The purposes of this study were to describe our 16-month experience with onabotulinumtoxinA (OnabotA) for the treatment of chronic migraine (CM) in the Spanish province of Segovia, evaluate its benefits, and determine clinical markers of good response to treatment.
Patients and methodsProspective study of patients with CM who received OnabotA for 16 months. The effectiveness of OnabotA was evaluated based on the reduction in the number of headache days, pain intensity, and side effects. We used two-way analysis of variance to assess the effects of treatment according to the time factor. We studied the correlation between treatment effects and other variables using a linear regression model to establish the clinical markers of good response to treatment.
ResultsWe included 69 patients who met the diagnostic criteria for CM. Patients underwent an average of two infiltrations. Mean age was 43 years; 88.4% were women. The number of headache days and pain intensity decreased significantly (P<.005); improvements remained over time. We found a negative correlation between the reduction in pain intensity and the number of treatments before OnabotA.
ConclusionThe beneficial effects of OnabotA for CM continue over time. OnabotA is a safe and well-tolerated treatment whose use for refractory CM should not be delayed since early treatment provides greater benefits.
Describir la experiencia con la administración de toxina botulínica tipo A (OnabotA) en el tratamiento de la migraña crónica (MC) en Segovia durante 16 meses, evaluar su beneficio y buscar marcadores clínicos que sirvan para predecir una mejor respuesta al tratamiento.
Pacientes y métodosEstudio prospectivo de pacientes con MC que recibieron infiltraciones con OnabotA durante 16 meses. Se evaluó la eficacia de OnabotA comparando la reducción en el número de días de cefalea, en la intensidad y efectos adversos. Se comparó el efecto del tratamiento con el factor tiempo mediante un análisis de la varianza de dos vías (ANOVA). Se estudió la correlación del efecto del tratamiento con el resto de las variables mediante un modelo de regresión lineal para buscar marcadores clínicos que sirvan para predecir una mejor respuesta.
ResultadosSe incluyó a 69 pacientes que cumplían criterios de MC. Se les realizó una media de 2 infiltraciones. La edad media fue de 43 años, el 88,4% fueron mujeres. La frecuencia de los días de cefalea y su intensidad se redujeron de forma significativa (p<0,005) y esta mejoría se mantuvo a lo largo del tiempo. Se encontró una correlación negativa entre la reducción de la intensidad y el número de tratamientos previos a la administración de la toxina.
ConclusiónEl efecto beneficioso de la OnabotA en la MC se mantiene en el tiempo, siendo un tratamiento seguro y bien tolerado. No debe retrasarse su uso en MC refractaria, ya que su beneficio podría ser mayor cuanto antes se administre.
Chronic migraine is an extremely complex and severely disabling neurological disorder which causes a profound impact on patients’ quality of life.1 The third edition of the International Classification of Headache Disorders (ICHD-3) defines chronic migraine as migraine occurring on at least 15 days per month for more than 3 months, which has the features of migraine headache with or without aura on at least 8 days per month or responds to migraine treatment. The complex pathogenic mechanisms of chronic migraine affect multiple sensory pathways, emotional networks, autonomic systems, and cortical functions. The condition has a prevalence of 1.4-2.2%.3
The use of botulinum toxin type A (OnabotA) for prophylaxis has considerably changed the management of chronic migraine. The efficacy of this treatment for chronic migraine was confirmed by the results of the PREEMPT programme. The programme demonstrated the efficacy of OnabotA in decreasing the frequency of headache days per month, reducing medication use, and improving quality of life. The PREEMPT trials also showed the treatment's safety and tolerability and the low incidence of associated adverse reactions.4–6 Subsequent studies have confirmed the efficacy, safety, and long-term effect of the drug, as well as its positive impact on quality of life and healthcare cost savings.7,8
Although the analgesic effect of OnabotA is not fully understood, the drug is thought to act not only by inhibiting acetylcholine release at the presynaptic level, relaxing the pericranial muscles, but also through an effect on other pathways. OnabotA inhibits the release of nociceptive mediators such as calcitonin gene-related peptide, glutamate, and substance P from afferent peripheral nerve fibres. This, in turn, inhibits neurogenic inflammation and consequently peripheral nociceptive fibre sensitivity, reducing the transmission of peripheral pain signals to the central nervous system. This decreases central sensitisation, which is responsible for progression to chronic migraine.9,10
In this study, we describe our experience with OnabotA for the treatment of chronic migraine at Complejo Asistencial de Segovia over a period of 16 months. We evaluate the treatment's capacity to reduce pain intensity and frequency and attempt to identify possible clinical markers of good response to treatment.
Patients and methodsStudy designWe conducted a prospective study with a sample of patients aged 18 years and older who visited our hospital's neurology department between 1 October 2013 and 1 April 2015; all participants met ICHD-3 diagnostic criteria for chronic migraine.2
All patients received OnabotA injections according to the PREEMPT injection paradigm (155U in 31 sites) every 3-4 months for 16 months. We gathered patients’ demographic and clinical data, including age, sex, history of psychiatric disorders, previous preventive treatments, number of headache days per month, and pain intensity. Patients were asked to complete a headache diary, in which they recorded the number of headache days and rated pain intensity using the visual analogue scale (VAS). We also recorded the number of injections each patient received and any adverse reactions to OnabotA (type of reaction, duration, and outcome).
The effect of OnabotA was evaluated by comparing the response after each cycle with the patients’ baseline status (status before first injection), and based on the following parameters: change in the number of headache days, subjective improvement (VAS scores), and adverse reactions to OnabotA.
Statistical analysisStatistical analysis was performed using SPSS statistics software, version 15.0.
We used a two-way analysis of variance (ANOVA) to evaluate the effect of the treatment, comparing values at baseline and after each treatment period. Statistical significance was set at P<.006. We used linear regression to analyse the correlation between the effect of the treatment and the remaining variables in order to determine which clinical markers may help predict good treatment response in clinical practice.
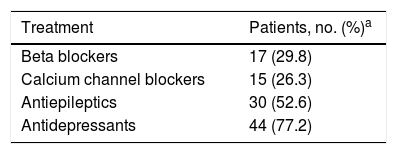
ResultsThe study included 69 patients meeting the diagnostic criteria for chronic migraine. The mean age was 43.2±15.2 years (range, 18-80); 88.4% were women. History of psychiatric disorders (including anxiety and depression) was present in 20.3% of participants; 82.6% had previously received treatment for chronic migraine. Table 1 summarises the treatments received previously to OnabotA injection; 12 patients (17.4%) had never received preventive treatment, 24 (34.8%) had received one, 21 (30.4%) had received two, 8 (11.6%) had received three, and 4 (5.8%) had received four treatments. Furthermore, eight patients (11.6%) had previously received botulinum toxin at a mean dose of 94.4±21.5U (95% CI, 76.4-112.3).
Distribution of preventive treatments received before OnabotA injection.
| Treatment | Patients, no. (%)a |
|---|---|
| Beta blockers | 17 (29.8) |
| Calcium channel blockers | 15 (26.3) |
| Antiepileptics | 30 (52.6) |
| Antidepressants | 44 (77.2) |
Patients in our sample received a mean of two OnabotA injections (range, 1-5). During the study period, 50 patients received at least two injections, with 28 receiving three and 14 receiving four. One patient who received 5 injections was not included in our study, since the last follow-up visit to assess the results of the fifth injection took place after the study period had concluded.
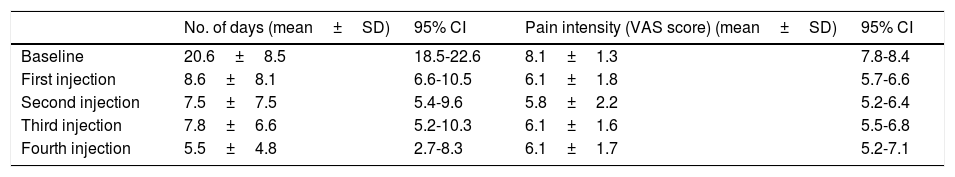
Our patients experienced 20.6±8.5 headache days per month at baseline. Frequency decreased to 8.6±8.1 headache days per month after the first injection, 7.5±7.5 after the second injection, 7.8±6.6 after the third injection, and to 5.5±4.8 after the fourth. At baseline, patients reported a pain intensity of 8.1±1.3 points on the VAS. Intensity decreased to 6.1±1.8 points after the first injection, 5.8±2.2 after the second, 6.1±1.6 after the third, and to 6.1±1.7 after the fourth. Changes in pain frequency and intensity are summarised in Table 2. The number of headache days per month decreased by 48.5%, and pain intensity by 20.7%.
Number of headache days per month and pain intensity after each injection.
| No. of days (mean±SD) | 95% CI | Pain intensity (VAS score) (mean±SD) | 95% CI | |
|---|---|---|---|---|
| Baseline | 20.6±8.5 | 18.5-22.6 | 8.1±1.3 | 7.8-8.4 |
| First injection | 8.6±8.1 | 6.6-10.5 | 6.1±1.8 | 5.7-6.6 |
| Second injection | 7.5±7.5 | 5.4-9.6 | 5.8±2.2 | 5.2-6.4 |
| Third injection | 7.8±6.6 | 5.2-10.3 | 6.1±1.6 | 5.5-6.8 |
| Fourth injection | 5.5±4.8 | 2.7-8.3 | 6.1±1.7 | 5.2-7.1 |
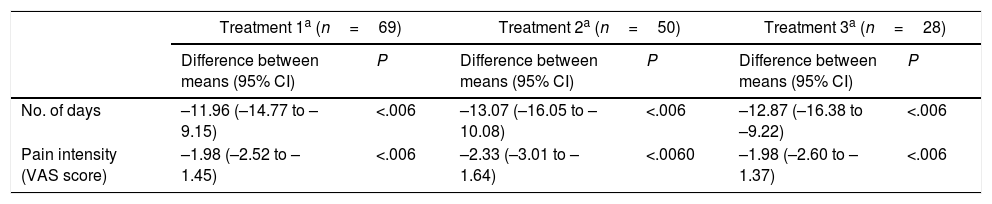
To evaluate the effect of the treatment in relation with time, we analysed the changes observed over the study period in the variables “number of headache days per month” and “pain intensity.” Both variables were observed to decrease significantly after treatment; decreases were uniform throughout the study period, with no significant differences between injections. These results are summarised in Table 3.
Effect of treatment with OnabotA over time.
| Treatment 1a (n=69) | Treatment 2a (n=50) | Treatment 3a (n=28) | ||||
|---|---|---|---|---|---|---|
| Difference between means (95% CI) | P | Difference between means (95% CI) | P | Difference between means (95% CI) | P | |
| No. of days | –11.96 (–14.77 to –9.15) | <.006 | –13.07 (–16.05 to –10.08) | <.006 | –12.87 (–16.38 to –9.22) | <.006 |
| Pain intensity (VAS score) | –1.98 (–2.52 to –1.45) | <.006 | –2.33 (–3.01 to –1.64) | <.0060 | –1.98 (–2.60 to –1.37) | <.006 |
The 28 patients who received three injections were analysed separately. In these patients, pain intensity and the number of headache days per month were observed to decrease significantly after treatment (sustained decrease over time, with no differences between the 3 injections; P<.005).
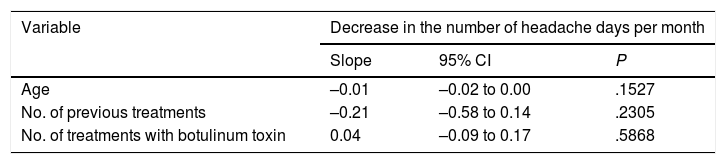
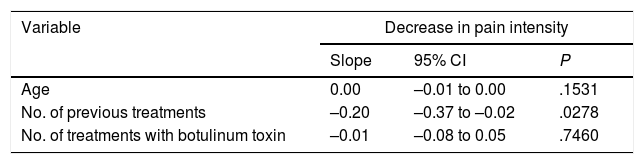
To evaluate the presence of markers of good treatment response, we analysed the correlation between the variable “decrease in the number of headache days per month” and the remaining variables using a linear regression model; we analysed the slope of the regression line. P values <.05 were considered statistically significant. We observed no association between the decrease in the number of headache days per month and any other variable (Table 4). However, we did find a negative correlation between the decrease in pain intensity and the number of treatments received previously (slope –0.20; 95% CI –0.37 to –0.02; P=.0278): the fewer treatments the patient had received before OnabotA injection, the greater the decrease in pain intensity (Table 5).
Correlation between the decrease in the number of headache days per month and the remaining variables.
| Variable | Decrease in the number of headache days per month | ||
|---|---|---|---|
| Slope | 95% CI | P | |
| Age | –0.01 | –0.02 to 0.00 | .1527 |
| No. of previous treatments | –0.21 | –0.58 to 0.14 | .2305 |
| No. of treatments with botulinum toxin | 0.04 | –0.09 to 0.17 | .5868 |
Over the study period, 26 patients were excluded for a number of reasons: 17 patients (24.63%) due to lack of improvement (after a mean of 2 injections), one due to pregnancy (after the second injection), 2 patients wishing to conceive (one after the second injection and the other after the third), 4 due to psychiatric disorders or cognitive impairment under evaluation (after the first injection), and 2 patients who were lost to follow up after the first injection.
Regarding adverse events, 3 patients experienced mild ptosis, resolving in less than a week in all cases.
DiscussionThe World Health Organization regards chronic migraine as one of the most disabling conditions.11,12
This complex disorder is characterised by persistent pain and resistance to preventive treatment, making it a severely disabling condition with a negative impact on patients’ quality of life.12 It is therefore essential to share clinical practice experiences with effective treatments. Studies into chronic migraine treatment should be designed with similar inclusion and treatment response criteria to avoid biases or non-conclusive data. Correct patient selection is key to ensure the success of botulinum toxin in treating chronic migraine.
OnabotA was approved in 2010 as a preventive treatment for chronic migraine in view of the results of the PREEMPT programme. The programme included 1354 patients, who received botulinum toxin injections every 12 weeks for 24 weeks, followed by a 32-week open-label phase. The PREEMPT programme, a multicentre, double-blind, placebo-controlled clinical trial, was conducted in 2 phases and demonstrated the efficacy, safety, and tolerability of OnabotA for preventing chronic migraine in adults.4–8,13
The present study is prospective and evaluates the effectiveness and tolerability of OnabotA in real-life conditions: in patients with chronic migraine visiting our neurology department. Following the PREEMPT injection paradigm, we injected patients with 155U of OnabotA at 31 sites distributed over the head and neck.13 We observed significant, sustained improvements: the number of headache days per month decreased by 48.5%, whereas pain intensity decreased by 20.7%. We measured pain intensity using the VAS, whereas the PREEMPT programme measured the number of days with severe headache pain and the condition's impact on the activities of daily living.
The demographic characteristics of our sample are similar, and therefore comparable, to those of the PREEMPT cohort6: our patients had a mean age of 43.2 years and were predominantly women (88.4%), vs a mean age of 41.1 years and 87.6% women in the PREEMPT cohort. In our study, and in line with the literature,13,14 chronic migraine was observed to be considerably more frequent among women.
The PREEMPT study included patients experiencing at least 20 headache days per month.6 Similarly, our patients experienced a mean of 20.6 headache days per month before starting treatment with OnabotA. In our study, the number of headache days decreased by 48.5%. The PREEMPT study evaluated treatment effectiveness differently, reporting a decrease of at least 50% in the number of headache days in 47.1% of patients.6
In line with the results of studies published in the last 2 years, which provide extensive data on clinical practice experience with this treatment, we not only achieved a significant decrease in the number of headache days per month after treatment with OnabotA, but also confirmed that treatment effect is maintained over time.10,14–18
A novel aspect of the present study is the correlation between clinical improvement due to OnabotA treatment and the number of previous treatments, whereby the sooner OnabotA is administered, the greater the benefits. Other recent studies have not identified clinical predictors of good response to OnabotA.19 Our results support early treatment with OnabotA for patients with refractory chronic migraine.
According to our findings and those of other studies,14,20,21 OnabotA has an excellent safety and tolerability profile. Only 3 patients in our sample developed mild ptosis (4.3%). This rate is similar to those reported by other studies, including the PREEMPT programme (3.3%).6 Furthermore, all of our cases resolved within a week. Our results also show that the benefits observed are maintained over time: successive injections increase the benefits achieved after the first session.22
OnabotA has been shown to be cost-effective and constitutes a promising preventive treatment option for chronic migraine.23,24
Our study does have certain limitations, including the small sample size and the lack of a control group, given that it includes a series of patients seen in clinical practice. The novel contribution of this study is the recommendation to administer OnabotA as early as possible, as its benefits for refractory chronic migraine may be greater with earlier administration.
ConclusionCorrect patient selection is key to ensuring the success of botulinum toxin for chronic migraine. The benefits of OnabotA for chronic migraine are maintained over time. This treatment shows a good safety and tolerability profile. For the greatest benefits, it should be administered as early as possible in cases of refractory chronic migraine.
FundingThis study was funded by the Foundation of the College of Physicians of Segovia.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Castrillo Sanz A, Morollón Sánchez-Mateos N, Simonet Hernández C, Fernández Rodríguez B, Cerdán Santacruz D, Mendoza Rodríguez A, et al. Experiencia con toxina botulínica en la migraña crónica. Neurología. 2018;33:499–504.
This study was presented as an oral communication at the 67th Annual Meeting of the Spanish Society of Neurology, 2015.