Contrast transcranial Doppler (c-TCD) has a high sensitivity for detecting right-to-left shunt (RLS), and is probably higher than transthoracic echocardiography (TTE) and comparable with transesophageal echocardiography (TEE).
ObjectiveTo evaluate the accuracy of echocardiography (TTE and TEE) to detect RLS compared to c-TCD.
Materials and methodsObservational study of patients <55 years old with cerebral ischaemia of undetermined origin (2007–2009). All underwent c-TCD monitoring to detect RLS, at rest and after Valsalva manoeuvre (VM). The TTE and TEE were performed when indicated by our cerebrovascular protocol. The accuracy of TTE and TEE for detecting RLS was calculated by comparing them with c-TCD.
ResultsA total of 115 patients with c-TCD, mean age 43.3 (SD 10.3) years, 51.3% male. The TTE was performed in 102, and TEE in 81, patients. RLS detection was higher with c-TCD than with TTE (67.6% vs. 22.5%, P=.001) or TEE (77.8% vs. 53.1%, P=.001). The TTE, compared with c-TCD after MV showed: sensitivity 31.8%, specificity 96.9%, positive predictive value (PPV) 95.6%, negative predictive value (NPV) 40.5% and accuracy 52.9% to detect RLS. TEE, compared with c-TCD after MV showed: sensitivity 63.4%, specificity 83.3%, PPV 93%, NPV 39.4% and accuracy 67.9%. The accuracy of TTE and TEE improved when they were compared with c-TCD at rest.
ConclusionsTTE and TEE show a considerable number of false negatives for RLS detection. Clinical studies should consider the c-TCD as the best technique to diagnose RLS when a paradoxical embolism is suspected.
El Doppler transcraneal con contraste (DTC-c) tiene una alta sensibilidad para la detección de comunicación derecha-izquierda (CDI), probablemente mayor que la del ecocardiograma transtorácico (ETT) y comparable con la del transesofágico (ETE). Objetivo: Evaluar la precisión del ecocardiograma (ETT y ETE) para detectar CDI, comparándolo con DTC-c.
Material y métodosEstudio observacional de pacientes <55 años con isquemia cerebral de origen indeterminado (2007–2009) a los que se les realizó una monitorización con DTC-c para detectar CDI, en reposo y tras maniobra de Valsalva (MV). El ETT y ETE se realizaron cuando estaba indicado según el protocolo de estudio cerebrovascular de nuestro centro. La precisión del ETT y ETE para detectar CDI fue calculada comparándolos con DTC-c.
ResultadosSe incluyeron 115 pacientes a los que se les realizó monitorización con DTC-c. Edad media 43,3 (DE 10,3) años, 51,3% hombres. El ETT se realizó en 102 y el ETE en 81 pacientes. La detección de CDI fue mayor con DTC-c que con ETT (67,6 vs. 22,5%, p=0,001) o con ETE (77,8 vs. 53,1%, p=0,001). El ETT, comparado con DTC-c tras MV, mostró: sensibilidad 31,8%, especificidad 96,9%, valor predictivo positivo (VPP) 95,6%, valor predictivo negativo (VPN) 40,5% y precisión 52.9% para detectar CDI. El ETE, comparado con DTC-c tras MV, mostró: sensibilidad 63,4%, especificidad 83,3%, VPP 93%, VPN 39,4% y precisión 67,9%. La precisión del ETT y ETE se incrementó cuando se compararon con el DTC-c en reposo.
ConclusionesEl ETT y ETE presentan un número elevado de falsos negativos para detección de CDI, cuando se comparan con el DTC-c. Los estudios clínicos deberían considerar al DTC-c como mejor técnica para diagnosticar CDI cuando se sospecha embolia paradójica.
Right-to-left shunt (RLS) occurs when there is a patent foramen ovale (PFO) or extracardiac arteriovenous fistula, and it is a risk factor for cerebral paradoxical embolism.1,2 It can be detected by various techniques, such as contrast-enhanced transcranial Doppler (c-TCD),3,4 transthoracic echocardiography (TTE)7,8 and transesophageal echocardiography (TEE).5,6 TEE is considered the “gold standard” for the diagnosis of RLS, especially because it enables direct visualisation of a PFO with more sensitivity and specificity than TTE.7,8 However, c-TCD detects the presence of RLS in a high percentage of patients with ischaemic stroke of undetermined origin,9,10 and this figure is comparable with that of TEE.3,11–13 Moreover, c-TCD detects up to 30% of RLS cases that are not detected by TEE, whilst RLS cases detected by TEE but not detected by c-TCD are only anecdotal.3,9,10,14 This divergence between c-TCD and echocardiography may be due to the presence of an extracardiac RLS. However, the exact intra- or extracardiac location of this short circuit should not be an obstacle that would justify RLS being detected less frequently with TTE or TEE than with c-TCD. On the other hand, the technical limitations of echocardiography, such as the difficulty to perform an adequate Valsalva manoeuvre (VM) or reduced visibility with this manoeuvre, could limit the sensitivity of this technique.
Although c-TCD is considered by most neurologists as the most sensitive technique for the detection of RLS,3 no studies have been conducted that evaluate the accuracy of TTE and TEE in its detection considering c-TCD as the “gold standard”.
Our objective was to evaluate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy of echocardiography (TTE and TEE) in detecting RLS, compared with c-TCD.
Materials and methodsThis was an observational study including patients younger than 55 years with ischaemic stroke treated at our stroke centre during a period of 3 years (2007–2009). Patients were recorded prospectively in a specific database for stroke, which included the results of c-TCD monitoring and echocardiographic studies. We retrospectively selected and studied those patients who had undergone monitoring with c-TCD, following the cerebrovascular study protocol, to investigate the presence of RLS. The performance of TTE and/or TEE followed the same cerebrovascular study protocol used at our centre, which is detailed below.
We analysed the following parameters: (a) demographic data; (b) prior vascular risk factors such as arterial hypertension (AHT), diabetes mellitus (DM), hyperlipidemia (hypercholesterolemia and hypertriglyceridemia), smoking, migraine (with or without aura), alcohol abuse, use of other drugs, peripheral arterial disease, prothrombotic coagulopathy, atrial fibrillation (AF), ischaemic heart disease (angina pectoris and myocardial infarction), valvular heart disease and prosthetic heart valve, haematocrit higher than 50%, treatment with oral contraceptives and pregnancy; c) aetiological subtype of stroke according to the classification by the Study Group for Cerebrovascular Diseases of the Spanish Neurology Society15 (cardioembolic, atherothrombotic and lacunar stroke, and stroke with unusual cause of undetermined origin).
During hospitalisation, all patients underwent the same cerebrovascular study protocol, which included at least 1 brain imaging study (computed tomography and/or MRI), a blood study, a chest radiograph, an electrocardiogram, a colour echo-Doppler study of the supra-aortic trunks, TCD and c-TCD monitoring for the detection of RLS. Patients with non-lacunar stroke without atherosclerotic disease or other non-atherosclerotic vascular disorders underwent a TTE study with echo-contrast. In cases where the latter revealed no structural heart disease due to embolism (including PFO or atrial septal aneurysm [ASA]), patients underwent a TEE study with echo-contrast to rule out the presence of atrial septal abnormalities. In 3 cases, the TEE study could not be performed due to low tolerance by patients.
c-TCD monitoring protocolThe presence of RLS was studied by monitoring the middle cerebral artery with TCD, using a 2-mHz probe and insonating through the temporal window. The contrast consisted of a mixture of saline solution (9ml) and air (1ml) formed by stirring the 2 components in 2 syringes of 10ml connected by a catheter. The solution was injected into the antecubital vein to produce boluses of air microbubbles (MBs). The procedure was performed 3 times at rest and 3 times during the VM, as described previously.2 The presence of RLS was established when MB were detected in the first 7s after contrast bolus infusion, and the number of MB recorded were counted. The magnitude of the RLS was established both at rest and after VM according to international criteria16: absent (no MB), small (<10MB), medium (>10MB in a shower pattern) and large (>10MB in a curtain pattern). RLS was considered late if MB were obtained beyond 7s.
Echocardiography protocolBoth TTE and TEE were conducted in the first 7 days after c-TCD monitoring, and always by cardiologists with experience in echocardiography at our hospital who followed the same protocol. Agitated serum was used as contrast in both tests. This was introduced as boluses into the antecubital vein. The mixture consisted of 9ml of normal saline solution and 1ml of air, shaken 10 times through 2 syringes connected to a stopcock. Contrast injection was performed at least twice at rest and twice after VM.
RLS was considered to exist when the passage of MB from the right atrium to the left was observed at any time. No standardised grading scale of RLS magnitude on echocardiography was established.
PFO was considered to exist when: (a) there was passage of MB to the left atrium in the first 3–5 heartbeats after the right atrium filled, or (b) a characteristic flap of the fossa ovalis membrane with flow passage was observed, as demonstrated by colour Doppler.17,18
The existence of ASA was considered when TEE revealed hypermobility of the fossa ovalis membrane with total displacement of at least 15mm and a diameter of the base of the domed area 15mm or greater.17,18
Patients who could not ccooperate by undergoing VM during the tests (c-TCD, TTE, TEE) (2 cases, due to coma) were excluded.
Statistical studyThe statistical study was performed using SPSS version 15.0 for Windows. Quantitative variables were expressed as mean (standard deviation, SD) or median (interquartile range, IQR) and qualitative variables as percentages. Univariate analysis was performed with the χ2 test or Fisher exact test for dichotomous variables. Continuous variables were analysed with the Student t test or Mann–Whitney test when normality could not be assumed. Significance was set for P values less than .05.
The sensitivity, specificity, PPV, NPV and accuracy of TTE and TEE for detecting RLS were calculated by comparing them with the results of c-TCD at rest and after VM.
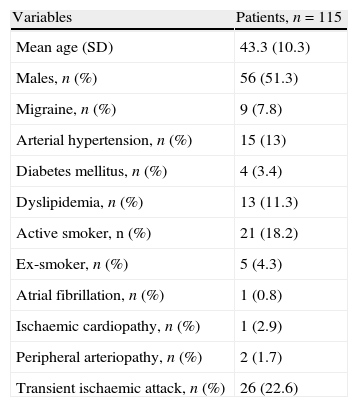
ResultsWe included 115 patients younger than 55 years with ischaemic stroke who had undergone c-TCD. The mean age was 43.3 (SD 10.3) years. A total of 51.3% were males. Table 1 shows the demographic data, risk factors and type of ischaemic stroke.
Baseline data.
| Variables | Patients, n=115 |
| Mean age (SD) | 43.3 (10.3) |
| Males, n (%) | 56 (51.3) |
| Migraine, n (%) | 9 (7.8) |
| Arterial hypertension, n (%) | 15 (13) |
| Diabetes mellitus, n (%) | 4 (3.4) |
| Dyslipidemia, n (%) | 13 (11.3) |
| Active smoker, n (%) | 21 (18.2) |
| Ex-smoker, n (%) | 5 (4.3) |
| Atrial fibrillation, n (%) | 1 (0.8) |
| Ischaemic cardiopathy, n (%) | 1 (2.9) |
| Peripheral arteriopathy, n (%) | 2 (1.7) |
| Transient ischaemic attack, n (%) | 26 (22.6) |
SD: standard deviation.
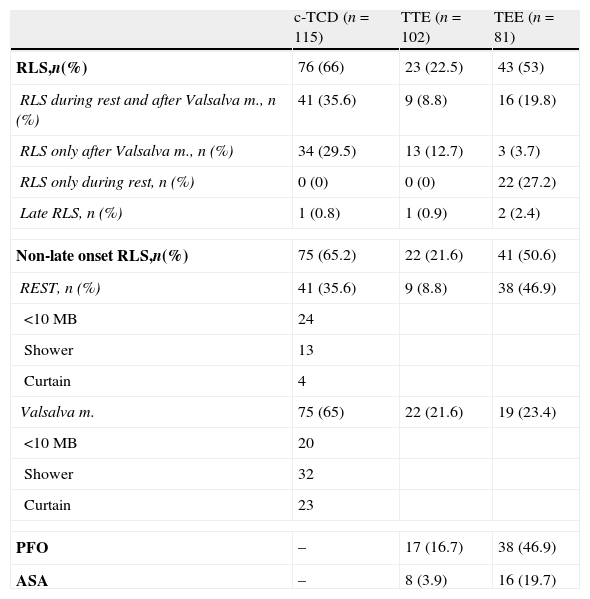
Of the 115 patients included who had undergone c-TCD, 102 patients had also undergone TTE. Of these 102 with c-TCD and TTE, 81 patients had also undergone TEE. The test results are shown in Table 2. Up to 66% of patients were shown to have RLS by the c-TCD and only in 1 case was it a late-onset RLS. Up to 35.6% were shown to have RLS in the c-TCD both at rest and after VM, 29.5% only after VM and no cases presented RLS only at rest but not after VM. TTE found RLS in 22.5% of cases, with 1 case being a late RLS. Up to 8.5% presented RLS at rest and after VM, 12.7% only after VM and no cases presented RLS only at rest and not after VM. In addition, TTE reported PFO in 17 patients and ASA in 8. TEE found RLS in 53% of cases, of which 2 cases were late RLS. Up to 19.8% presented RLS at rest and after VM, 3.7% only after VM and 27.2% presented RLS only at rest but not after VM. PFO was observed in 46.9% and ASA in 19.7% of the patients who underwent TEE.
Results of c-TCD, TTE and TEE.
| c-TCD (n=115) | TTE (n=102) | TEE (n=81) | |
| RLS,n(%) | 76 (66) | 23 (22.5) | 43 (53) |
| RLS during rest and after Valsalva m., n (%) | 41 (35.6) | 9 (8.8) | 16 (19.8) |
| RLS only after Valsalva m., n (%) | 34 (29.5) | 13 (12.7) | 3 (3.7) |
| RLS only during rest, n (%) | 0 (0) | 0 (0) | 22 (27.2) |
| Late RLS, n (%) | 1 (0.8) | 1 (0.9) | 2 (2.4) |
| Non-late onset RLS,n(%) | 75 (65.2) | 22 (21.6) | 41 (50.6) |
| REST, n (%) | 41 (35.6) | 9 (8.8) | 38 (46.9) |
| <10MB | 24 | ||
| Shower | 13 | ||
| Curtain | 4 | ||
| Valsalva m. | 75 (65) | 22 (21.6) | 19 (23.4) |
| <10MB | 20 | ||
| Shower | 32 | ||
| Curtain | 23 | ||
| PFO | – | 17 (16.7) | 38 (46.9) |
| ASA | – | 8 (3.9) | 16 (19.7) |
ASA: atrial septal aneurysm; c-TCD: contrast transcranial Doppler; MB: microbubbles; PFO: patent foramen ovale; RLS: right-to-left shunt; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography.
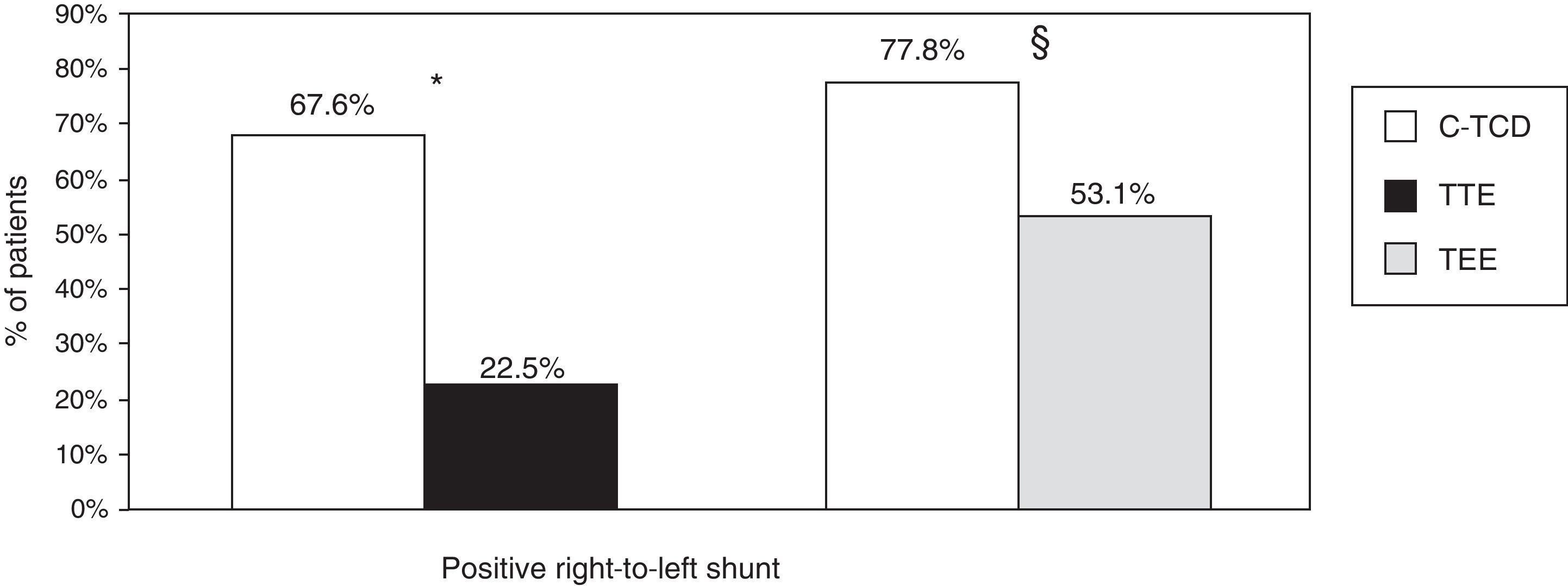
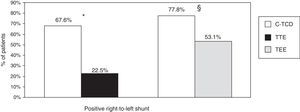
In the group of patients with c-TCD and TTE (n=102), we observed that the detection of RLS was higher with c-TCD than with TTE (67.6 vs. 22.5%, P=.001). In the 81 patients who underwent c-TCD and TEE, we also observed a higher detection of RLS with c-TCD than with TEE (77.8 vs. 53.1%, P=.001) (Fig. 1).
Detection of right-to-left shunt by c-TCD, TTE and TEE. * Percentages refer to the group of patients who underwent c-TCD and TTE (n=102). P=.001 for the detection of right-to-left shunt with c-TCD and TTE. § Percentages refer to the group of patients who underwent c-TCD and TEE (n=81). P=.001 for the detection of right-to-left shunt with c-TCD and TEE. c-TCD: contrast-enhanced transcranial Doppler; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography.
One patient with negative c-TCD presented RLS in TTE and TEE. In addition, 3 other patients with negative c-TCD presented RLS in TEE.
We analysed whether detection of RLS by echocardiography varied with the size of the RLS in c-TCD after VM. TTE was performed in 69 of the 75 cases of RLS after VM observed by c-TCD. After analysing these 69 cases, TTE detected RLS in 31.6% (6/19) of cases with small RLS (<10MB), in 25% (7/28) of cases with medium-sized RLS (>10MB, shower pattern) and in 50% (11/22) of patients with large RLS (>10MB, curtain pattern) according to the c-TCD. TEE was performed in 61 of the 75 cases with RLS after VM by c-TCD. After analysing these 61 cases, TEE detected RLS in 36.8% (7/19) of cases with small RLS, 59.1% (13/22) of cases with medium-sized RLS and 100% (20/20) of cases with large RLS by c-TCD.
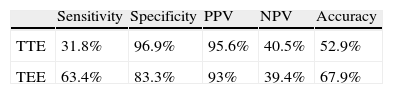
Compared with c-TCD after VM, TTE presented: sensitivity 31.8%, specificity 96.9%, PPV 95.6%, NPV 40.5% and accuracy of 52.9% for the detection of RLS. Compared with c-TCD after VM, TEE presented: sensitivity 63.4%, specificity 83.3%, PPV 93%, NPV 39.4% and accuracy of 67.9% for the detection of RLS (Table 3).
Accuracy parameters for the detection of right-to-left shunt in TTE and TEE, compared with c-TCD after Valsalva manoeuvre.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| TTE | 31.8% | 96.9% | 95.6% | 40.5% | 52.9% |
| TEE | 63.4% | 83.3% | 93% | 39.4% | 67.9% |
NPV: negative predictive value; PPV: positive predictive value; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography.
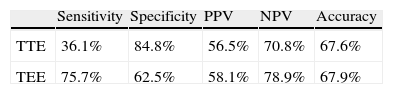
Compared with c-TCD at rest, TTE presented: sensitivity 36.1%, specificity 84.4%, PPV 56.5% and NPV of 70.8% for the detection of RLS. However, TEE presented: sensitivity 75.7%, specificity 62.5%, PPV 58.1%, NPV 78.9% and accuracy of 67.9% for the detection of RLS (Table 4).
Accuracy parameters for the detection of right-to-left shunt in TTE and TEE, compared to c-TCD during rest.
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
| TTE | 36.1% | 84.8% | 56.5% | 70.8% | 67.6% |
| TEE | 75.7% | 62.5% | 58.1% | 78.9% | 67.9% |
NPV: negative predictive value; PPV: positive predictive value; TEE: transesophageal echocardiography; TTE: transthoracic echocardiography.
This study represents one of the largest series comparing 3 diagnostic techniques (c-TCD, TTE and TEE) for the detection of RLS in patients under 55 years with ischaemic stroke. It is also the first to use c-TCD as a “gold standard”. The results show that although echocardiogram is the only diagnostic test that identifies PFO from the anatomical point of view, it is also very limited in the detection of RLS compared with c-TCD. This limitation is even greater in the case of TTE.
The presence of RLS in our series, as assessed by c-TCD, was high (66%). Given that patients were young and had suffered a stroke of undetermined origin at the time of the test, the data were similar to those observed in previous studies. A meta-analysis revealed the prevalence of PFO in 55% of patients younger than 55 years with cryptogenic cerebral stroke.19 In addition, a study evaluating RLS detection with c-TCD and TEE simultaneously reported a frequency of 66% in young patients with stroke of undetermined origin,9 similar to that reported in this study.
The c-TCD is a very sensitive technique for the detection of RLS, with sensitivity figures close to 100% when compared with TEE.8,14 However, despite c-TCD detecting the presence of RLS more frequently, TEE and not c-TCD is considered the most reliable test for establishing the diagnosis. This is probably due to the fact that most comparative studies have been conducted by cardiologists.7,8,11 Moreover, a recent study highlighted these differences when comparing the sensitivities of c-TCD and TEE in a series of 100 young ischaemic stroke patients.14 The study reported 8 cases of RLS detected by c-TCD that were not confirmed by TEE, and were instead considered false positives. Conversely, echocardiography only detected 2 RLS cases not confirmed by c-TCD.
On the other hand, previous studies have focused on anatomically demonstrating PFO by echocardiography, when the mere presence of RLS is considered a risk factor for paradoxical stroke.1,2 In some cases, RLS can be located in the lung, and this is suspected through the detection of a late passage of bubbles.20,21 The incidence of late RLS in the present study was very small, with only 1 case being observed by c-TCD and TTE, and 2 cases by TEE. This suggests that the most frequent location of RLS is the intracardiac.
The differences obtained in the 3 tests (c-TCD, TTE and TEE) for the detection of RLS were striking. The c-TCD detected more than double the cases found with TTE and 25% more than with TEE. The lower accuracy of TTE with respect to TEE for the detection of atrial septal abnormalities and RLS was already known.8 In fact, heart visibility may be limited by a suboptimal thoracic window and, in addition, the VM further diminishes the quality of the image. Nevertheless, few studies show TEE to be inferior to c-TCD for the detection of RLS, as measured by the passage of bubbles from the right atrium to the left. The limitations of TEE to adequately detect RLS are also well known. They include sedation, which, along with other technical difficulties, may prevent an effective patient VM.8,22 In fact, in the present study we observed a higher frequency of RLS by TEE at rest than after VM. A comparative study of c-TCD, TTE and TEE noted that in all cases in which RLS was detected by ultrasound, it was also detected by c-TCD.8 Additionally, in 4 cases RLS was observed by c-TCD but not by TTE or TEE. In these cases, RLS was observed after VM and its intracardiac origin was questioned. The present study registered only 4 cases of negative c-TCD in which RLS was detected by TEE (1 of them also by TTE), whilst cases of negative echocardiography with positive c-TCD were much more numerous, as noted previously.
Perhaps the technical limitations of echocardiography, rather than the exact location of the short circuit, were responsible for this test's reduced sensitivity in detecting of RLS when compared to c-TCD. In the present study we observed a lower sensitivity of TTE, and especially TEE, for the detection of RLS when it appeared after VM in c-TCD. These data support the presence of a high number of false negatives for the detection of RLS and possibly FOP in echocardiographic techniques. Furthermore, we also observed that the detection of RLS by TTE and TEE was better with respect to c-TCD for larger RLS. This finding is important when we consider that RLS with larger sizes have been linked to ischaemic stroke.2 From all these data, after obtaining discordant results between the techniques, we believe that a good option would be to repeat the echocardiogram to increase its sensitivity, since the presence of RLS could modify the therapeutic approach.
This study had several limitations. Being a retrospective study, it was difficult to ensure that the studies were performed in a blinded fashion in all cases. Another limitation was not having categorised the degree of RLS on the echocardiography in a standard manner. However, the goal of the study was to establish the performance of TTE and TEE in the detection of RLS, regardless of its size, and to compare the results with those of c-TCD, at rest and after VM.
The diagnosis of RLS is important in investigating the causes of ischaemic stroke, especially in stroke of undetermined origin, which has a high prevalence of PFO and ASA. In this study, which considered c-TCD as the “gold standard” for the detection of RLS, TTE presented low sensitivity and specificity, especially if the RLS was observed following VM, although these figures increased for TEE. Therefore, we recommend that a TEE should be performed following completion of c-TCD, especially if it shows RLS. Furthermore, we believe that, in the future, the possibility of conducting a second echocardiogram should be explored in cases where the first is negative, so as to improve the performance of this test.
In conclusion, TTE and TEE present a high number of false negatives in the detection of RLS. Clinical studies should consider c-TCD as the best technique to diagnose RLS in suspected paradoxical embolism.
Conflict of interestsThe authors have no conflicts of interest to declare.
This work was presented as an oral communication at the 62nd Annual Meeting of the Spanish Society of Neurology in 2010.
Please cite this article as: Martínez-Sánchez P, et al. Bajo rendimiento del ecocardiograma, comparado con el Doppler transcraneal, en la detección de la comunicación derecha-izquierda. Neurología. 2011;27:61–7.