In recent years, there has been increasing recognition of the benefits offered by rapid-access transient ischemic attack (TIA) clinics for the early assessment of patients with suspected TIA. These clinics, designed to deliver specialized diagnoses and treatments, play an important role in mitigating the risk of stroke recurrence. Most of these clinics benefit from using ultrasound diagnostic imaging conducted by qualified neurologists, which guides the treatment and management of TIA patients. This consensus document, developed by a working group from the Spanish Society of Neurosonology, introduces a novel concept for point-of-care ultrasound (POCUS), specifically focusing on optimizing the diagnostic process for TIA patients in the outpatient setting. The aim is to encourage experienced neurovascular clinicians to adopt a standardized, disease-oriented POCUS that can identify ultrasonographic findings related to the underlying cause of the TIA. Additionally, the document seeks to centralize the recommended diagnostic evaluations for TIA patients. By doing so, the goal is to optimize the diagnostic workup and subsequent treatment performed by the neurologist, fostering a more cohesive and effective approach to managing TIA cases.
En los últimos años, ha ido creciendo el reconocimiento de los beneficios que aporta el acceso rápido a clínicas especializadas en ataques isquémicos transitorios (AIT) para la evaluación precoz de pacientes con sospecha de este tipo de eventos. Estas clínicas, concebidas para proporcionar diagnósticos y tratamientos especializados, juegan un papel esencial en la reducción del riesgo de recurrencia de ictus. La mayoría de estas clínicas utilizan la ecografía, realizada por neurólogos cualificados, que orienta el tratamiento y el abordaje de los pacientes con AIT. Este documento de consenso, desarrollado por un grupo de trabajo de la Sociedad Española de Neurosonología, introduce el nuevo concepto de point-of-care ultrasound (POCUS, ecografía en el punto de atención), que se centra específicamente en optimizar el proceso diagnóstico de los pacientes con AIT en el contexto ambulatorio. El objetivo es promover entre los especialistas con experiencia en el campo neurovascular la realización de POCUS centrado en la patología y estandarizado para identificar hallazgos ecográficos relacionados con la causa subyacente del AIT. Además, este documento pretende proporcionar un compendio de las pruebas diagnósticas recomendadas para pacientes con AIT. De esta forma, nuestro objetivo es optimizar la labor diagnóstica y terapéutica del neurólogo, promoviendo un enfoque más cohesionado y efectivo del manejo del AIT.
Transient ischemic attack (TIA) is a common medical emergency associated with a high risk of ischemic stroke recurrence, which is 10–20% during the first 90 days and even greater within the first 48h following transient neurological symptoms.1 The pathophysiological mechanisms underlying TIA vary between patients and influence the specific recurrence risk profile and the most appropriate secondary prevention strategies.2,3 Specialized early assessment of TIA patients and the initiation of individualized secondary prevention strategies have proved to reduce the risk of recurrence by 80%.4 To achieve these objectives, dedicated outpatient TIA clinics have increased in many hospitals, offering patients a multidimensional assessment that includes clinical evaluation, ultrasound (US), other imaging diagnostic techniques, and health education programs.5 These outpatient TIA clinics have proven to be cost-effective, preventing prolonged hospital stays for asymptomatic patients and expediting the diagnostic and therapeutic processes for TIA patients.6,7
Concurrently, over the past two decades, neurosonology—a branch of neurology focused on US examination—has experienced significant growth. This expansion includes the introduction of new diagnostic procedures and the development of increasingly portable, higher-quality devices, seamlessly integrating neurosonological techniques into routine clinical practice.8 Due to its high diagnostic accuracy, noninvasiveness, widespread availability, and low cost, sonographic examination is often the first choice for evaluating TIA patients.3,9 In response to the growing demand and mounting evidence supporting the utility of US, point-of-care ultrasound (POCUS) has emerged. POCUS represents a simplified yet high-quality protocol designed to rapidly identify or rule out the main suspected conditions underlying a patient's symptoms. In this approach, the treating physician conducts the US evaluation in real-time, enabling a direct correlation of imaging findings with the patient's clinical characteristics and facilitating prompt therapeutic decisions.10,11 Ultimately, POCUS serves as a rapid and targeted tool, offering timely answers to specific clinical questions.12,13
A neuro-POCUS has recently been developed by the European Academy of Neurology Scientific Panel of Neurosonology, the European Society of Neurosonology Centers in Neurosonology and Cerebral Hemodynamics, and the European Reference working group. This innovative approach consolidates various US examinations, guided by the patient's symptoms, to aid in both diagnosis and patient care.14 In addition to traditional neurosonological techniques, there is a growing emphasis on incorporating other evaluations, such as focused cardiac ultrasound (FoCUS) within neurovascular units. Administered by trained neurologists, FoCUS serves as a screening method for identifying potential embolic sources. This simplified point-of-care cardiac evaluation can be quickly learned and implemented, enhancing diagnostic confidence and increasing the quality of outpatient care.15 Various professional societies and training agreements have provided guidance on using FoCUS to optimize diagnostic yield and ensure patient safety.15,16 A recent consensus between the Spanish Society of Neurology and Cardiology envisages using FoCUS as a screening tool, establishing the training standards to facilitate its implementation.17
This study seeks to introduce a specialized US protocol, termed TIA-POCUS, tailored for the assessment of TIA patients. The primary goal is to encourage neurologists to adopt a standardized, disease-oriented point-of-care ultrasound. The TIA-POCUS is designed to detect key ultrasonographic findings related to the etiology of TIA. Another objective of this study is to streamline diagnostic evaluations by assigning responsibility to the overseeing neurologist. The results will then guide the neurologist to assess the need for further diagnostic tests, which may improve patient care.
What is TIA-POCUS?TIA-POCUS is a focused multi-dimensional US protocol conducted by the attending neurologist for patients presenting with TIA. This protocol combines the patient's medical history with a physical examination to identify the primary etiology of the ischemic event and identify relevant risk factors. The objective is to empower clinicians to guide the diagnostic assessment effectively, aiding in selecting the most appropriate tests for accurate diagnosis and subsequent treatment. TIA-POCUS involves a series of focused ultrasonographic examinations, providing dynamic and immediate information. This approach can potentially detect conditions closely linked to TIA mechanisms, such as severe carotid stenosis or significant systolic dysfunction, which profoundly impact the patient's prognosis and treatment.10 Additionally, the protocol may reveal other pertinent data related to TIA pathophysiology, such as left atrium enlargement (a risk factor for paroxysmal atrial fibrillation) or the presence of microangiopathy. These findings are valuable in guiding the etiological workup for each patient. When used appropriately, TIA-POCUS has the potential to offer real-time diagnosis, and in selected cases, it may complement or even replace more advanced imaging techniques.10
The optimal timing for conducting the TIA-POCUS examination is immediately after the onset of symptoms, preferably within the first 24h following TIA symptoms. This examination is ideally performed in TIA clinics, stroke units, or emergency departments. Successful completion of the TIA-POCUS examination requires a combination of theoretical knowledge of TIA physiopathology and proficiency in technical and sonographic skills. Concerning technical requirements, a portable duplex US device capable of studying extracranial and intracranial vessels, the heart, orbits, and temporal arteries is essential. Transcranial Doppler may also be useful, especially in cases where the transtemporal window is suboptimal. The main US evaluations recommended for assessing a patient with suspected TIA are listed in Table 1. Depending on the patient's characteristics, duplex evaluation of extracranial and intracranial vessels may be completed with FoCUS, orbital or temporal artery examination, right-to-left shunt detection, or hemodynamics-cerebral autoregulation tests.
Evaluation of supra-aortic arteriesDuplex US of the cervical arteries is recommended to assess steno-occlusive lesions in extracranial cerebral arteries such as the internal carotid artery (ICA) and the vertebral artery (VA). Color-coded duplex US has a sensitivity of 95.3% and a specificity of 84.4% compared to angiography, particularly in detecting carotid stenosis greater than 70%.18 For this examination, a linear-array transducer with an operating frequency of 5–12MHz, optimally 3–15MHz, is required.19,20
Each routine examination should include a B-mode evaluation of the vessel wall, typically conducted before the color-coded Doppler evaluation. The aim is to characterize atheromatous plaques and detect signs of instability associated with cerebral ischemia. Lipid-rich plaques and intra-plaque hemorrhages are associated with more echolucent (lower echogenicity) plaques. In contrast, fibrous tissue and calcium, both related to stable plaques, are present in more echogenic (high echogenicity) plaques.21,22 For the qualitative characterization of atheromatous plaque, a classification system has been proposed.23 This includes Type I, uniformly hypoechoic; Type II, fundamentally hypoechoic (>50% of the area); Type III, fundamentally echogenic (>50% of the area); Type IV, homogeneous; and Type V, not classifiable, calcified. This classification system has shown predictive value in assessing the risk of ipsilateral stroke, independent of the degree of stenosis, as evidenced in a previous randomized clinical trial where Type I and II plaques were associated with an increased risk.24
Complications such as ulceration and thrombosis can be identified through visual analysis of the atheroma plaque surface.25 Recent years have seen a growing use of contrast-enhanced ultrasound techniques, offering improved visualization of plaque irregularities and ulcers. Moreover, these techniques enable the visualization of microvascularisation within the carotid atheromatous plaque.26,27
The Spanish Society of Neurosonology has established the key parameters for assessing carotid stenosis28 according to validation studies published in the literature.29–33 These are listed in Table 2.
Regarding the vertebral artery, the most common location for steno-occlusive lesions after the carotid bifurcation, there is no consensus regarding the quantitative criteria for diagnosis. Qualitative parameters such as the number, morphology, and size of plaques imaged at the origin of the vertebral artery should be considered. An aliasing effect at the origin and an altered Doppler flow profile characterized by elevated velocities and spectral broadening can be observed in stenoses starting at 50–60%. In a recent study by Zhang et al.,34 the proposed cutoff values for ≥50% and ≥70% V1 segment stenosis were peak systolic velocity≥146cm/s and ≥184cm/s and V1/V2 peak systolic velocity ratio≥2.2 and ≥3.5, respectively. The cutoff values for predicting ≥50% and ≥70% V2 segment stenosis were peak systolic velocity≥80cm/s and ≥111cm/s and V2/V1 peak systolic velocity ratio≥1.2 and ≥1.7, respectively.
A distal extracranial VA occlusion may cause a high pulsatile flow signal with an absent end-diastolic flow.35,36 Another finding to be considered in the posterior circulation is the subclavian steal phenomenon, a pathognomonic sign of proximal high-grade subclavian artery stenosis or occlusion characterized by a distinctive flow pattern within the ipsilateral VA. Depending on the degree of subclavian stenosis, various flow patterns may be observed within the ipsilateral VA, including reduced systolic flow (systolic deceleration, grade 1), alternating flow (grade 2, incomplete), or even retrograde flow (grade 3, complete).35,36
Arterial dissection should be suspected when the duplex US assessment shows: (a) echolucent intramural hematoma; (b) an intimal flap with abnormal flow waves; (c) direct visualization of a double lumen and its corresponding flow waves; (d) stenosis or occlusion of an arterial segment typically unaffected by atherosclerosis (the distal part of the ICA≥2cm after the carotid bifurcation or the V2–V4 segment of the VA). In addition to these direct indicators, several indirect findings may be related to arterial dissection. These include increased or decreased pulsatility upstream or downstream of the suspected arterial lesion in the absence of atheromatous lesions, >50% difference in the blood flow velocity compared to the unaffected side, and the presence of intracranial collateral flow.37
Evaluation of intracranial arteriesTranscranial Doppler (TCD) or transcranial color-coded duplex (TCCD) is highly effective in detecting intracranial large vessel occlusion or stenosis. A low-frequency (2–2.5MHz) sector transducer is required, while transtemporal, suboccipital, and transorbital examinations are also recommended.
The primary indicator of an intracranial occlusion assessed by TCD is an abnormal flow waveform at the presumed location of the thrombus, which includes both the absence and the decrease of flow signals.38 These variations in arterial flow have been classified using the Thrombolysis in Brain Ischemia (TIBI) flow grades.39
The TCCD offers added value over TCD by enhancing the accuracy of the evaluation through the visualization of color and flow signals in the specific anatomical location of interest. In the case of a proximal occlusion of the middle cerebral artery (MCA), the absence of color and flow signals is sufficient. However, in some instances, these findings may be due to a very high degree of stenosis (preocclusive) of the MCA itself or an insufficient temporal window. The visualization of other arteries of the Circle of Willis ipsilateral to the MCA is considered a highly specific criterion for distinguishing an occlusion from the absence of a temporal window.40 Additionally, a reduction ≥21% in the mean velocities of the MCA compared to the contralateral side suggests the presence of a distal occlusion in the main trunk of the MCA or one of its distal branches.41 A summary of intracranial arteries’ peak systolic normal velocity values and the gradation of intracranial stenosis for intracranial circulation assessed by TCD and TCCD is available in Table 3.
The assessment of the intracranial collateral arteries is also of interest, especially when ICA occlusion or stenosis is suspected.42-46 Three types of collateral flow can be identified on TCD and TCCD:
- a)
Extracranial to intracranial collateral communication: Reverse ophthalmic artery, present in up to 80% of severe ICA stenosis or occlusion cases. The ophthalmic artery can be insonated through the orbital window as a low pulsatility flow away from the probe at a depth of 40–60mm.47,48
- b)
Circle of Willis collateral flow:
- -
Anterior communicating artery (AcomA). The first sign of ICA critical stenosis or occlusion on TCCD is the reversal of the ipsilateral anterior cerebral artery due to anterior cross-filling through the AcomA from the contralateral anterior cerebral artery.49 In addition, AcomA can be identified on TCD through the temporal window as a turbulent flow signal at the midline at a depth of 70–75mm or at a depth of 60–65mm through the orbital window.50,51
- -
Posterior Communicating Artery (PcomA): This plays a major role as a posterior collateral blood flow in the presence of ICA critical stenosis or occlusion (flow from posterior to anterior circulation) or basilary artery occlusion (flow from anterior to posterior circulation). The TCCD will show a stenotic signal with turbulence flow at 55–70mm depth through the temporal window aiming at the midline.46 On TCD, the blood flow in the PcomA is usually low-resistance and directed toward the probe, located posterior to the ICA bifurcation, when insonated through the temporal window at a depth of 58–68mm.52 The PcomA can be defined indirectly53 by the presence of increased flow velocity of 50% or greater in the P1 segment of the posterior cerebral artery ipsilateral to the ICA stenosis compared to the contralateral.
- -
Reverse basilar artery: In proximal basilar or acute bilateral VA occlusions, flow reversal from the ICA through the PcomA bilaterally to the basilar artery will be present.54
- -
- c)
Leptomeningeal collateral blood flow. Although it cannot be visualized directly, it can be evaluated indirectly by a ≥30% increase of flow velocity and low resistance (pulsatility index<1.2) in the ipsilateral anterior and posterior cerebral arteries compared to the analogous contralateral arteries.55 Flow diversion or compensatory velocity increase can be distinguished from increased flow velocities due to stenosis or communicating arteries because it preserves its laminar flow morphology.
The orbital blood vessels can be assessed using color and pulsed Doppler with 2–3MHz sectoral probes and 7.5MHz linear probes. Before the evaluation, the mechanical index should be reduced to 0.23, and the scanning time should be kept as short as possible to avoid damage to the vitreous and crystalline lens.
The ophthalmic artery can be visualized in color mode as the most prominent vessel, with a reference peak systolic velocity value of 37.5±7.1cm/s. It is recommended to assess the direction of the flow to verify whether it is inverted (collateral) at the level of the apex of the orbit.46,56,57 However, flow directions away from the transducer might occasionally be observed due to the vessel's elongated course. In cases of giant cell arteritis, the ophthalmic artery may also appear inverted or absent.58
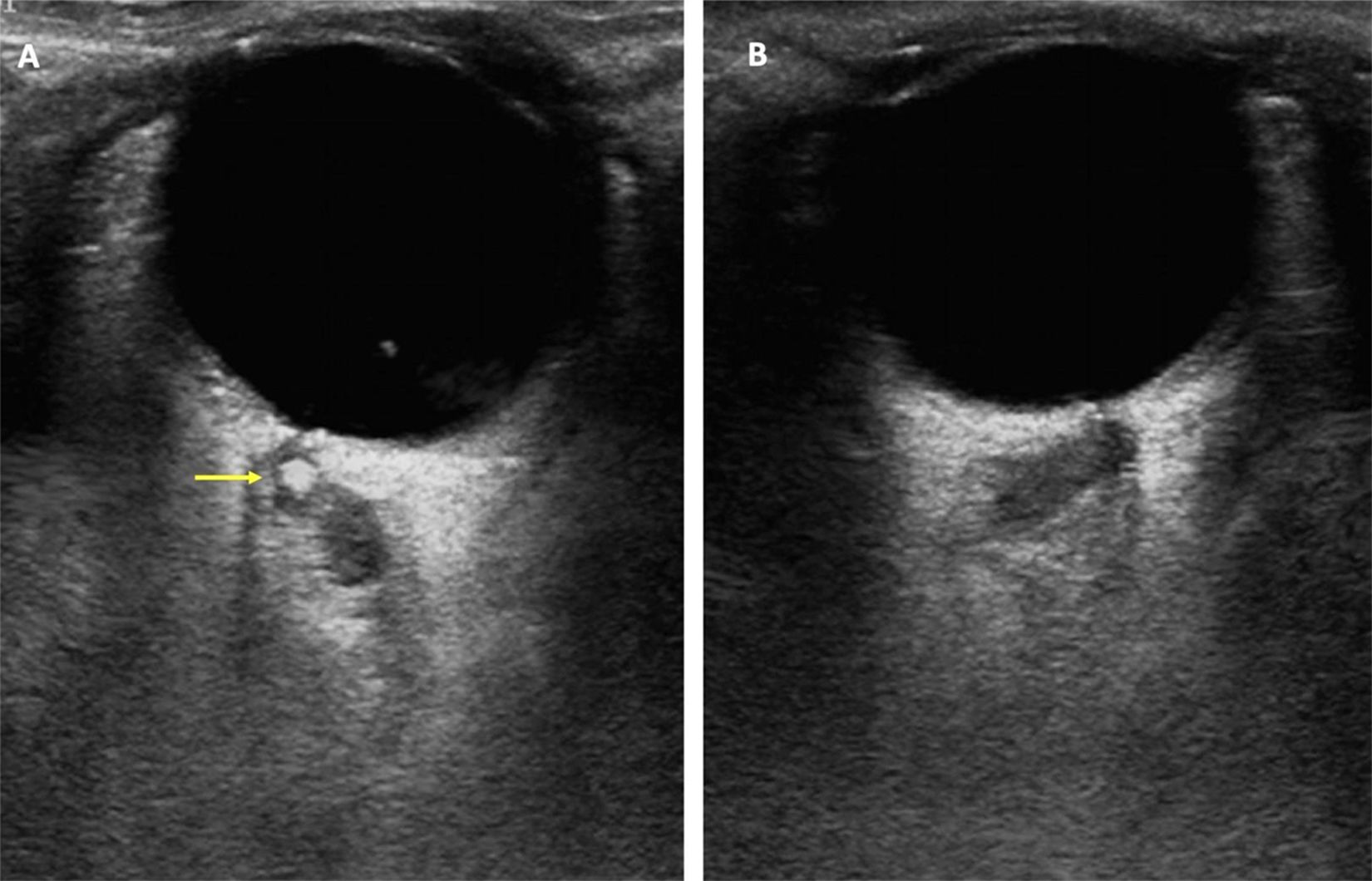
The central retinal artery and vein are typically observed within the optic nerve, very close to the eyeball.57 The key indicator of central retinal artery occlusion is the absence or marked decrease in flow.59 In instances of calcium-rich emboli, a spot sign—defined as a hyperechoic spot within the central retinal artery—may be visible, indicating a carotid atheromatous plaque as the cause of the occlusion, as illustrated in Fig. 1.60
Spot sign found at the transorbital sonographic evaluation of the central retina artery in a patient with vision loss in the right eye. (A) Right eye. A hyperechogenic spot (yellow arrow), compatible with calcium-rich emboli, can be found at the central retina artery. (B) Left eye. No pathological findings.
Sudden and transient unilateral vision loss, resembling a TIA, could be the first manifestation of anterior ischemic optic neuropathy,61 particularly in the context of giant cell arteritis.
For a thorough examination of the temporal artery, it should be assessed in a sagittal and transversal projection from its proximal portion in the tragus toward the temple, following its two main branches: anterior and posterior. The use of a high-frequency probe (≥15MHz) is recommended.
Recently, the European Alliance of Associations for Rheumatology has incorporated US findings into the diagnostic criteria for giant cell arteritis.62 The halo sign, characterized by a homogenous, hypoechoic intima-media thickness, is highly suggestive of this condition. Another indication arising from the thickening of the arterial wall is the absence of compressibility of the artery. The evaluation of axillary arteries is also recommended.58,63,64
Right-to-left shunt detectionA right-to-left shunt refers to the presence of abnormal communication between the systemic and pulmonary circulation, commonly caused by patent foramen ovale—communication between the right and left atria at the level of the fossa ovalis. This assessment is especially important in patients under 55 years of age with suspected ischemic stroke, as patent foramen ovale has been associated with an increased risk of ischemic stroke at younger ages. TCD imaging is employed to detect gas microemboli in the MCA by injecting intravenous agitated saline solution. These microemboli appear as high-intensity transient signals (HITS) in the presence of a right-to-left shunt. The suboccipital approach targeting the V4 segment of the VA is also valid for shunt monitoring in case of a suboptimal transtemporal window.65 The test should be conducted with the patient sitting or lying down at rest and after the Valsalva maneuver. Different patterns based on the maximum number of HITS detected in the intracranial artery following the injection of agitated saline solution can be observed, reflecting the size of the right-to-left shunt: absent (0 HITS), low-grade (<10 HITS), medium-grade (>10 HITS, shower pattern) and high-grade (curtain pattern: uncountable HITS).66
Focused cardiac ultrasoundThe diagnostic goals of FoCUS are broadly aligned with those of standard echocardiographic studies, with a primary focus on ruling out cardioembolic sources in patients with suspected transient cerebral ischemia. Although the recommended elements of a FoCUS assessment may vary slightly across specialized organizations, there is general agreement on its core components.17,67–70 As with other imaging techniques, it is advisable to standardize the sequence for acquiring views and the goals of the examination. The ideal standard set of views for FoCUS includes parasternal long axis, parasternal short axis, apical four and two-chambers, subcostal long axis, subcostal inferior vena cava, and suprasternal, the latter being specifically useful for assessment of aortic arch atheromatosis. A 2.5–5MHz sector transducer is required.67,71
The main objectives of FoCUS in the evaluation of TIA patients are:
- a)
Qualitative assessment of left ventricle systolic function: FoCUS provides a visual estimate of overall systolic function., relying on a qualitative endocardial excursion and myocardial thickening using multiple windows, including parasternal, subcostal, and apical views. A semiquantitative classification can be visually provided as normal, probably reduced, and severely reduced systolic function.17
- b)
Heart-chamber dimensions: FoCUS can screen for Left ventricular dilatation,72 moderate-to-severe left ventricular hypertrophy,73 and enlargement of the left atrium,74 adding value to the assessment of the TIA patient. Left ventricle and atrium size are recommended to be obtained in the parasternal long-axis and four and two-chamber apical views.75 Left atrial enlargement is widely linked to atrial disease and may be a risk factor for atrial fibrillation, encouraging cardiac monitoring if a positive finding.76 Recent guidelines recommend indexed biplane volume calculation (Simpson disk method) as a preferred method for left atrial remodeling estimation.75 The most relevant measurements in FoCUS for TIA patients are listed in Table 4.
- c)
Distinguishing left ventricular contraction patterns: Evaluation of segmental wall motion abnormalities can be challenging and should be assessed by performing a comprehensive echocardiogram. However, in patients with an optimal window, it is possible to identify some alterations in segmental motility, such as akinesia or even aneurysms of the apical region, which are associated with a high risk of intraventricular thrombus formation, potentially indicating the need for anticoagulation.
- d)
Detection of significant mitral or aortic valve abnormalities. FoCUS operators should be able to visually identify decreases in valve aperture combined with color aliasing suggestive of stenosis and the presence or regurgitation with color-Doppler evaluation, as well as the presence of valve degenerative signs (annular calcification, valve thickening).77 Patients with more than mild valve lesions should be referred for a conventional echocardiographic examination.17
- e)
Large intracardiac masses and aortic arch plaques. FoCUS can detect erratic movements indicative of intracardiac thrombi and tumors, distinguishing them from cardiac structures (tendons and trabeculations). Cardiac tumors most frequently associated with stroke include myxoma, commonly located in the left atrium, and fibroelastoma, typically located in the aortic valve.78 Although transesophageal echocardiography is the modality of choice for detecting aortic arch atheromatosis, FoCUS from the suprasternal window can rapidly assess the aortic in some patients. Additionally, aortic arch plaques may be inaccessible for transesophageal echocardiography due to the interposition of the left bronchus.79 Plaques that are ≥4mm thick or contain mobile elements are considered complex aortic plaques.80
- f)
Intracardiac shunt. Cardiac ultrasound can confirm the presence of an intracardiac right-to-left shunt using intravenous agitated saline. Moreover, it can identify structures associated with the presence of large patent foramen ovale or those with a higher risk of recurrence, such as atrial septal aneurysm, prominent Eustachian valve, or Chiari network.
The recommended approach for investigating cardioembolic sources in TIA patients involves a comprehensive assessment. This includes evaluating the movement of the left ventricle to rule out severe systolic dysfunction. Additionally, the mobility of mitral valves and the Doppler pattern should be assessed to rule out mitral stenosis, while evaluation of the aortic arch is also recommended to identify complex aortic plaques. In cases where no major cardioembolic source is identified, the size and characteristics of the left atrium are of significant interest for assessing the risk of paroxysmal atrial fibrillation.81
DiscussionThe integration of focused sonographic assessment through POCUS emerges as a valuable tool in the clinical management of patients with conditions requiring early diagnosis and guided therapeutical strategies. POCUS protocols have been widely adopted among physicians across various specialties, demonstrating efficacy in emergency and intensive care settings.82,83 Consequently, developing a POCUS protocol specifically aimed at assessing TIA patients, capable of identifying the main underlying processes linked to TIA symptoms, holds significant promise for improving clinical care. TIA-POCUS, particularly suitable for implementation in TIA clinics, offers the advantage of a comprehensive evaluation, including both clinical and neurological assessments by expert neurologists and a focused neurosonological examination in a single visit. In this respect, tailoring the assessment based on the patient's symptoms and clinical profile enables the targeting of specific clinical questions.
The evaluation of extracranial and intracranial arteries by TCD and TCCD by a trained sonographer has widely demonstrated its potential to detect steno-occlusive lesions of large vessels.43,84 More recently, there is a growing body of evidence supporting the diagnostic value of FoCUS evaluation in stroke patients to rule out major cardiac embolic sources, which further improves traditional neurosonological assessments.85,86 In this regard, neurologist-driven FoCUS has shown a good correlation with standard cardiology examinations.87 Additional data, such as left atrial enlargement or significant valvulopathy, may provide decisive information in the diagnostic process of the patient.81,86
In addition to its portability, fast learning curve, and high availability, US is a safe and cost-effective imaging modality compared to alternatives such as magnetic resonance imaging and computed tomography. However, it is important to note that US diagnostic methods are operator-dependent, and the reliability and validity of the achieved results depend on the sonographer's skills and experience. Specifically, learning protocols and consensus are being developed to standardize the recommended requirements for acquiring the necessary technical skills as a sonographer.17,69 Ensuring adequate training and certification of the operator is essential to guarantee the highest quality and reliability of patient assessments. To this end, the Spanish Society of Neurosonology, in collaboration with the Spanish Society of Neurology and the Spanish Society of Cardiology, offers regular training and certification programs for sonologists, covering both standard neurosonological techniques and FoCUS.17
The incorporation of POCUS into routine clinical practice has the potential to provide physicians with immediate access to US examinations, thereby enhancing diagnostic efficiency and overall patient care. To ensure the validity of TIA-POCUS, seamless communication with cardiac imaging specialists is crucial, along with the availability of complementary diagnostic techniques to confirm suspicions derived from the initial assessment. Any abnormal finding on FoCUS should be confirmed by a standard echocardiogram.88
Compared to traditional management involving standard neuroimaging examinations, adopting a specific and standardized TIA-POCUS expedites the diagnostic process at the patient's bedside. However, to establish its clinical utility, further studies are needed to determine whether the implementation of TIA-POCUS leads to improved patient outcomes and is a cost-effective tool.
FundingThis work is part of the Spanish Health Outcomes-Oriented Cooperative Research Networks (RICORS-ICTUS), Instituto de Salud Carlos III (Carlos III Health Institute), Ministerio de Ciencia e Innovación (Ministry of Science and Innovation), RD21/0006/0010 (Torrecardenas University Hospital).
Conflict of interestThe authors have no conflicts of interest to declare.