Alexander disease is a rare disorder caused by mutations in the gene coding for glial fibrillary acidic protein (GFAP). In a previous study, differentiation of neurospheres transfected with these mutations resulted in a cell type that expresses both GFAP and NG2.
ObjectiveTo determine the effect of molecular marker mutations in comparison to undifferentiated glioma cells simultaneously expressing GFAP and NG2.
MethodsWe used samples of human glioblastoma (GBM) and rat neurospheres transfected with GFAP mutations to analyse GFAP and NG2 expression after differentiation. We also performed an immunocytochemical analysis of neuronal differentiation for both cell types and detection of GFAP, NG2, vimentin, Olig2, and caspase-3 at 3 and 7 days from differentiation.
ResultsBoth the cells transfected with GFAP mutations and GBM cells showed increased NG2 and GFAP expression. However, expression of caspase-3-positive cells was found to be considerably higher in transfected cells than in GBM cells.
ConclusionsOur results suggest that GFAP expression is not the only factor associated with cell death in Alexander disease. Caspase-3 expression and the potential role of NG2 in increasing resistance to apoptosis in cells co-expressing GFAP and NG2 should be considered in the search for new therapeutic strategies for the disease.
La enfermedad de Alexander es una enfermedad rara causada por mutaciones en el gen que codifica la proteína glial ácida fibrilar (GFAP). En un estudio previo hemos observado que la diferenciación de neuroesferas transfectadas con estas mutaciones genera un tipo celular que comparte la expresión de GFAP y NG2.
ObjetivosDeterminar el efecto de las mutaciones en marcadores moleculares en comparación con células de glioma diferenciados que expresan simultáneamente GFAP y NG2.
MétodosSe utilizaron muestras de glioblastoma humana (GLM) y neuroesferas procedentes de rata transfectadas con mutaciones de GFAP para el análisis de la expresión tras diferenciación de GFAP y NG2, así como el análisis inmunocitoquímico de diferenciación de ambos tipos celulares y detección de ambas proteínas, junto a nestina, vimentina, Olig2 y caspasa 3 a los 3 y 7 días de diferenciación.
ResultadosTanto las células transfectadas con mutaciones de GFAP como las células procedentes de GLM mostraron un incremento de NG2 y GFAP. Sin embargo, la expresión de células caspasa 3 positiva era marcadamente mayor entre las células transfectadas que entre las células procedentes de GLM.
ConclusiónNuestros resultados parecen indicar que la expresión de GFAP no es el único factor que condiciona la muerte celular en la enfermedad de Alexander y que la expresión de caspasa 3 y el potencial papel de la NG2 en incrementar la resistencia a la apoptosis en las células que coexpresan GFAP y NG2 deben ser considerados en la búsqueda de acciones terapéuticas en esta enfermedad.
The pathophysiology of Alexander disease (AxD) involves the formation of Rosenthal fibres,1 cytoplasmic inclusions within glial cells that have also been observed in other diseases. These cytoplasmic inclusions contain glial fibrillary acidic protein (GFAP). AxD has been associated with mutations in the gene coding for GFAP, although the exact mechanism is yet to be determined.2,3 It has been hypothesised that GFAP mutations may result in accumulation of GFAP; elevated levels of the protein may damage cells as a result of structural abnormalities and gain-of-function4 and aggregation mechanisms.5 Other hypotheses explaining the pathophysiology of the disease have also been proposed.6–8 In a previous study, our research group showed that GFAP mutations may cause GFAP expression during differentiation into oligodendrocytes, generating a cell type that persistently coexpresses GFAP and NG2, inhibiting differentiation of oligodendrocyte precursors9; this may explain the myelin alterations observed in most patients with AxD. NG2 glial cells coexpress GFAP and NG2; these cells can migrate to the corpus callosum, where they differentiate into oligodendroglial progenitor cells. Presence of GFAP and NG2 expression excludes other cell populations.10,11 However, GFAP and NG2 coexpression has also been described in cells of some types of glioma (glioblastoma multiforme; GBM), both in the tumour12,13 and in surrounding tissue.14 These NG2-expressing gliomas may originate from the so-called cancer stem cells, which are undifferentiated,15 although other types of glioma have also been found to express NG2. NG2 expression in these gliomas seems to cause chemoresistance, protect against cell death, and promote tumour invasion, as NG2 promotes migration.16 Increased resistance of NG2-expressing glioma cells to chemotherapy and radiotherapy has been attributed to cis interaction between NG2 and α3β1 integrin, which activates the PI3K/Akt signalling pathway.17 GBM may express GFAP and display Rosenthal fibres, as occurs in AxD,18 but does not cause myelin alterations; it may therefore be interesting to compare both entities when NG2 is coexpressed. To address this question, we analysed differentiation of undifferentiated GBM cells and neurospheres transfected with GFAP mutations to evaluate the differences between the resulting cell types.
MethodsCollection of glioblastoma multiforme cellsAfter receiving participants’ informed consent, we obtained samples by stereotaxic surgery; samples were placed in Dulbecco's Modified Eagle Medium for cell disaggregation, and cultured in Dulbecco's Modified Eagle Medium – low glucose (supplemented with 2mM l-glutamine, 1% non-essential amino acids, 10% foetal bovine serum, penicillin [1.00U/mL], and streptomycin [0.1g/mL]) at 37°C in a humid atmosphere of 5% CO2. All culture media and supplements were purchased from Sigma-Aldrich. For differentiation (passage 8), cells were disaggregated and cultured in pre-coated 4-well chamber slides (Fisher) at a concentration of 25×103cells/cm3. At days 3 and 7, cells were fixed in 4% paraformaldehyde for subsequent immunocytochemical analysis.
Collection of plasmids of GFAP mutationsExperiments were conducted using plasmids of the most frequent GFAP mutations. Dr Michael Brenner (NINDS, NIH, Maryland, USA) provided us with constructs hGFAPR79H, hGFAPR239H, and hGFAPR416W. Site-directed mutagenesis was performed to obtain construct hGFAPR88C. Neurospheres were transfected with hGFAPwt (WT), hGFAPR88C, hGFAPR79H, hGFAPR239H, and hGFAPR416W using Pfu turbo polymerase (Stratagene). After 6-7 passages, neurospheres were transfected for 5 days by nucleofection in vitro (programme A-33, Amaxa Nucleofector II, Lonza). Neurospheres were placed in 75-cm2 cell culture flasks, transfected with 4g plasma DNA per flask, and cultured in complete medium. Transfection efficiency was estimated using pMAX-GFP plasmid supplied in the Mouse Neural Stem Cell Amaxa Nucleofector® kit (Lonza). The methodology used is described in detail in a previous article.9
DifferentiationAfter 72hours in complete medium, neurospheres were seeded in a differentiation medium composed of control medium supplemented with 1.5% foetal bovine serum (Ref. 16000; Gibco). The neurospheres contained in each 9-cm2 well were seeded in 3 wells of 2cm2 with poly-d-lysine-coated coverslips (Ref. P1024; Sigma). We obtained a total of 6 wells of 2cm2 from each transfection. Prior to seeding in differentiation medium, cells were washed twice to remove residual growth factors from the complete medium. Cell differentiation was studied at 2 different time points (at 3 and 7 days), using 3 wells for each time point. We performed an immunocytochemical analysis of the neurospheres adhered to coverslips.
ImmunocytochemistryThe following primary antibodies were used for immunocytochemistry: mouse anti-hGFAP (1:500; Sternberger Monoclonals), chicken anti-Vimentin (1:200; Millipore), rabbit anti-NG2 (1:200; Millipore), mouse anti-Olig2 (1:200; Millipore), mouse anti-Nestin (1:200; Millipore), and rabbit anti-active caspase-3 (1:200; Abcam). Coverslips were incubated in secondary antibodies (goat anti-mouse, chicken, or rabbit Alexa-Fluor 405, 488, 555, and 647; 1:500; Invitrogen) for 2hours, then incubated in DAPI solution (1:1000; Sigma) for 10minutes, and washed before being mounted on glass slides with FluorSave Reagent (Calbiochem). Analysis was performed with a Nikon 80i fluorescence microscope at 40× or 63× magnification.
Statistical analysisFor statistical analysis, samples were processed in triplicate; a Nikon 80i fluorescence microscope at 40× or 63× magnification was used to count cells on coverslips from 2 independent experiments for each condition. A minimum of 150 transfected cells were counted per coverslip; results from transfected cells were compared against those from GBM cells. In GBM cell cultures, we analysed at least 300 cells per condition and experiment. Results are shown as means and standard error.
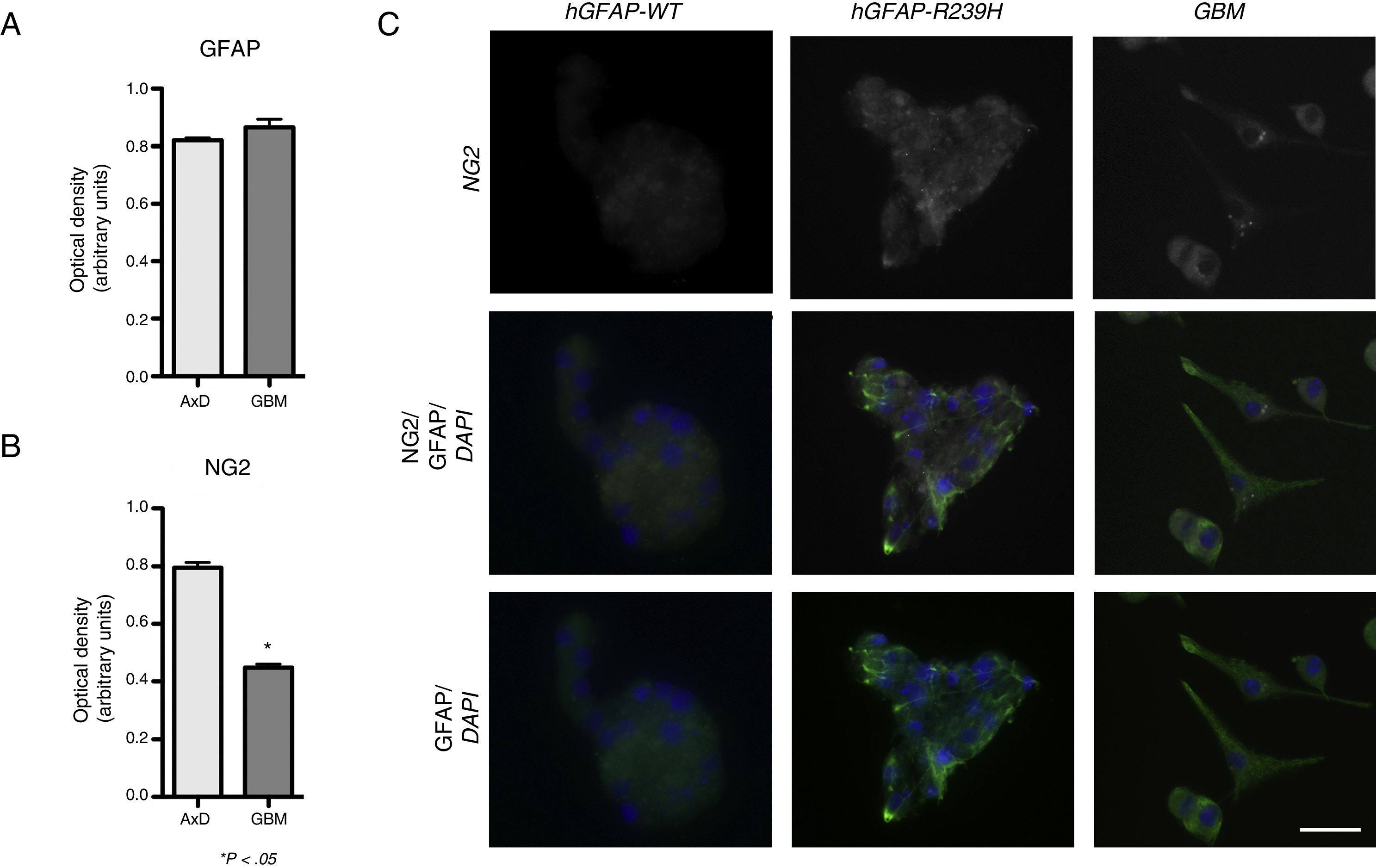
ResultsElevated GFAP expression was observed in undifferentiated GBM cells (mean=0.339 OD; range, 0.333-0.342) compared to undifferentiated WT cells (mean=0.272; range, 0.256-0.287). Considerable increases in GFAP expression were observed during differentiation. At 3 days of differentiation, WT cells displayed a mean GFAP expression of 0.664 OD (range, 0.632-0.691); at day 7, mean GFAP expression was 0.865 OD (range, 0.822-0.918). No significant differences in GFAP expression were observed between WT and transfected cells, with transfected cells exhibiting a GFAP expression of 0.648 OD (range, 0.578-0.705) at 3 days of differentiation, and 0.820 OD (range, 0.797-0.834) at 7 days (Fig. 1A). GFAP expression was increased in both mutant GFAP-transfected and GBM cells during differentiation compared to WT cells, both at 3 days (0.357 OD; range, 0.315-0.391) and at 7 days of differentiation (0.511 OD; range, 0.491-0.521). We also detected differences in NG2 expression between undifferentiated GBM cells and WT cells (0.339 OD; range, 0.333-0.345 vs 0.184 OD; range, 0.178-0.189). During differentiation, NG2 expression increased in GBM cells (0.411 OD; range, 0.388-0.435) compared to WT cells (0.271 OD; range, 0.225-0.282), although NG2 expression was more marked in cells transfected with the mutant gene than in GLM and WT cells (0.772 OD; range, 0.625-0.868). These differences are also seen at 7 days of differentiation: although NG2 expression is slightly higher in GBM cells (0.423 OD; range, 0.401-0.467) than in WT cells (0.201 OD; range, 0.125-0.229), it seems to be lower than that observed in mutant GFAP-transfected cells (0.807 OD; range, 0.745-0.888) (Fig. 1B).
GFAP and NG2 expression. (A and B) GFAP and NG2 expression at 7 days of differentiation. (C) Immunofluorescence images of WT, transfected, and GBM cells showing different patterns of protein expression and types of cell groups. AxD neurospheres show greater GFAP+ filament aggregation and atypical NG2 expression in the cell membrane. In GBM cells, NG2 expression is observed in the cell membrane and in small perinuclear areas, whereas GFAP is expressed in the cytoskeleton and in the apical part of filopodia, where expression is more intense.
Scale bar: 25μm. Graphs show means and standard error. *P<.05. GBM: glioblastoma multiforme.
Fig. 1 compares GFAP and NG2 expression. NG2 is expressed in the form of aggregates and shows greater labelling density. NG2 expression is observed in cells forming neurospheres of atypical shape (not displaying the uniform, spherical structure observed in WT cells). In GBM cells, in contrast, NG2 is expressed in isolated cells or small groups of cells, which are star-shaped or fusiform and show greater marker density in apical branches and small perinuclear areas (Fig. 1C). Mutant GFAP-transfected neurospheres and GBM cells show similar GFAP expression; expression is more marked in areas coexpressing NG2.
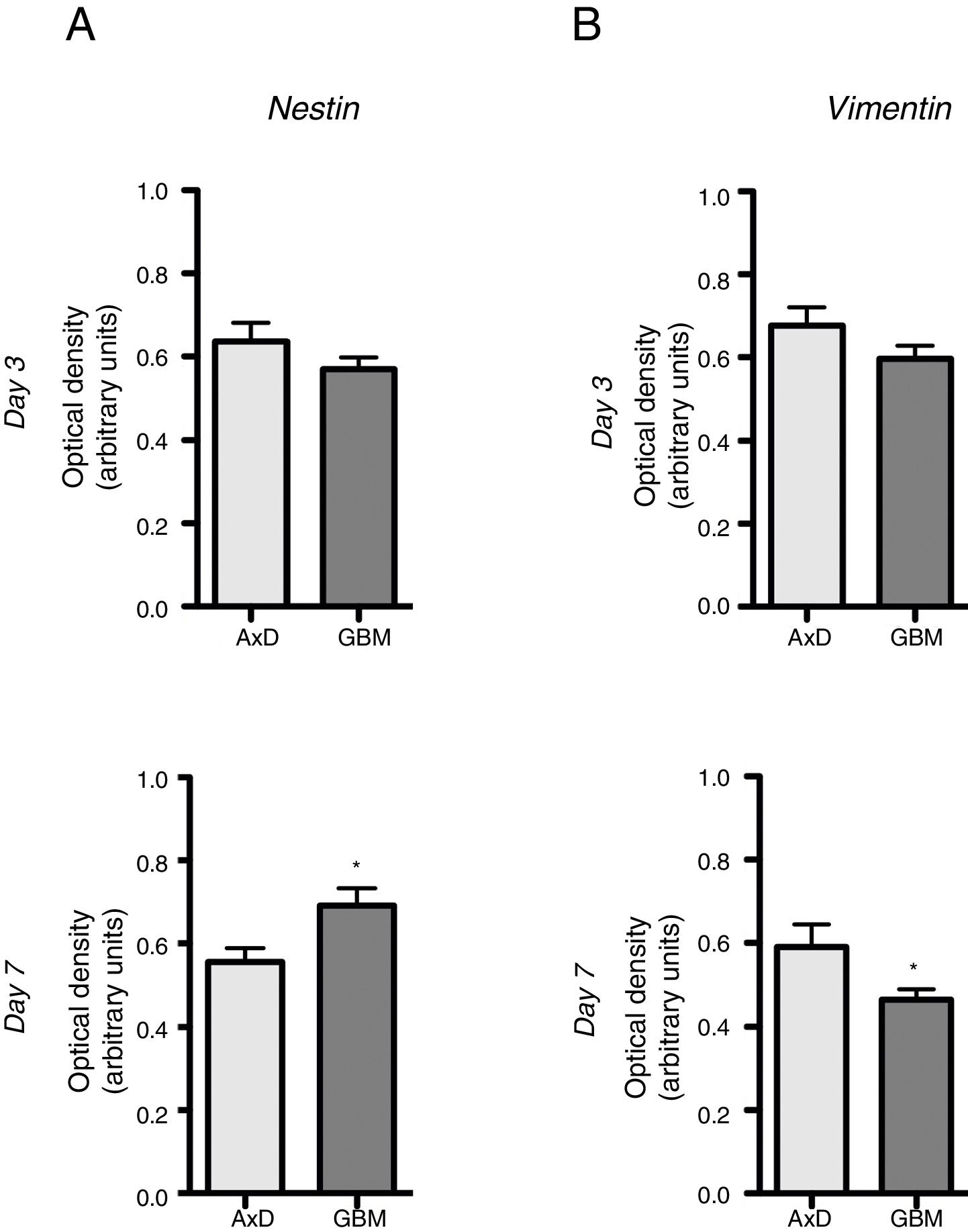
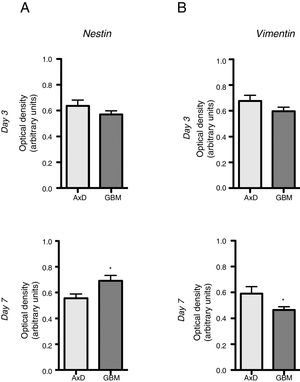
No significant differences were observed in nestin expression between mutant GFAP-transfected cells and GBM cells, either at day 3 (0.636 OD; range, 0.429-0.764 vs 0.569 OD; range, 0.490-0.670) or at day 7 of differentiation (0.555 OD; range, 0.408-0.671 vs 0.691 OD; range, 0.589-0.865) (Fig. 2A). We did observe, however, that nestin expression increased with differentiation, whereas the opposite trend was seen in mutant GFAP-transfected cells. Likewise, no differences in vimentin (Vim) expression were observed between mutant GFAP-transfected cells and GBM cells, either at day 3 (0.676 OD; range, 0.494-0.870 vs 0.596 OD; range, 0.521-0.665) or at day 7 of differentiation (0.590 OD; range, 0.310-0.847 vs 0.463 OD; range, 0.412-0.513); vimentin expression showed a tendency to decrease in both cell types (Fig. 2B). During differentiation, the percentage of cells coexpressing vimentin and NG2 was slightly higher in mutant GFAP-transfected cells than in GBM cells: at day 3, 25.5% of transfected cells and 16% of GBM cells were Vim+ NG2+, whereas 37.5% and 33%, respectively, were Vim+ NG2−. After 7 days of differentiation, 24% of transfected cells and 19% of GBM cells were Vim+ NG2+, whereas 37.75% and 31%, respectively, were Vim+ NG2−. Regarding Olig2 expression, which indicates differentiation of NG2+ cells, after 3 days of differentiation 15.1% of transfected cells and 13.2% of GBM cells expressed Olig2; at 7 days, Olig2 expression was observed in 16.9% of transfected cells and 8% of GBM cells.
Nestin and vimentin expression in transfected and GBM cells. Both cell types showed similar levels of nestin and vimentin expression at day 3 of differentiation; differences in marker expression were observed at day 7. These findings reflect transfected cells attempting to compensate for altered differentiation, and confirm the glial nature of GBM cells.
Graphs show means and standard error. *P<.05. GBM: glioblastoma multiforme.
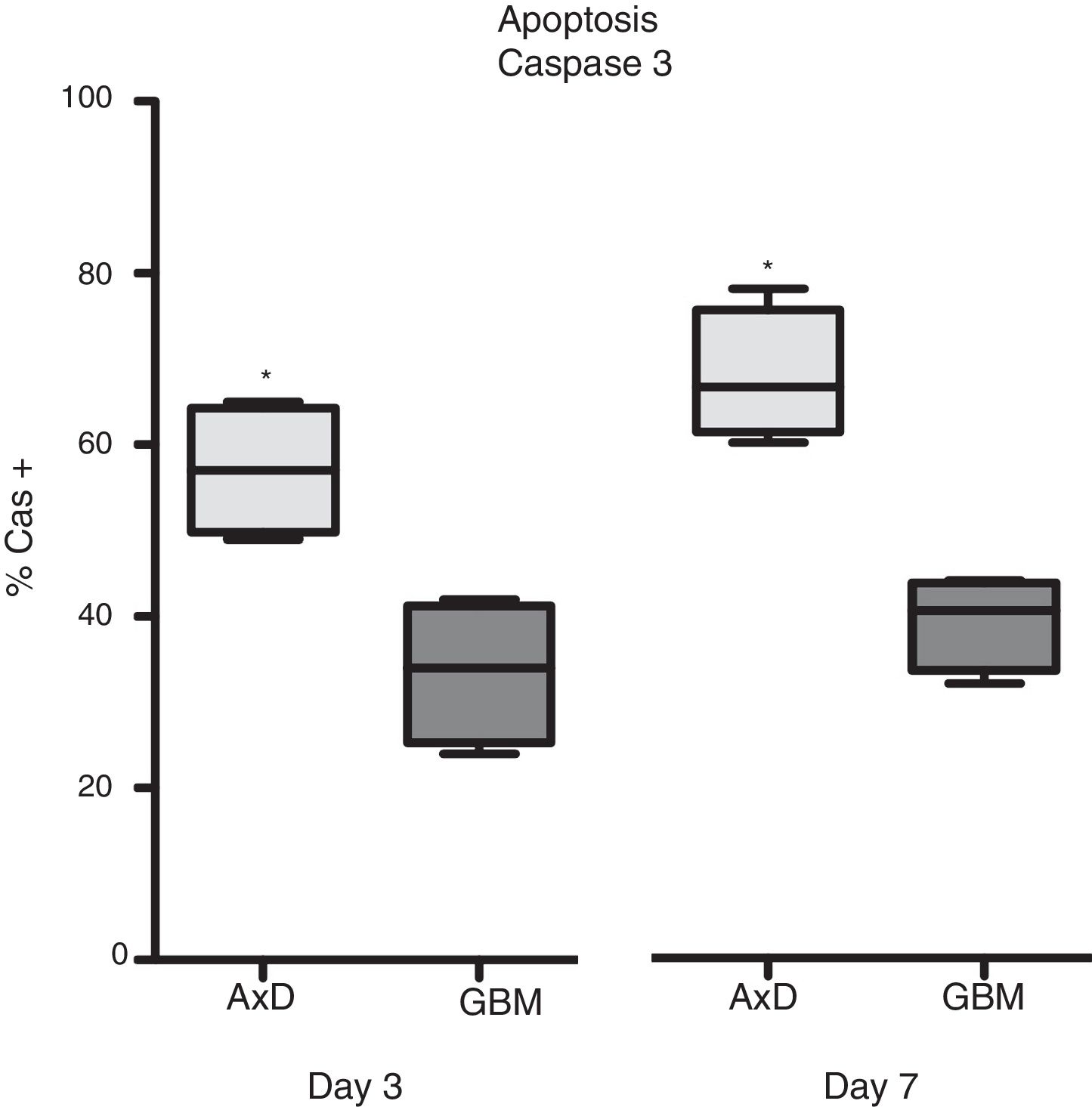
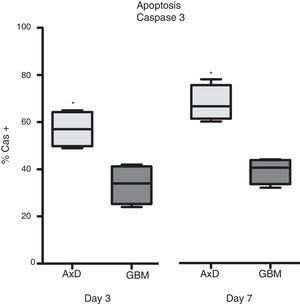
Caspase 3 (Cas3) expression was also analysed in all 3 cell types: 57% of transfected cells and 33.5% of GBM cells were Cas3+ at day 3, with percentages increasing on day 7 (transfected: 67.75%; GBM: 39.25%) (Fig. 3).
Caspase 3 expression. Cas3 expression was considerably higher in transfected cells than in GBM cells at day 3 of differentiation. This effect was more marked at day 7, with nearly twice as many Cas3+ transfected cells than Cas3+ GBM cells.
Graphs show means and standard error. *P<.05. GBM: glioblastoma multiforme.
In the adult brain, GFAP-expressing cells are different from NG2-expressing cells,19,20 except in the case of some embryonic and adult cell populations.21–23 After transfecting neurospheres with plasmids carrying GFAP mutations associated with AxD and promoting differentiation, we obtained a cell type expressing both proteins; this suggests that the pathophysiology of AxD may be associated with alterations in differentiation. To increase our understanding of GFAP and NG2 coexpression, we compared various markers in cells transfected with mutations associated with AxD and a cell type also coexpressing these proteins (a type of GBM cells). GFAP+ NG2+ cells extracted from surgically resected GBM underwent the same in vitro procedure. Both cell types showed increased NG2 and GFAP expression compared to WT cells.12,13
Some GBM cells express such oligodendrocyte progenitor cell (OPC) markers as NG2 and PDGFαR, both of which are markers of NG2 glia24–26; this supports the hypothesis of NG2 playing a role in GBM.27 However, these cells also express nestin and GFAP, and thus cannot be identified as NG2 glia.28 Coexpression of nestin and NG2 has also been described in GBM.29 Glioma cells, which have the ability to differentiate, have been regarded as glioma-initiating cells30,31; this was the reason for comparing these cells against transfected neurospheres. In our model, GBM cell differentiation involves increased NG2 and GFAP expression, similarly to the situation described in AxD.9 Given that GFAP mutations increase expression of GFAP but not nestin, we observed a correlation between GFAP and NG2: while nestin expression increases with differentiation in GBM cells, the opposite is observed in transfected cells. This is not the case with GFAP or vimentin expression, which tends to decrease in both cell types, although it is still present, probably because increased expression of one intermediate filament is accompanied by expression of other intermediate filaments.31,32 This has previously been observed in models of AxD.33 While differentiated transfected cells show very high levels of Olig2 expression, GBM cells express Olig2 at lower levels. More interesting is the difference between both cell types in Cas3 expression. Transfected cells express Cas3, with 67.75% of these cells being Cas3+ after 7 days of differentiation, compared to only 39.25% of GBM cells. The association between AxD and increased Cas3 expression has previously been described.34 Procaspase 3 expression plays a role in oxidative stress resistance in OPCs35; increased Cas3 expression may reduce resistance. At some level, however, increased Cas3 expression may have a neuroprotective effect.36,37 Oxidative stress may also lead to OPC death in multiple sclerosis lesions.38 Oxidative stress is observed in astrocytes expressing mutant GFAP and may increase GFAP transcription by an unknown mechanism, which would further contribute to GFAP accumulation.33 NG2 is protective against apoptosis: apoptotic factors from the intrinsic pathway appear as a result of oxidative stress39 and bind to the cytoplasmic PDZ-binding motif of NG2, increasing resistance in oligodendrocyte lineage cells.40 These data point to the need for further exploration of the role played by Cas3 expression in AxD. Our study analysed the effects of GFAP and NG2 coexpression in 2 very different cell types, with a view to determining whether they show different mechanisms. Our results should be interpreted with caution for 2 main reasons. Firstly, one of the cell lines was from neurospheres derived from rats, whereas the other cell line was from undifferentiated gliomas from human patients. Differences between species should be taken into consideration when interpreting our results. Secondly, both cell types were treated with the same differentiation procedure in vitro; however, human GBM cells do not receive this treatment in normal conditions. Despite these methodological limitations, our study does enable us to draw some interesting conclusions about the pathophysiology of AxD, which would be very hard to reach otherwise. Firstly, both cell types coexpress GFAP and NG2; protein expression increases with differentiation; nestin behaves differently, which further supports the hypothesis that GFAP plays a role in NG2+ GFAP+ cells obtained in vitro after transfection of mutant genes associated with AxD. Secondly, differentiation led to higher Cas3 expression in transfected cells compared to GBM cells, which has potential implications for AxD management.
Author contributionsStudy design: UGP, SSP, JMGV, JAB, and JAMG.
Glioma sample collection: JAB.
Plasmid hGFAPR88C construction and transfection: SSP.
Microscopy and molecular study: UGP, MDM, SSP, and JMGV.
Statistical analysis: UGP, JAMG, and JMG.
Analysis of results: all authors.
Figures: UGP.
Manuscript drafting: JAMG, UGP, and JMG.
Manuscript revision and approval: all authors.
Conflicts of interestThe authors have no financial or commercial relationships which could create conflicts of interest with regard to this article.
The authors would like to thank Irene Borreda for her expert technical assistance; the confocal microscopy department at Príncipe Felipe Research Centre and Universidad Complutense de Madrid; and Dr Guillermo Velasco of the biological sciences school at Universidad Complutense de Madrid, for his help in the culture of GBM samples. We also wish to thank Dr Michael Brenner for providing us with the AxD mutant plasmids, and the Ayuda Juanma Foundation for providing funding for this study.
Please cite this article as: Gómez-Pinedo U, Sirerol-Piquer S, Durán-Moreno M, Matias-Guiu JA, Barcia JA, García-Verdugo JM, et al. Coexpresión de NG2/GFAP tras la diferenciación en células transfectadas con las mutaciones de GFAP y en células procedentes de gliomas indiferenciados. Neurología. 2020;35:479–485.