Cognitive impairment, a clinical entity causing complete or partial intellectual dysfunction, is a major public health concern that poses a challenge for health and social services. The purpose of this study is to estimate the prevalence of this disorder in people aged 65 and older visiting the primary care physician in 5 health sentinel networks.
MethodA sample of patients visiting their primary care doctor on 4 randomly selected days completed the Mini-Cog screening test. Diagnosis of cognitive impairment was confirmed with the Mini-Mental State Examination and the Alzheimer's Questionnaire. We estimated raw and adjusted rates using demographic and social variables.
ResultsWe included 4624 patients from 5 autonomous communities and representing a population of 1723216 inhabitants. The adjusted prevalence rate was 18.5% (95% CI 17.3-19.7], with differences between sentinel networks. Women showed higher adjusted rates than men: 18.5 vs. 14.3%. The highest prevalence rate was observed in people aged 85 and older (45.3%); prevalence rates vary depending on education level and marital status.
ConclusionsCognitive impairment is a frequent reason for consultations in primary care. Its prevalence is higher in women and increases exponentially with age. A number of sensitive, validated tools have been proven useful in screening for and confirming cognitive impairment. Using these tools in primary care settings enables early treatment of these patients.
El deterioro cognitivo es una entidad clínica en la que las funciones intelectuales están parcial o totalmente alteradas y supone un problema de salud pública y un reto para los servicios sanitarios y sociales. El objetivo de este trabajo es estimar la prevalencia de estos trastornos en la población mayor de 65 años que consulta en atención primaria en 5 redes centinelas sanitarias.
MétodoSe realizó el test Mini-Cog de cribado de deterioro cognitivo en una muestra de pacientes que acudieron a su médico de familia en 4 días seleccionados al azar. La confirmación se hizo con el test Mini-Mental y el Alzheimer's Questionnaire. Se estimaron tasas brutas y ajustadas por las variables demográficas y sociales.
ResultadosSe estudiaron 4.624 pacientes, que representan a una población de 1.723.216 personas de 5 comunidades autónomas. La prevalencia ajustada para el conjunto de la población estudiada fue del 18,5% (IC 95% 17,3-19,7), con diferencias entre las redes centinelas. Las mujeres presentan tasas ajustadas significativamente más elevadas que los hombres: 18,5 y 14,3%, respectivamente. La prevalencia por grupo de edad alcanza el 45,3% por encima de los 85 años y presenta diferencias por nivel de estudios alcanzado y tipo de convivencia.
ConclusionesEl deterioro cognitivo es un motivo de consulta frecuente en atención primaria. Es mayor en mujeres y aumenta exponencialmente con la edad. Tanto la sospecha como la confirmación del deterioro cognitivo pueden realizarse por el médico de familia con instrumentos sensibles y validados, lo que permite iniciar un tratamiento precoz.
Cognitive impairment is a clinical entity associated with partial or total alteration of intellectual function (memory, reasoning, orientation, etc.) which develops throughout life, ranging from mild cognitive impairment (MCI) to full dementia (as in Alzheimer disease or other types of dementia).1
Cognitive impairment may be associated with other conditions, including depression, mood disorders, tumours, metabolic disorders, and cerebrovascular disease.
MCI is an intermediate state between age-dependent cognitive alterations and dementia, and has no significant impact on the activities of daily living. MCI is observable and persistent, and involves a more pronounced cognitive decline than expected for the patient's age and education level,2 but it is less severe than dementia.3
MCI is a common reason for primary care consultation, with an estimated prevalence of 15%-20% in people older than 60 years.4 A longitudinal study conducted in Leipzig reported a global incidence of MCI of 7.6% and found a significant association with older age, subjective memory complaints, difficulty with instrumental activities of daily living, and a history of reduced cognitive performance.5
Several studies conducted in Spain report prevalences ranging from 14.5% (range, 12.4%-16.8%)6 to 17.6% (range, 14.3%-20.9%)7 among people older than 65 years; prevalence increases with age. The DERIVA study reports a prevalence of 11.6% (range, 4.0%-19.1%) among individuals aged 65-69, increasing to 22.9% (range, 11.0%-34.8%) among those aged 85 years and older.8 A meta-analysis of 9 Spanish studies into different types of dementia, with considerable methodological differences, found a prevalence of 3.2%-12.3% in people older than 70 years; prevalence was higher among women and increased with age.9
Risk factors for MCI include older age, low level of education (illiteracy), and poor social life. MCI has also been associated with family history of the condition and with such genetic factors as mutations in the amyloid precursor protein gene and the ¿4 allele of the APOE gene. MCI has also been associated with high vascular risk,10 cerebral microbleeds,11 and with some endocrine and metabolic disorders and infections. Vascular disease and such neurological lesions as amyloid plaques, neurofibrillary tangles, and atrophy appear at older ages.12
Progression depends on the aetiology of cognitive impairment. Around 8%-15% of cases of MCI progress to dementia annually4,13 and over half of cases revert to a normal state, although some authors suggest that only 20% of patients recover normal cognitive function.14
Progression to dementia is associated with a low level of education,15 older age, and presence of multiple chronic diseases and depression,16 which is a frequent condition among elderly people.
Detecting MCI in certain age groups is especially relevant: increasing evidence suggests that early treatment may alter the natural history of dementia.17 Vascular risk prevention has been found to decrease the prevalence of dementia by 50% in people aged 65 and older. Vascular risk factors also promote the cognitive deficits observed in preclinical stages of cognitive impairment.11
MCI is detected by assessing memory, orientation, language, and ability to communicate. Multiple tools have been created for this purpose, ranging from such short tests as the Mini-Cog (3-word repetition, clock drawing, and 3-word recall)18 to complex assessment tools and interviews aiming both to diagnose cognitive impairment and to determine the underlying condition. One of these more complex tools is the Alzheimer's Questionnaire (AQ),19,20 which explores clinical symptoms known to have a high predictive value for detecting Alzheimer disease.
A recent systematic review of 149 studies identified a wide range of dementia screening tests, with the most widely used being the Mini-Mental State Examination (MMSE), with 81% sensitivity and 89% specificity.21 Among the short tests evaluated, the Mini-Cog and the Addenbrooke's Cognitive Examination-Revised had similar levels of sensitivity (91% and 92%, respectively) and specificity (86% and 89%).
The Gómez de Caso study aims to estimate the prevalence of cognitive impairment in people aged 65 years and older attending primary care consultations, to analyse the possible risk factors, and to establish Mini-Cog scores that are predictive of MCI, for use in primary care. This study presents only the main results obtained from the tests used in the 2 phases of the study; we estimate the prevalence of diagnosed and undiagnosed cognitive impairment in the study population.
Patients and methodsData were gathered from the Gómez de Caso study into the prevalence of acquired cognitive impairment and the factors associated with the condition. This descriptive observational study analysed data from the reference population and from patients aged 65 years and older who visited their primary care physicians for any reason during the study period. A total of 179 primary care physicians and nurses participated in the study. These came from the health sentinel networks of Castile-Leon, the autonomous city of Ceuta, Extremadura, the autonomous city of Melilla, and the Valencian Community, which use a common methodology and the organisation and functioning standards of health sentinel networks.22 The population assigned to these sentinel healthcare professionals is representative of the population covered by the healthcare system of the corresponding autonomous city or community in terms of age, sex, and type of area (urban/rural). The study was conducted between February 2014 and March 2015.
PatientsThe frame of reference included all patients aged 65 years and older who consulted with sentinel network physicians. We randomly selected 4 days per year (one day per trimester), and gathered data from a maximum of 15 patients per day. We also selected alternative days to avoid potential sample losses (physicians were absent or had no consultations scheduled, the day selected was a local public holiday, etc.) and sample selection biases (evening consultations); in these cases, we gathered data from patients attended the previous or the following day. We selected 4 additional days from different months to avoid holiday periods.
The reference population of the participating sentinel healthcare professionals included 45967 people aged 65 years or older, representing 2.7% of the over 1700000 people aged 65 years and older who live in the autonomous cities and communities included in the study.
Inclusion criteriaWe included all patients aged 65 years and older who, on the selected day, either:
- •
attended a consultation scheduled by the physician,
- •
attended a patient-requested consultation,
- •
had a home visit by the primary care physician, or
- •
consulted the primary care physician via a third party (requesting prescriptions, etc.).
All sentinel physicians used the same standard forms (health questionnaire) to gather patients’ clinical and epidemiological data, and the same cognitive assessments.
Sentinel physicians informed patients about the test and requested oral consent. They recorded patient refusals in the health questionnaire; this information was necessary for correctly calculating prevalence.
The study included 2 phases:
Phase 1: sentinel physicians completed the health questionnaires and evaluated patients with the Mini-Cog. The Mini-Cog is scored from 0 to 5, with scores below 3 indicating cognitive impairment.
Phase 2: to detect any degree of cognitive impairment, all patients with positive Mini-Cog results completed the MMSE (administered by the sentinel physician) and the AQ (completed by an informant).
The MMSE is scored from 0 to 30; scores below 24 are indicative of cognitive impairment.
The AQ is scored from 0 to 27; scores below 5 are considered negative, scores 5 to 14 are suggestive of cognitive impairment, and scores above 14 indicate Alzheimer disease.
In phase 2, diagnosis was confirmed either at the same consultation or during a subsequent visit (the patient was accompanied by the informant, who had to complete the AQ), as determined by the sentinel physician.
All data were recorded in a database (Epi Info) and validated by the coordinating centre of each health sentinel network and subsequently by the general coordinating centre.
Patients were considered to have cognitive impairment if they obtained positive Mini-Cog results and suspicion was confirmed by the MMSE or the AQ.
To calculate the prevalence of cognitive impairment in our sample, we also included the patients with a history of dementia who had not completed the Mini-Cog or obtained positive results in phase 2, given that patients with Alzheimer disease or other types of dementia were often unable to complete the tests. We also calculated crude prevalence for the total population, adjusting for each sentinel network's reference population.
We calculated prevalences and 95% confidence intervals by sentinel network, age, and sex using Poisson regression. Age and sex were included in the Poisson model to calculate prevalences by marital status, education level, and household structure. Statistical analysis was performed using SAS 9.3.
The study was approved by the research committee and the clinical research ethics committee of the west Valladolid health district.
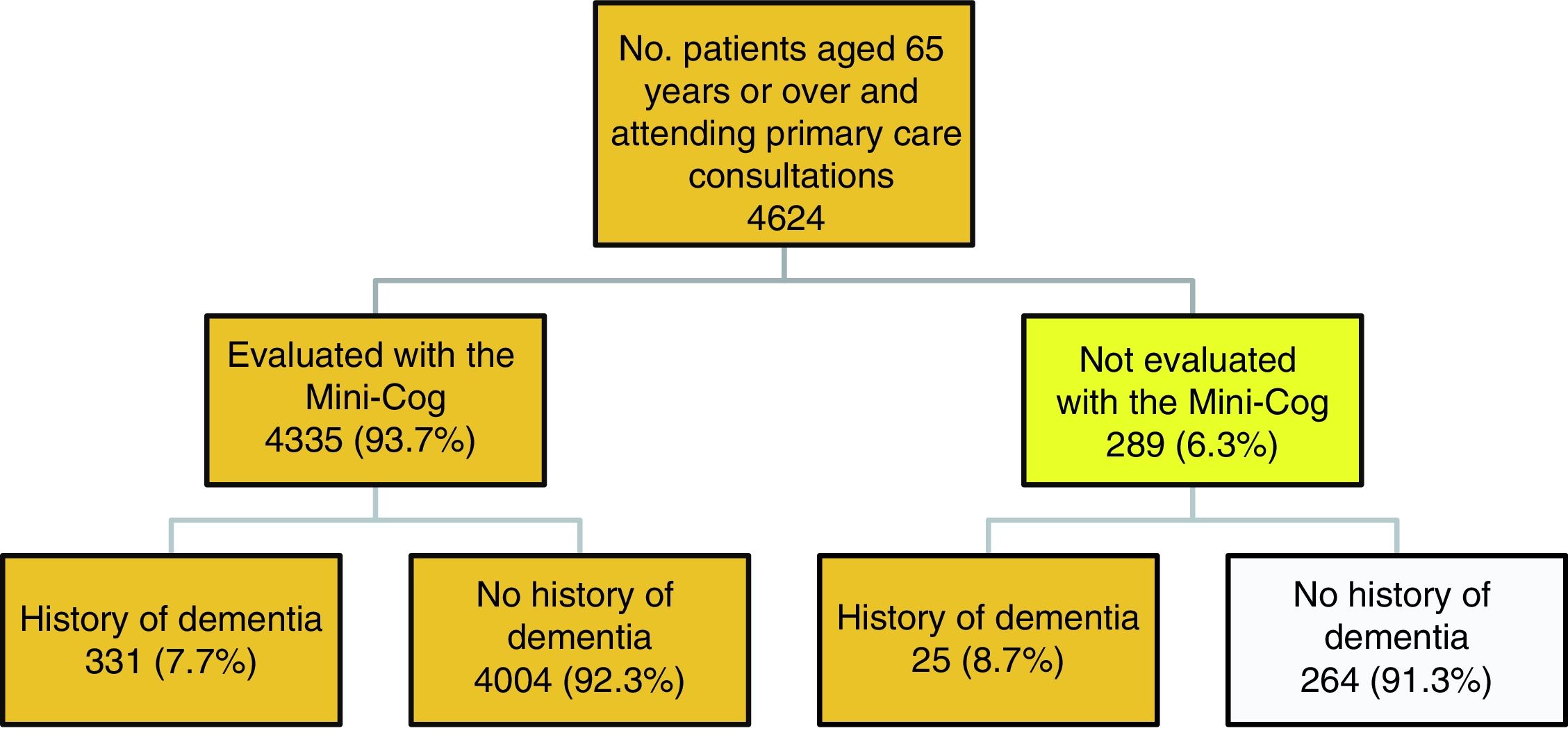
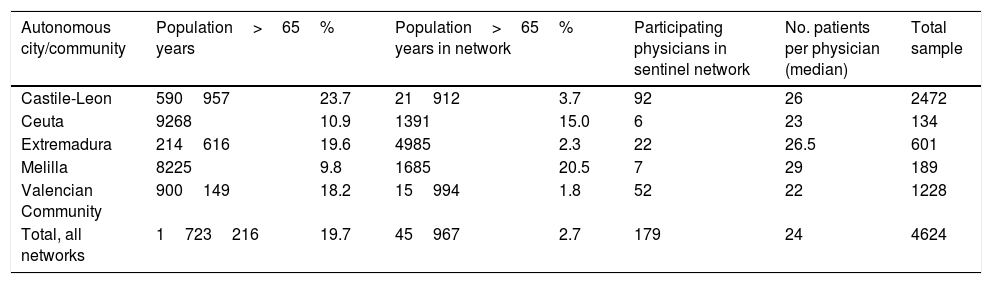
ResultsThe study included 4624 patients aged 65 years or older after excluding repeat visits by patients consulting on successive days. The distribution of our sample by sentinel network is heterogeneous, as we would expect from populations with different sizes and age structures. Table 1 shows the total population represented in this study (1723216 people): the percentage of patients aged 65 and older ranges from 23.7% in Castile-Leon to 9.8% in Melilla. Each sentinel physician recorded a mean of 24 cases, with little variation between sentinel networks.
Characteristics of the population, participants, and study sample from each health sentinel network.
| Autonomous city/community | Population>65 years | % | Population>65 years in network | % | Participating physicians in sentinel network | No. patients per physician (median) | Total sample |
|---|---|---|---|---|---|---|---|
| Castile-Leon | 590957 | 23.7 | 21912 | 3.7 | 92 | 26 | 2472 |
| Ceuta | 9268 | 10.9 | 1391 | 15.0 | 6 | 23 | 134 |
| Extremadura | 214616 | 19.6 | 4985 | 2.3 | 22 | 26.5 | 601 |
| Melilla | 8225 | 9.8 | 1685 | 20.5 | 7 | 29 | 189 |
| Valencian Community | 900149 | 18.2 | 15994 | 1.8 | 52 | 22 | 1228 |
| Total, all networks | 1723216 | 19.7 | 45967 | 2.7 | 179 | 24 | 4624 |
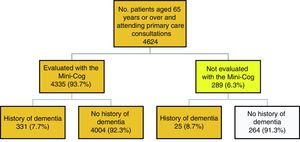
Fig. 1 describes the algorithm of patient selection. A total of 289 patients (6.3%) did not complete the Mini-Cog, this was due to severe dementia in 25 cases. The other reasons for not completing the test are as follows: the consultation was made by a relative (12.8%); the patient did not agree to participate (20.8%); the patient had physical health issues, such as blindness, being unable to move or leave bed, being admitted to hospital, or severe diseases rendering them unable to complete the test (19.6%); the patient had mental health problems (6.4%); and other reasons, including the patient being illiterate, not speaking Spanish, or being from a different health district (40.4%).
A total of 4360 people were eligible for inclusion in the study: 331 completed the evaluation and had a history of dementia, 4004 completed the evaluation and had no history of dementia, and 25 had a history of dementia but could not be evaluated with the Mini-Cog.
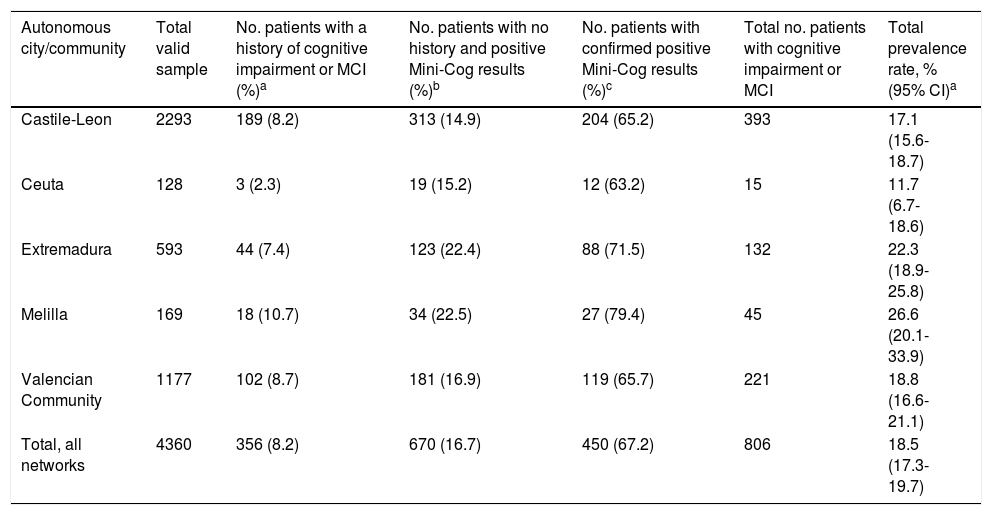
A total of 356 patients had a history of dementia or MCI, representing a known prevalence of 8.2% for the total sample, with marked differences between sentinel networks (Table 2). Among the patients with no history of dementia, 16.7% scored positive on the Mini-Cog, with Castile-Leon and Ceuta showing the lowest percentages (14.9% and 15.2%, respectively) and Melilla and Extremadura showing the highest percentages (22.5% and 22.4%, respectively). Cognitive impairment was confirmed with the MMSE or the AQ in 67.2% of cases. This percentage was higher in the sentinel networks with more patients obtaining positive Mini-Cog results (Melilla and Extremadura).
Prevalence of cognitive impairment in our sample, by autonomous city or community.
| Autonomous city/community | Total valid sample | No. patients with a history of cognitive impairment or MCI (%)a | No. patients with no history and positive Mini-Cog results (%)b | No. patients with confirmed positive Mini-Cog results (%)c | Total no. patients with cognitive impairment or MCI | Total prevalence rate, % (95% CI)a |
|---|---|---|---|---|---|---|
| Castile-Leon | 2293 | 189 (8.2) | 313 (14.9) | 204 (65.2) | 393 | 17.1 (15.6-18.7) |
| Ceuta | 128 | 3 (2.3) | 19 (15.2) | 12 (63.2) | 15 | 11.7 (6.7-18.6) |
| Extremadura | 593 | 44 (7.4) | 123 (22.4) | 88 (71.5) | 132 | 22.3 (18.9-25.8) |
| Melilla | 169 | 18 (10.7) | 34 (22.5) | 27 (79.4) | 45 | 26.6 (20.1-33.9) |
| Valencian Community | 1177 | 102 (8.7) | 181 (16.9) | 119 (65.7) | 221 | 18.8 (16.6-21.1) |
| Total, all networks | 4360 | 356 (8.2) | 670 (16.7) | 450 (67.2) | 806 | 18.5 (17.3-19.7) |
CI: confidence interval; MCI: mild cognitive impairment.
The total number of known cases plus cases confirmed in the study was 806, representing a crude prevalence rate of 18.5% (95% CI, 17.3-19.7) in our sample (18.7% after adjusting for the population size of each autonomous city or community). The highest rate was seen in Melilla (26.6%) and the lowest in Ceuta (11.7%).
By sex, the prevalence of cognitive impairment was 21.3% in women (95% CI, 19.7-23.0) and 14.8% in men (95% CI, 13.2-16.5). The prevalence of cognitive impairment increases with age, reaching maximum values at ages 85 and older (42.3%; 95% CI, 38.6-46.1).
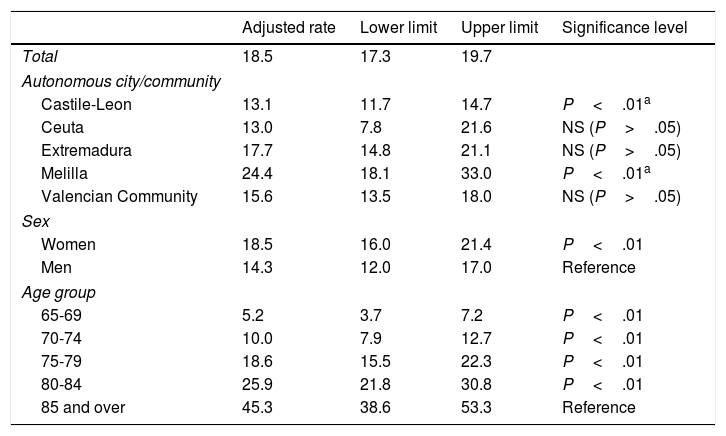
Table 3 shows the prevalence rates adjusted for autonomous city or community, sex, and age (Poisson model). The Melilla sentinel network continues to have the highest prevalence rate, at 24.4% (95% CI, 18.1-33.0), whereas the lowest corresponds to Ceuta, at 13.0% (95% CI, 7.8-21.6). Adjusted rates in the sentinel networks of Extremadura and especially Castile-Leon, at 13.1% (95% CI, 11.7-14.7), are lower than crude rates. Only the Melilla and Castile-Leon sentinel networks showed statistically significant differences from the mean prevalence observed for all autonomous cities and communities.
Prevalence rates for cognitive impairment, adjusted for autonomous city or community, sex, and age.
| Adjusted rate | Lower limit | Upper limit | Significance level | |
|---|---|---|---|---|
| Total | 18.5 | 17.3 | 19.7 | |
| Autonomous city/community | ||||
| Castile-Leon | 13.1 | 11.7 | 14.7 | P<.01a |
| Ceuta | 13.0 | 7.8 | 21.6 | NS (P>.05) |
| Extremadura | 17.7 | 14.8 | 21.1 | NS (P>.05) |
| Melilla | 24.4 | 18.1 | 33.0 | P<.01a |
| Valencian Community | 15.6 | 13.5 | 18.0 | NS (P>.05) |
| Sex | ||||
| Women | 18.5 | 16.0 | 21.4 | P<.01 |
| Men | 14.3 | 12.0 | 17.0 | Reference |
| Age group | ||||
| 65-69 | 5.2 | 3.7 | 7.2 | P<.01 |
| 70-74 | 10.0 | 7.9 | 12.7 | P<.01 |
| 75-79 | 18.6 | 15.5 | 22.3 | P<.01 |
| 80-84 | 25.9 | 21.8 | 30.8 | P<.01 |
| 85 and over | 45.3 | 38.6 | 53.3 | Reference |
NS: not significant.
Adjusting for sex reduced the differences between women (18.5%; 95% CI, 16.0-21.4) and men (14.3%; 95% CI, 12.0-17.0), although these continued to be statistically significant.
A statistically significant, age-dependent increase in the incidence of cognitive impairment was also observed after adjusting for autonomous city or community and sex: the adjusted rate nearly doubled every 5 years from the age of 65. All age groups displayed statistically significant differences with regard to the oldest age group.
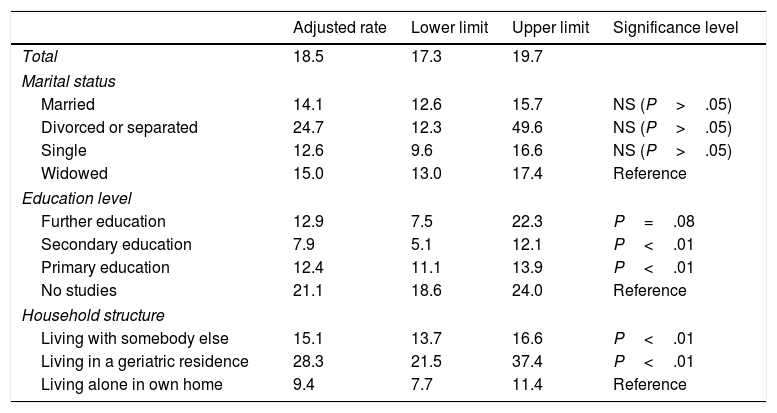
The Poisson model (Table 4) shows a high prevalence of cognitive impairment among people who are separated or divorced (24.7%), compared to singles (12.6%); however, these differences were not statistically significant after adjusting for age and sex. We observed statistically significant differences between patients with no studies (21.1%) and those with primary or secondary studies; the differences between patients with no studies and those with further education were on the verge of significance. As may be expected, patients living alone in their homes showed a significantly lower prevalence of cognitive impairment than those living in geriatric residences (28.3%; 95% CI, 21.5-37.4) or with somebody else (15.1%; 95% CI, 13.7-16.6); these differences are statistically significant.
Prevalence rates for cognitive impairment, adjusted for marital status, education level, and household structure.
| Adjusted rate | Lower limit | Upper limit | Significance level | |
|---|---|---|---|---|
| Total | 18.5 | 17.3 | 19.7 | |
| Marital status | ||||
| Married | 14.1 | 12.6 | 15.7 | NS (P>.05) |
| Divorced or separated | 24.7 | 12.3 | 49.6 | NS (P>.05) |
| Single | 12.6 | 9.6 | 16.6 | NS (P>.05) |
| Widowed | 15.0 | 13.0 | 17.4 | Reference |
| Education level | ||||
| Further education | 12.9 | 7.5 | 22.3 | P=.08 |
| Secondary education | 7.9 | 5.1 | 12.1 | P<.01 |
| Primary education | 12.4 | 11.1 | 13.9 | P<.01 |
| No studies | 21.1 | 18.6 | 24.0 | Reference |
| Household structure | ||||
| Living with somebody else | 15.1 | 13.7 | 16.6 | P<.01 |
| Living in a geriatric residence | 28.3 | 21.5 | 37.4 | P<.01 |
| Living alone in own home | 9.4 | 7.7 | 11.4 | Reference |
NS: not significant.
Mild cognitive impairment and dementia constitute a major public health problem and a challenge for the healthcare system. The prevalence of these conditions is expected to increase exponentially in the coming years, with the current prevalence rate expected to triple by 2050, increasing from the 35.6 million patients currently diagnosed with these conditions to 115.4 million patients in under 40 years. The available evidence suggests that dementia is an issue not only in developed countries, but also in developing countries, where prevalence rates are increasing considerably.23
The overall prevalence of cognitive impairment in our study (18.5% of patients aged 65 and older attending primary care consultations) is higher than that estimated by Luck et al.5 in Leipzig and by Gavrila et al.6 in Spain, and similar to the prevalence rates reported by the DERIVA study8 and by Petersen4 for MCI. The significant age-dependent increase in the prevalence of cognitive impairment and the higher prevalence among women found in our study are also consistent with the literature.9 According to our results, prevalence is 8 times higher in the 65-69 age group than in the group of patients aged over 85. The prevalence of cognitive impairment in women is 29% higher than in men.
One limitation of our study is that estimations are based on a sample of patients attending primary care consultations, rather than on the general population. However, the fact that individuals aged over 65 years attend primary care consultations over 5 times per year24 means that nearly all individuals of advanced age consult with the primary care physician at least once, and would therefore fall within the sampling frame. We paid particular attention to the days selected for data gathering in order to represent all patterns of consultation seen throughout the year. The distribution of our sample by day selected was homogeneous: 28.6% of the sample was drawn from day 1, 28.1% from day 2, 22.0% from day 3, and 21.3% from day 4. All participating physicians recorded a similar number of consultations, ranging from 22 to 29. Although our study only includes data from 3 autonomous communities and 2 autonomous cities, the geographic, social, economic, and cultural diversity of over 1.7 million patients aged 65 years and over guarantees the heterogeneity of the study population, which is likely to be similar to a great extent to a larger sample of the Spanish population.
To determine the number of patients with a history of dementia, we reviewed the reasons for not completing the test or participating in the study. We confirmed that the physician had no data on the type of dementia for those cases where he or she had reported no history of dementia. We also checked whether information on type of dementia was available for those cases where the physician had reported a history of dementia.
Brief cognitive assessment in primary care is a challenge as it requires adaptation and validation of tools for the study population and the use of tests with transcultural validity and adequate sensitivity for detecting MCI.25
Given the lack of a gold standard tool for detecting cognitive impairment, assessment is performed with short tests, such as the Mini-Cog, which provide a quick, preliminary diagnosis to be confirmed in subsequent, more thorough examinations, using such tools as the AQ. The latter test evaluates the patient's clinical symptoms and has high sensitivity and specificity for detecting Alzheimer disease (98% and 96%, respectively) and MCI (87% and 94%, respectively).19 Primary care physicians have traditionally used Lobo's Spanish-language version of the MMSE, which was used in phase 2 of our study; this test has been criticised for being highly dependent on patients’ level of education. Shorter tests, such as the Mini-Cog, which mainly assess memory and language, are currently available.26,27
The Mini-Cog, used in phase 1 of the study, requires only basic language skills and can therefore be used with patients of any language, ethnicity, and socioeconomic status. Minimal interviewer training is required, which makes this test suitable for routine screening in primary care. The Mini-Cog includes 3 items assessing recall plus a clock-drawing test, which helps interpret unclear 3-item recall results; this tool is useful both for epidemiological studies and in clinical practice, and is able to detect moderate cognitive impairment and full dementia. In our study, 67.2% of the patients with positive Mini-Cog results were confirmed to have cognitive impairment after completing the MMSE and/or the AQ. It was not possible to determine whether the remaining 32.8% of this group were false positives or were at very early stages of disease progression, or whether the confirmation tests failed to detect cognitive impairment.
Indiscriminate screening is not free from controversy due to potential anxiety or depression caused by positive test results in people without dementia. On some occasions, as indicated by Panegyres et al.,17 a false positive result may cause the individual to lose his or her social status, employment, or driving license, and may also lead to stigmatisation, with the resulting loss of quality of life.
The annual rate of progression from MCI to dementia ranges from 8% to 15%. However, not all cases of MCI are due to Alzheimer disease; early detection may therefore result in early treatment of the underlying disease, increasing the patient's chances of recovering or at least maintaining cognitive function.4
This collaborative study of several Spanish healthcare sentinel networks demonstrates the major public health problem cognitive impairment poses for older populations such as that of Spain, as well as the critical role played by primary care physicians in early detection and treatment of the condition. Further studies should establish the most suitable procedure for early detection and confirmation of cognitive impairment, and for follow-up of patients with a high risk of progression to dementia.
FundingThis study was funded by the healthcare administrations involved.
Conflict of interestThe authors have no conflicts of interest to declare.
We would like to thank the patients included for agreeing to participate.
We also wish to thank the participating primary care physicians and nurses for their support, dedication, and commitment to the project.
We are also grateful to all the participants, technicians, administrative staff, and support staff involved in the management and coordination of the healthcare system in each autonomous community.
This study is called “Gómez de Caso study” in honour of the late Dr José Ángel Gómez de Caso, head of the epidemiology department of the Segovia regional service of health and social welfare. Dr Gómez de Caso played a central role in launching this project, defining the aims of the study, and designing the protocol. This study would not have been possible without his dedication and enthusiasm.
Please cite this article as: Vega Alonso T, Miralles Espí M, Mangas Reina JM, Castrillejo Pérez D, Rivas Pérez AI, Gil Costa M, et al. Prevalencia de deterioro cognitivo en España. Estudio Gómez de Caso en redes centinelas sanitarias. Neurología. 2018;33:491–498.