the curve was established using the Kolmogorov–Smirnov test.
ResultsA total of 184 studies were performed on 92 patients; 81 of 92 individuals (88%) showed concordant results between the initial and follow-up studies; 51 of 81 (62.9%) had normal results on both the initial and the follow-up studies (N–N). In 30 of 81 patients, results from both studies were considered pathological (P–P). Lastly, results were discordant in 11 of the 92 cases (11.9%). In this group, initial results were normal and follow-up results were pathological (N–P) in 7 of 11 cases; in the remaining 4 cases, the sequence was reversed (P–N).
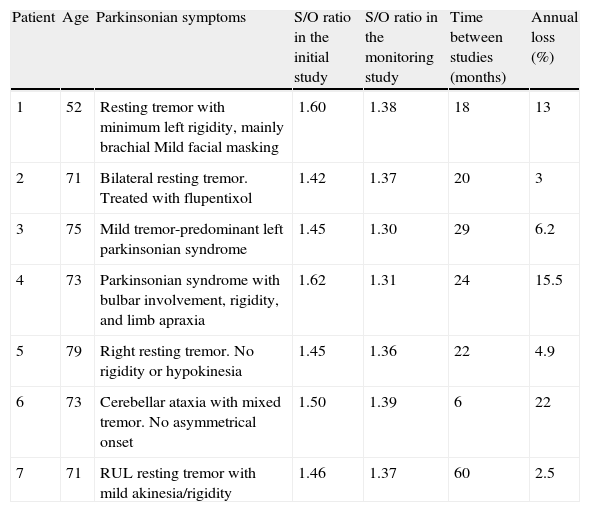
The semi-quantitative assessment provided the following data: mean S/O ratio corresponding to patients with concordant results from both studies, N–N sequence, was 1.58±0.10 (initial); 1.56±0.11 (final); P>.05. P–P sequence: 1.26±0.11 (initial); 1.20±0.12 (final); P>.05. P–N sequence: 1.32±0.10; 1.48±0.12; P>.05. N–P sequence: 1.50±0.12; 1.35±0.10; P>.05. Estimated mean annual loss rate in this last group was 8.15%±7.5%. The mean S/O ratio for the control group is 1.60±0.11. Fig. 1 shows all these data. Table 2 displays the predominant clinical sign in the N–P group as well as relevant data on the semi-quantitative assessment.
Semi-quantitative assessment of patients with a normal/pathological sequence on serial FP-CIT studies and their predominant symptoms.
| Patient | Age | Parkinsonian symptoms | S/O ratio in the initial study | S/O ratio in the monitoring study | Time between studies (months) | Annual loss (%) |
| 1 | 52 | Resting tremor with minimum left rigidity, mainly brachial Mild facial masking | 1.60 | 1.38 | 18 | 13 |
| 2 | 71 | Bilateral resting tremor. Treated with flupentixol | 1.42 | 1.37 | 20 | 3 |
| 3 | 75 | Mild tremor-predominant left parkinsonian syndrome | 1.45 | 1.30 | 29 | 6.2 |
| 4 | 73 | Parkinsonian syndrome with bulbar involvement, rigidity, and limb apraxia | 1.62 | 1.31 | 24 | 15.5 |
| 5 | 79 | Right resting tremor. No rigidity or hypokinesia | 1.45 | 1.36 | 22 | 4.9 |
| 6 | 73 | Cerebellar ataxia with mixed tremor. No asymmetrical onset | 1.50 | 1.39 | 6 | 22 |
| 7 | 71 | RUL resting tremor with mild akinesia/rigidity | 1.46 | 1.37 | 60 | 2.5 |
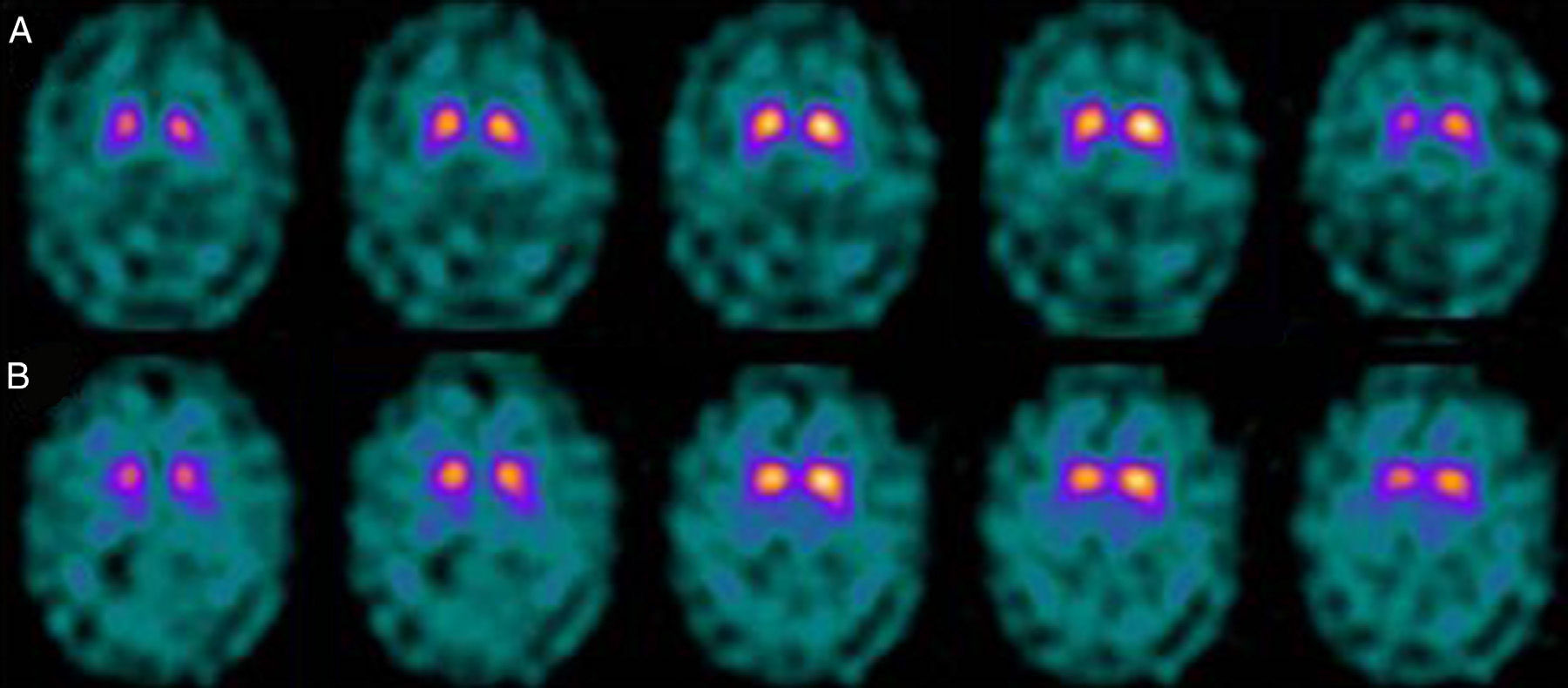
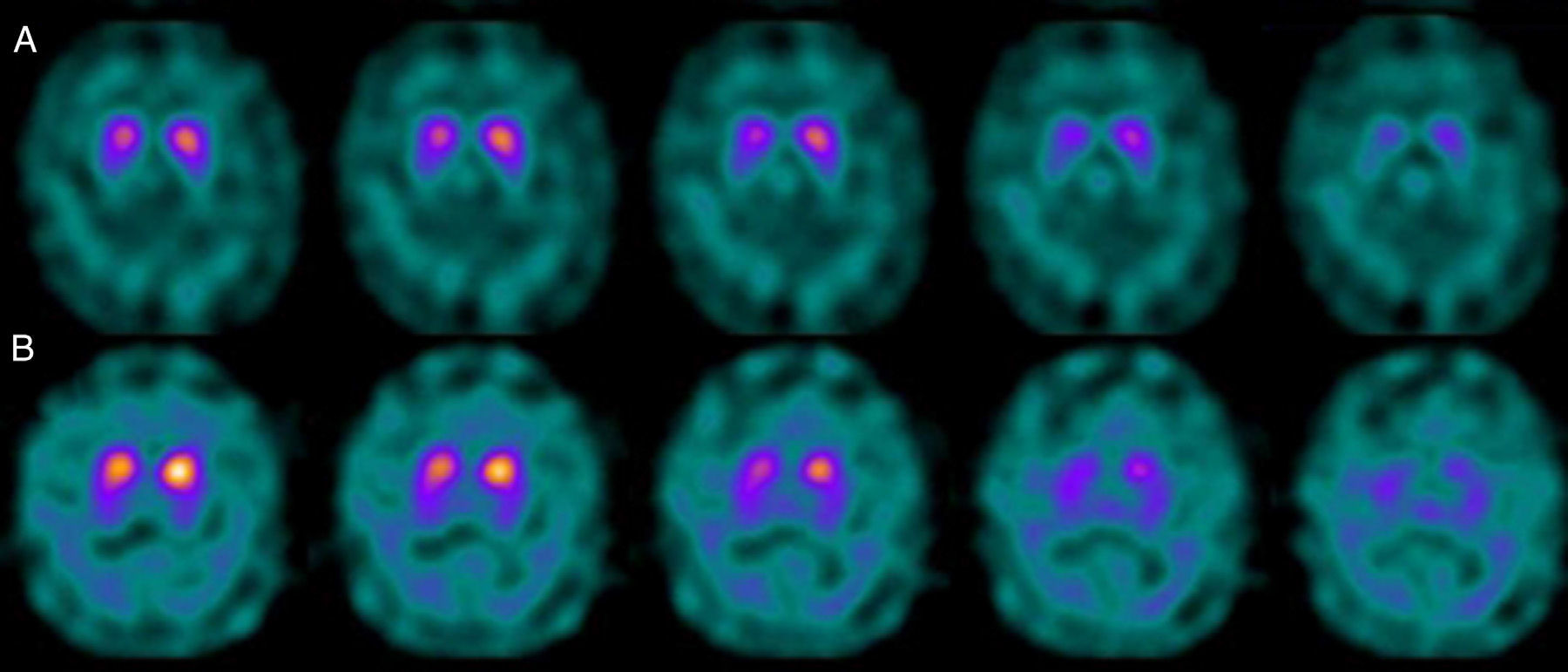
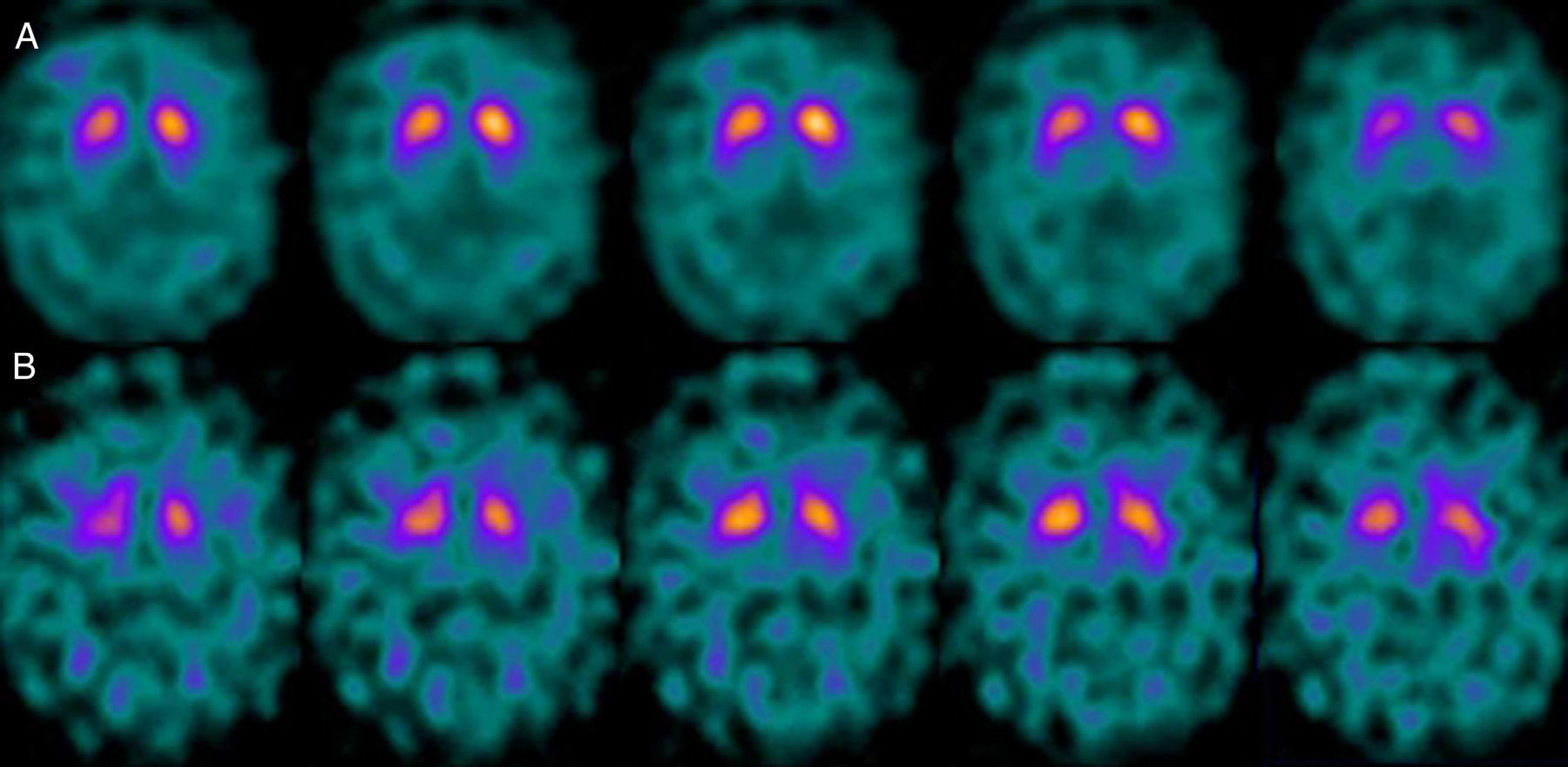
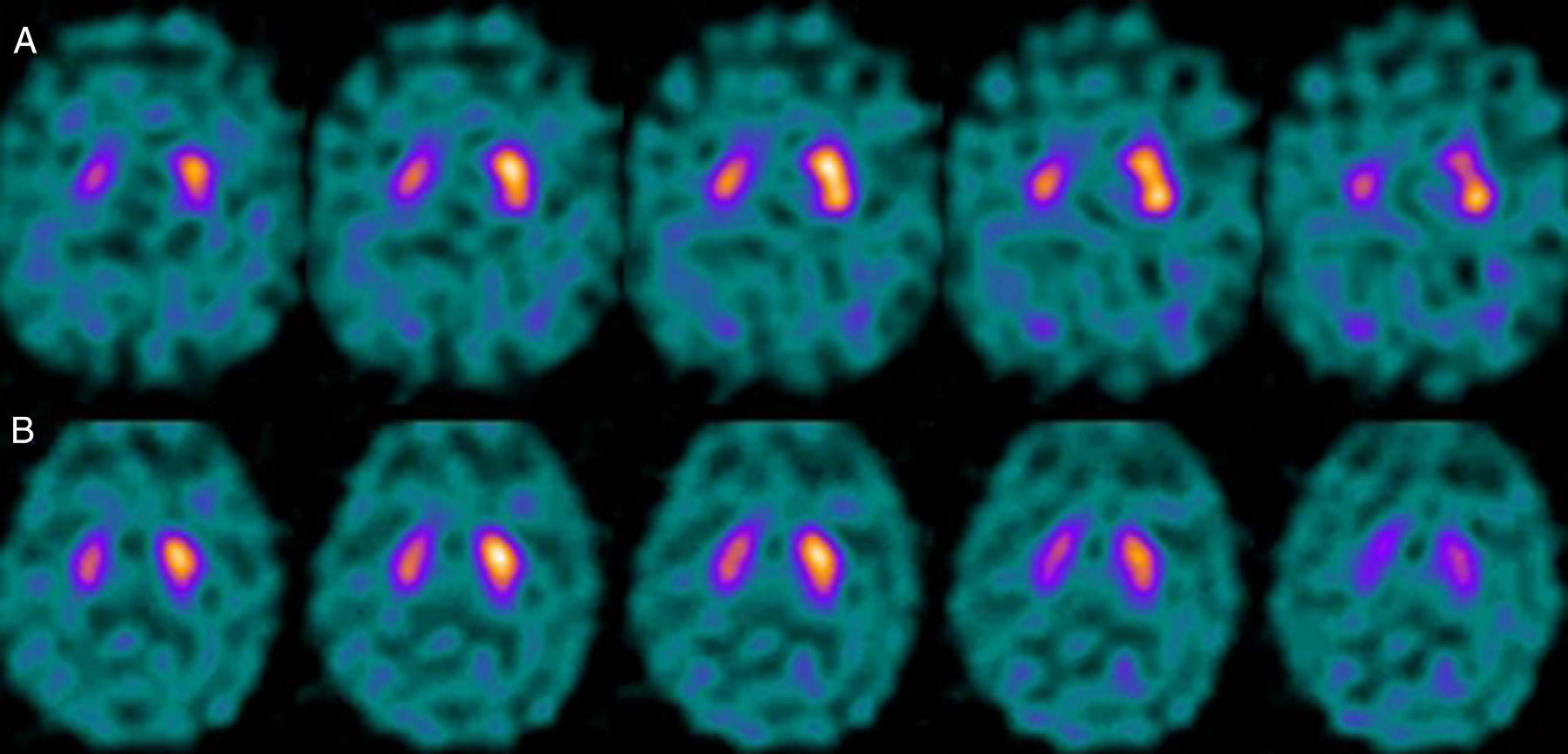
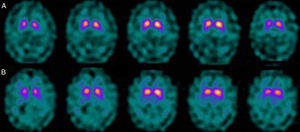
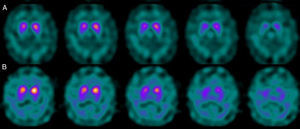
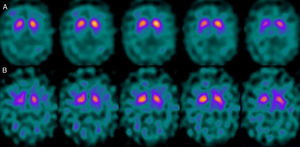
If we analyse the predominant clinical signs of patients with a normal initial study and a pathological follow-up study (N–P), using earlier studies as a reference, we learn that possible causes of these findings may be related to the tremor-dominant signs with little to no rigidity/hypokinesia that presented in 4 of 7 individuals (Fig. 2). One patient in 7 presented PS, classified as stage I according to the Hoehn and Yahr (H&Y) scale. He was being treated concomitantly with an antidopaminergic drug (flupentixol) (Fig. 3). Another individual was classified as a possible case of multiple system atrophy of cerebellar type (MSA-C) (Fig. 4), while yet another patient presented clinical signs indicative of atypical PK but we did not find an explanation for these discrepant results.
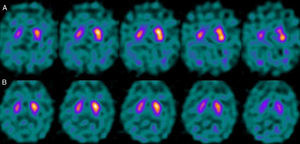
For patients with a P–N sequence, we confirmed that 3 out of 4 were treated with a selective serotonin reuptake inhibitor (SSRIs) at the time of the study with FP-CIT+. After one patient's treatment was suspended, we measured a significant increase in the S/O ratio (due to decreased background activity). Nevertheless, general low uptake persisted in the right striatum with no alterations in the left. After clinical follow-up, the patient was diagnosed with vascular PK (Fig. 5). One patient was being treated with bupropion as a smoking cessation aid.
DiscussionThe results obtained show that in 88.04% of patients, results from initial and subsequent follow-up studies were concordant. Discordant results were found for 11 patients, including 7 cases with an N–P sequence. In the initial study, these 7 patients showed an S/O ratio within the normal range. The mean value was lower than that of the control group and clearly higher than the mean in the pathological group. This supports our first assumption that there are clinical situations that initially give rise to less functional involvement of the nigrostriatal pathway than might be expected.
When we analysed their clinical histories, we observed the following data of interest. Leaving individual peculiarities aside, 4 patients presented PS with initial tremor-predominant signs and little to no rigidity/hypokinesia. Several studies have shown that there is little correlation between tremor severity and striatal uptake of FP-CIT.8,9 This has also been demonstrated when F-DOPA is used in PET techniques.10 Some authors have suggested that morphological patterns of involvement in akinetic-rigid PK differ from those in tremor-predominant forms. While akinetic-rigid PK is characterised by neuronal loss mainly in the ventrolateral part of the substantia nigra (which features projections to the putamina), the tremor-predominant type shows more restrained neuronal loss throughout the substantia nigra.11 In this context, and using FP-CIT as presynaptic marker, 2 recent studies corroborated these neuropathological models. Rossi et al.12 assess the pattern of affectation in several clinical phenotypes of initial PD. The tremor-predominant variant demonstrates better preservation of the putamen than akinetic-rigid variants; this indicates that neurotransmission systems other than the dopaminergic system are involved in tremor genesis. On the other hand, Eggers et al.13 describe a more severe loss of projection to the dorsal putamen in the akinetic-rigid types and to the lateral putamen in the tremor-dominant PD. From the clinical point of view, some observations also support the idea that the origin of tremor may differ from that of bradykinesia, based on the slow progression of tremor-dominant PD,14 bradykinesia/rigidity's better response to levodopa, and the fact that thalamotomy elicits improvements in tremor and not in other symptoms. Other authors have contemplated the possibility that tremor is not directly related to dopaminergic neuronal loss.15
One of the 7 discordant cases, a man whose main clinical signs were mixed-type tremor, cerebellar ataxia, and erectile dysfunction was diagnosed with probable MSA-C. MSA exerts variable effects on several systems (pyramidal, cerebellar, autonomic nervous, and nigrostriatal). In the case of MSA-C, the only consistent finding is cerebellar cortical degeneration. Nigral degeneration in this entity is not as obvious as in MSA-P, a condition in which parkinsonian signs predominate. A recent study performed by our group16 shows that FP-CIT scan cannot rule out the presence of MSA-C, at least not in initial stages.
Returning to the patients with an N–P sequence, one individual previously treated with an antidopaminergic drug presented initial parkinsonian signs (H&Y score I). Occupancy of D2 receptors after this treatment might lead to signs of early onset parkinsonism if there is a subclinical dopaminergic deficit at the synaptic level, even when dopaminergic neuronal cell loss in the nigrostriatal pathways has not reached the minimum threshold to be detected by the FP-CIT scan. The review by Kägi et al.17 of the role of FP-CIT in movement disorders points to evidence suggesting that antidopaminergic drugs can reveal PD in patients who are still in the pre-symptomatic stage. Kim et al.18 followed up on 20 patients diagnosed with drug-induced PK for 2 years and studied them using DAT marker with the PET technique and MIBG scintiscan. They highlighted that the initial study with DAT marker was normal in 2 out of 20 patients, although patients showed altered myocardial sympathetic innervation. Two months after antipsychotic treatment was suspended, both patients’ parkinsonian symptoms improved, but they developed new PK symptoms after 2 years with pathological DAT scan findings.
Lastly, one patient with atypical PS and progressive bulbar involvement, rigidity, and limb apraxia also presented a normal initial study and pathological follow-up study, and we could not determine an explanation for this profile.
Regarding the patient group with P–N sequence, we should mention that treatment with SSRIs produces an increase in non-specific activity, resulting in a decrease in the uptake ratio of specific and non-specific activity (S/O ratio).19 FP-CIT does not show selective affinity for DAT receptors given that it also binds to serotonin receptors. Although blocking the serotonin receptors has no effect on striatal uptake, it results in an increase in available substrate that will augment non-specific activity due to an increase in the vascular pool. In contrast, bupropion binds to the DAT receptor and therefore directly competes with FP-CIT for binding sites, resulting in altered uptake at the striatal level.20
Part of a study similar to this one also assessed dopaminergic neuron loss using sequential FP-CIT scans; it was designed on a larger scale since it was a European multicentre study, but it contained a similar number of patients.21 Out of 99 patients, there were only 2 cases of discordant results between the initial study and the follow-up study at 36 months. One of the patients with an N–P sequence identified after 36 months of follow-up was listed as a possible case of PD, while the other patient with P–N sequence had a final diagnosis of probable PD. However, the study provides no potential explanations for discrepancies between initial and final results.
Another very interesting point raised by this study is the high level of inter-individual variability in the rate of disease progression. This phenomenon remains poorly understood, although it has been reported in other longitudinal studies as well as our own.22 On possible explanation could be that in pathological studies, incidence of dementia with Lewy bodies is 14 to 16 times that of PD.23 This subclinical nigrostriatal dysfunction could explain cases of individual susceptibility/variability.
Among our study's limitations, we are aware of the inaccuracies that may result from using an ROI that does not exclusively circumscribe the area of specific activity and which includes areas of non-specific activity, as in the case of the ROI for striatal structures. This fact might deliver a more or less inaccurate estimation of semi-quantitative parameters, above all when there is a marked loss of dopamine receptors. Furthermore, we should stress that knowing the results of the FP-CIT study before the final diagnosis creates a bias, but that this is difficult to avoid in retrospective studies on imaging techniques.
In conclusion, although performing serial FP-CIT studies in the context of PK is unnecessary in the vast majority of cases, they may be justified in certain clinical situations. Reviewing the clinical literature when interpreting these studies can provide relevant information.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Martínez-Valle Torres MD, Ortega Lozano SJ, Gómez Heredia MJ, Amrani Raissouni T, Ramos Moreno E, Moya Espinosa P, et al. Evaluación longitudinal con FP-CIT en pacientes con parkinsonismo. Neurología. 2014;29:327–333.