The self-administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) scale is a tool designed to identify patients with pain with neuropathic features.
ObjectiveTo assess the validity and reliability of the Spanish-language version of the S-LANSS scale.
MethodsOur study included a total of 182 patients with chronic pain to assess the convergent and discriminant validity of the S-LANSS; the sample was increased to 321 patients to evaluate construct validity and reliability. The validated Spanish-language version of the ID-Pain questionnaire was used as the variable criterion. All participants completed the ID-Pain, the S-LANSS, and the Numerical Rating Scale for pain. Discriminant validity was evaluated by analysing sensitivity, specificity, and the area under the receiver-operating characteristic curve (AUC). Construct validity was assessed with factor analysis and by comparing the odds ratio of each S-LANSS item to the total score. Convergent validity and reliability were evaluated with Pearson's r and Cronbach's alpha, respectively.
ResultsThe optimal cut-off point for S-LANSS was ≥12 points (AUC=0.89; sensitivity=88.7; specificity=76.6). Factor analysis yielded one factor; furthermore, all items contributed significantly to the positive total score on the S-LANSS (P<.05). The S-LANSS showed a significant correlation with ID-Pain (r=0.734, α=0.71).
ConclusionThe Spanish-language version of the S-LANSS is valid and reliable for identifying patients with chronic pain with neuropathic features.
La escala autoadministrada de Evaluación de Signos y Síntomas Neuropáticos de Leeds (S-LANSS) es un instrumento diseñado para identificar a pacientes con dolor de características neuropáticas.
ObjetivoEvaluar la validez y fiabilidad de la versión española del S-LANSS.
MétodosSe incluyó un total de 182 pacientes con dolor crónico para evaluar la validez discriminante y convergente del S-LANSS, incrementándose la muestra hasta 321 pacientes para valorar la validez de constructo y la fiabilidad de la escala. Se utilizó como variable criterio la versión validada al español del ID-Pain. Todos los participantes cumplimentaron el cuestionario ID-Pain, el S-LANSS, y la Escala Numérica del Dolor. La validez discriminante se evaluó mediante el análisis del área bajo la curva de características operativas para el receptor, y la sensibilidad y especificidad. La validez de constructo se evaluó mediante un análisis factorial y mediante el análisis del odds-ratio de cada ítem del S-LANSS respecto a la puntuación total. La validez convergente y la fiabilidad se valoraron con la R de Pearson y el alfa de Cronbach respectivamente.
ResultadosEl punto de corte óptimo del S-LANSS fue ≥12 puntos (área bajo la curva=0,89; sensibilidad=88,7; especificidad=76,6). El S-LANSS presentó un factor y, además, cada ítem contribuyó significativamente a la puntuación total positiva del S-LANSS (p<0,05). El S-LANSS mostró una relación significativa con el ID-Pain (R=0,734) y un alfa de Cronbach de 0,71.
ConclusiónLa versión española del S-LANSS es válida y fiable para identificar pacientes con dolor crónico con características neuropáticas.
Pain is defined as an unpleasant sensory and emotional experience associated with real or potential tissue damage, or described in relation to that damage.1 It has a considerable impact on quality of life.2,3 We can distinguish acute from chronic pain; the latter constitutes a diagnostic challenge in terms of aetiology and pathophysiology.2 From a pathophysiological perspective, pain can be classified as nociceptive or neuropathic; neuropathic pain is defined as any pain caused by a lesion to or disease of the somatosensory nervous system.4
Although the prevalence of neuropathic pain is not known with precision, approximately 2 million people in Spain are thought to be affected.5 According to several recent studies, neuropathic pain may account for up to 25% of primary care consultations for chronic pain and 51% of consultations at pain units.6,7
Several diagnostic questionnaires, validated in different languages, are available for assessing neuropathic pain. Examples include the Douleur Neuropathique 4 (DN4) questionnaire, the PainDETECT scale, the ID-Pain questionnaire, and the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS).8–14 Bennett created the LANSS pain scale with the aim of identifying patients with pain with neuropathic characteristics; a validated Spanish-language version of the scale is available.13,15 The scale is not without limitations, as it requires a clinical examination to be performed by a physician, despite the sensory exploration comprising only 2 tests (allodynia, tested by stroking the affected area with cotton wool, and mechanical hyperalgesia, tested with a monofilament); it also increases the duration of patient examinations.16 Perhaps for this reason, Bennett also created the self-administered LANSS (S-LANSS).17 This scale addresses the limitations of the original LANSS: being a self-administered instrument, it does not need to be completed by a physician; it may also take less time to complete, as it includes the same number of items as the original scale.17
Different language versions of the S-LANSS have been demonstrated to be valid and reliable for diagnosing individuals with pain with neuropathic characteristics17–20; however, no validated Spanish-language version is currently available. Previously validated Spanish-language tools, such as the DN4,11 PainDETECT,14 and ID-Pain12 questionnaires, can be used to identify patients with pain with neuropathic characteristics. However, the S-LANSS has the following advantages: (1) the scale is self-administered, whereas the DN4 must be completed by a physician, which is also more time-consuming; (2) it has fewer items than the PainDETECT, thereby reducing administration time; and (3) unlike the ID-Pain, the S-LANSS contains items for self-assessment of allodynia and hyperalgesia, targeting the affected region, potentially enabling more accurate identification of the pathophysiology of the patient's pain.
The present study aimed to create a Spanish-language version of the S-LANSS scale and to demonstrate its validity and reliability for identifying patients experiencing chronic pain with neuropathic characteristics.
MethodsInstrumentsThe self-administered LANSS scaleThe S-LANSS is a self-administered scale for identifying pain with neuropathic characteristics.17 The instrument comprises 7 items, 5 addressing the patient's experience of pain in the previous week and 2 addressing clinical signs, in which the patient performs a self-examination to detect allodynia and hyperalgesia. All items elicit dichotomous responses (yes/no). Total score ranges between 0 and 24 points, with scores of 12 or higher indicating neuropathic pain.
Description of the ID-PainThe ID-Pain questionnaire is a 6-item self-administered questionnaire intended to distinguish nociceptive pain from pain with neuropathic characteristics. All items refer to the pain experienced in the last week and elicit dichotomous responses. Each affirmative answer scores 1 point, with the exception of item 6, which is worth –1 point; total score therefore ranges between –1 and 5 points. In the validated Spanish-language version of the ID-Pain, scores of 3 or higher are considered to indicate pain with neuropathic characteristics. The test also has acceptable psychometric properties (sensitivity: 0.81; specificity: 0.84).12
Description of the Numeric Pain Rating ScaleThe Numeric Pain Rating Scale (NPRS) was used to assess the perceived intensity of patients’ pain in the last week. Patients select a number from a scale from 0 to 10 that best fits the intensity of their pain. A score of 0 indicates “no pain,” whereas 10 indicates “worst possible pain.” The NPRS has been shown to be valid and reliable for measuring pain intensity.21,22
PatientsAll study participants were required to read an information sheet and sign an informed consent form. The following selection criteria were applied: (1) age over 18; (2) presence of chronic pain (progression longer than 3 months) scoring at least 3 on the NPRS; and (3) ability to read and understand Spanish. As a validated Spanish-language version of the ID-Pain questionnaire is already available, this test was also administered to all patients in order to diagnose whether pain had a neuropathic component; in the absence of consensus criteria for the diagnosis of neuropathic pain, we decided to use the ID-Pain scale as a reference test or criterion value.
Patients with cancer of unknown aetiology, comorbidities with potential to interfere in the diagnosis, and fibromyalgia were excluded from the study. We also excluded patients with any psychological condition or cognitive deficits or who were taking therapeutic or recreational drugs that, in the specialist's opinion, may compromise their comprehension of the S-LANSS scale.
Study designWe performed an observational, cross-sectional study with the participation of the Hospital Universitario La Paz (Madrid, Spain) pain unit and the Physiotherapy Department of the Miraflores Primary Care Centre (Alcobendas, Madrid, Spain). The project was approved by the Hospital Universitario La Paz Clinical Research Ethics Committee (registry number, HULP: PI-2072).
We sampled consecutive patients attending both centres. All patients meeting the selection criteria were initially diagnosed with nociceptive pain or pain with neuropathic characteristics, according to the criterion variable. Patients were then led to a separate room, where a researcher blinded to the diagnosis established with the ID-Pain administered the NPRS and invited them to complete (with no assistance of any kind) the following information: (1) demographic and anthropometric variables (age, sex, height, and weight) and (2) the S-LANSS.
Sample size was calculated in order to perform an adequate factor analysis, establishing a minimum of 10-15 patients per item (total sample size: 70-105 patients, as the scale comprises 7 items).23 As the S-LANSS scale is intended to distinguish between pain with and without neuropathic characteristics, we deemed it beneficial to include at least 70 patients with each type of pain, yielding a final sample size of at least 140 patients.
Linguistic adaptationDr Michael Bennett, who created the S-LANSS, authorised the translation of the questionnaire into Spanish. The linguistic adaptation of the S-LANSS was performed according to the conventional recommendations published by Wild et al.24 for the translation and cultural adaptation of questionnaires based on patient-reported outcomes measures. Firstly, the original English version of the S-LANSS was translated into Spanish by 2 independent, bilingual, native Spanish speakers, aiming to maintain the original meaning of each item and to use the clearest possible language. A panel of pain specialists (with proficiency in both languages) evaluated, approved, and merged both translations of the scale. Two bilingual, native English speakers who were unfamiliar with the original version of the scale then back-translated the S-LANSS into English. The expert panel then compared the 2 back-translations to the original scale, verifying that semantic equivalence was preserved. Finally, the Spanish translation of the S-LANSS was assessed for clarity and feasibility of use in a pilot study including 30 patients with chronic pain. The results of the pilot study were used as a basis to modify the instrument; this was the final step in the linguistic adaptation process. The definitive version used in the study can be consulted in the Appendix.
PsychometricsAnalysis of the scale's psychometric properties focused on validity and reliability. The analysis was performed as described below.
ValidityWe studied the following types of validity:
- –
Discriminant validity: this parameter was assessed by comparing the S-LANSS’ capacity to diagnose pain with neuropathic characteristics against the criterion variable (diagnosis established using the ID-Pain). Firstly, we evaluated the area under the receiver operating characteristic (ROC) curve in order to determine what proportion of patients were correctly classified. The largest possible value for this parameter is 1; therefore, diagnostic usefulness increases as the area under the ROC curve approaches 1. Diagnostic accuracy is considered to be high for values above 0.9, moderate between 0.71 and 0.9, and low between 0.51 and 0.7; values ≤0.5 are considered to depend on chance alone.25
After using the Youden index to establish the optimal cut-off point, we calculated the following measures of diagnostic accuracy for the resulting score: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (PLR), and negative likelihood ratio (NLR).
We also used the Cohen kappa coefficient to calculate the level of agreement between the diagnoses established by the ID-Pain and the S-LANSS, based on the previously calculated optimal cut-off point. According to the criteria established by Landis and Koch,26 the kappa coefficient was classified as follows: almost perfect agreement (>0.80), substantial agreement (>0.60 and ≤0.80), moderate agreement (>0.40 and ≤0.60), fair agreement (>0.20 and ≤0.40), and slight agreement (≤0.20).
- –
Construct validity: we performed an exploratory factor analysis with an Oblimin rotation to check the unidimensionality of the scale. We determined the number of factors to be extracted using the Kaiser criterion (with eigen value ≥1) and a scree plot.27 We used the Bartlett test to evaluate homogeneity of variance and the Kaiser–Meyer–Olkin (KMO) test to evaluate the suitability of the data for factor analysis. The Bartlett test of sphericity tests the hypothesis that the correlation matrix is an identity matrix; the result must therefore be <0.05.28 The KMO test measures the degree of multicollinearity, with values varying between 0 and 1 (0.9-1: marvellous, 0.8-0.9: meritorious, 0.7-0.8: middling, 0.6-0.7: mediocre, 0.5-0.6: miserable, and <0.5: unacceptable).29
We also analysed how each S-LANSS item was related with the criterion variable and with total S-LANSS score, establishing the odds ratio. This enabled us to confirm each item's contribution to the discriminative validity, construct validity, and total scale score.
- –
Convergent validity: we used the Pearson correlation coefficient to analyse the convergent validity between the S-LANSS and the ID-Pain scale. Pearson correlation coefficient values higher than 0.60 reflect a strong correlation; values between 0.30 and 0.60 reflect a moderate correlation, and values below 0.30 indicate a weak or very weak correlation.30
The Cronbach alpha test was used to determine the internal consistency of the S-LANSS. In order for a questionnaire to be considered internally consistent, the Cronbach alpha value must be >0.7.31
We also performed a descriptive analysis of our sample's demographic characteristics and pain intensity. Continuous variables are expressed as mean±standard deviation (SD) and 95% confidence intervals (CI); discrete variables are expressed as numbers (n) and percentages. Based on the central limit theorem, we decided to use parametric tests (normal distribution) for comparisons between groups, as the sample size in both groups was >30.32,33 The t-test was used for quantitative variables, and the χ2 test was used for qualitative variables, with a significance threshold of P<.05. Data analysis was performed using version 21.0 of the SPSS statistics software (SPSS Inc., Chicago, IL, USA).
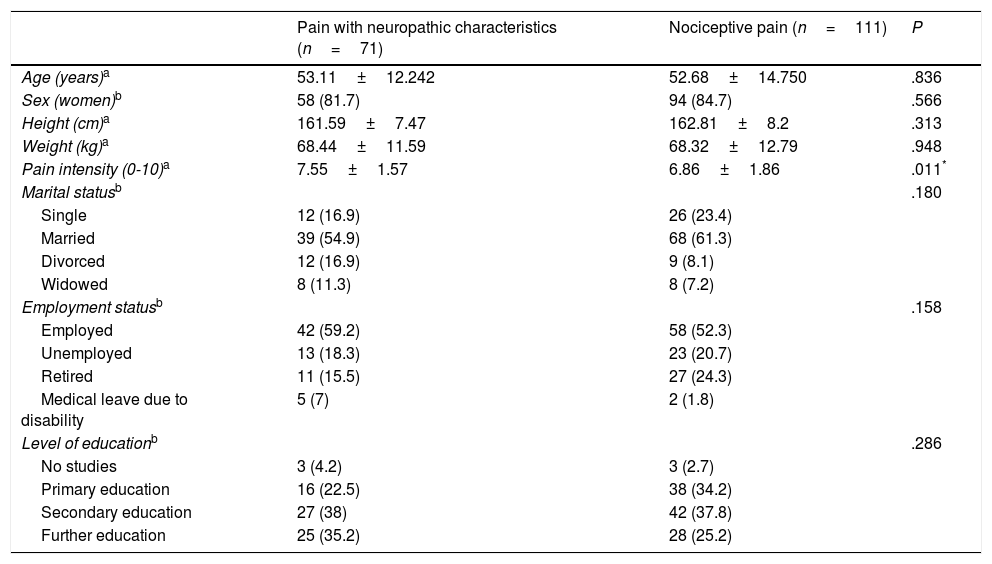
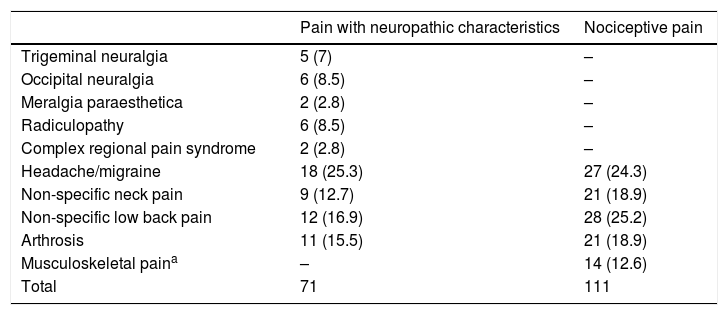
ResultsThe study included a total of 182 patients with chronic pain, with a mean age of 52.85±13.79 years and a larger proportion of women (83.5%). The ID-Pain was used to classify patients according to type of pain, with 71 assigned to the group of patients with pain with neuropathic characteristics, and 111 assigned to the group of patients with nociceptive pain. We considered the groups to be homogeneous, as no significant differences were observed between groups for sociodemographic variables. However, the group of patients with pain with neuropathic characteristics did experience higher pain intensity than the patients with nociceptive pain. Table 1 shows the clinical and demographic characteristics of each group. Pain aetiology is shown in Table 2. It should be noted that this sample (n=182) was used for the evaluation of discriminant validity, convergent validity, and some elements of construct validity (the relationship of each S-LANSS item with total ID-Pain score).
Data on sociodemographic characteristics and pain intensity for patients experiencing pain with and without neuropathic characteristics.
| Pain with neuropathic characteristics (n=71) | Nociceptive pain (n=111) | P | |
|---|---|---|---|
| Age (years)a | 53.11±12.242 | 52.68±14.750 | .836 |
| Sex (women)b | 58 (81.7) | 94 (84.7) | .566 |
| Height (cm)a | 161.59±7.47 | 162.81±8.2 | .313 |
| Weight (kg)a | 68.44±11.59 | 68.32±12.79 | .948 |
| Pain intensity (0-10)a | 7.55±1.57 | 6.86±1.86 | .011* |
| Marital statusb | .180 | ||
| Single | 12 (16.9) | 26 (23.4) | |
| Married | 39 (54.9) | 68 (61.3) | |
| Divorced | 12 (16.9) | 9 (8.1) | |
| Widowed | 8 (11.3) | 8 (7.2) | |
| Employment statusb | .158 | ||
| Employed | 42 (59.2) | 58 (52.3) | |
| Unemployed | 13 (18.3) | 23 (20.7) | |
| Retired | 11 (15.5) | 27 (24.3) | |
| Medical leave due to disability | 5 (7) | 2 (1.8) | |
| Level of educationb | .286 | ||
| No studies | 3 (4.2) | 3 (2.7) | |
| Primary education | 16 (22.5) | 38 (34.2) | |
| Secondary education | 27 (38) | 42 (37.8) | |
| Further education | 25 (35.2) | 28 (25.2) |
Results are expressed as mean±standard deviation or n (%).
Classification of chronic pain aetiology according to the criterion variable (ID-Pain).
| Pain with neuropathic characteristics | Nociceptive pain | |
|---|---|---|
| Trigeminal neuralgia | 5 (7) | – |
| Occipital neuralgia | 6 (8.5) | – |
| Meralgia paraesthetica | 2 (2.8) | – |
| Radiculopathy | 6 (8.5) | – |
| Complex regional pain syndrome | 2 (2.8) | – |
| Headache/migraine | 18 (25.3) | 27 (24.3) |
| Non-specific neck pain | 9 (12.7) | 21 (18.9) |
| Non-specific low back pain | 12 (16.9) | 28 (25.2) |
| Arthrosis | 11 (15.5) | 21 (18.9) |
| Musculoskeletal paina | – | 14 (12.6) |
| Total | 71 | 111 |
Values are expressed as n (%).
The construct validity and reliability of the S-LANSS scale were analysed with a larger sample size; we added 139 patients included in databases created prior to the study period. The databases used included patients with non-specific chronic neck pain. This resulted in a final sample of 321 patients with chronic pain for this analysis. Mean age in the sample was 48.85±14.94 years; 82.5% of patients were women.
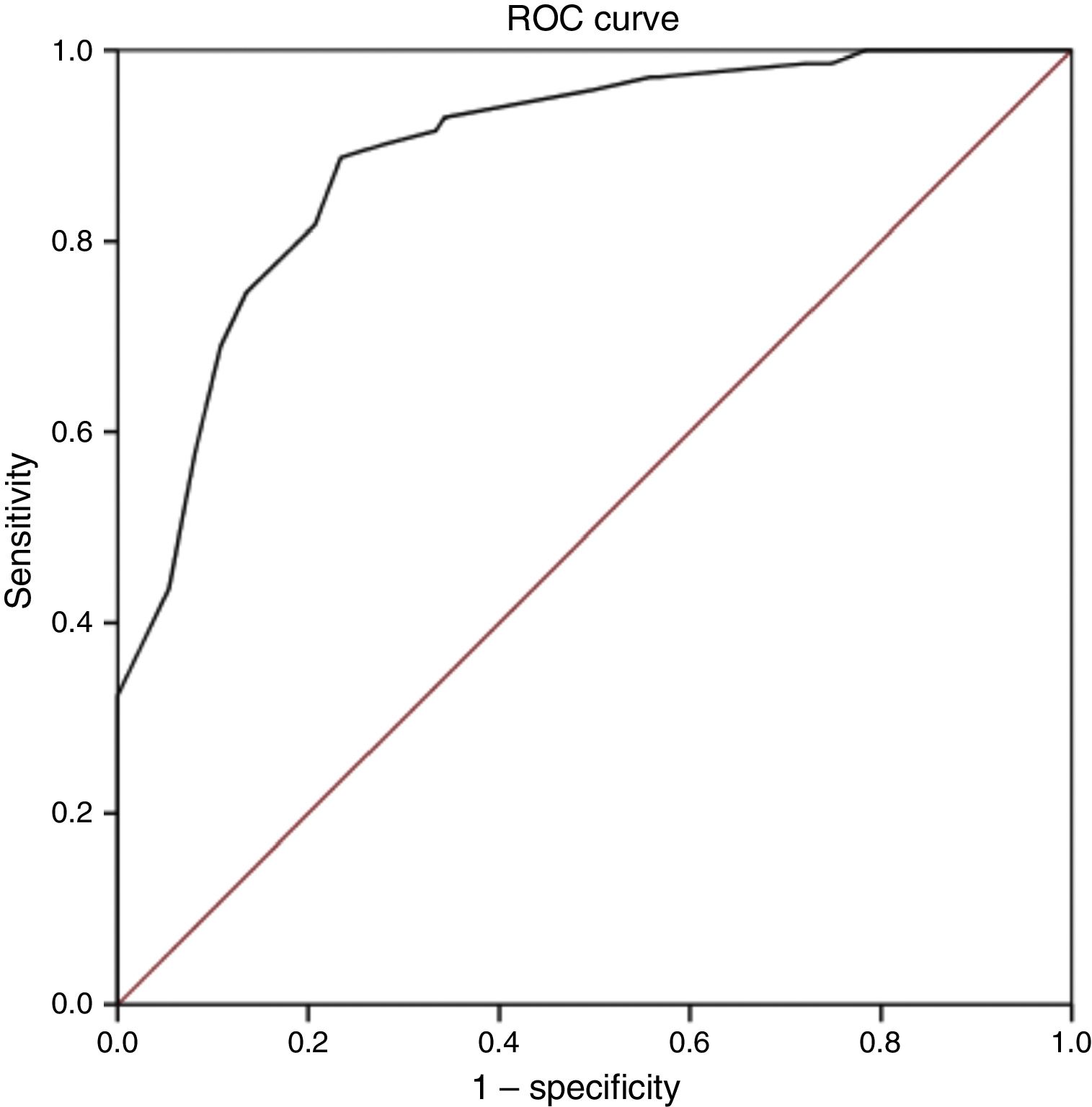
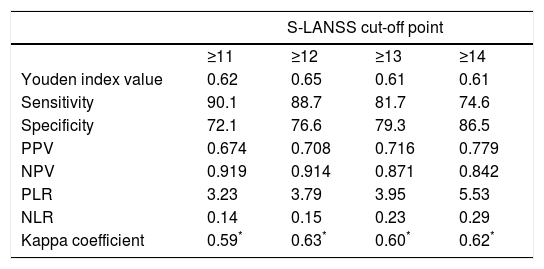
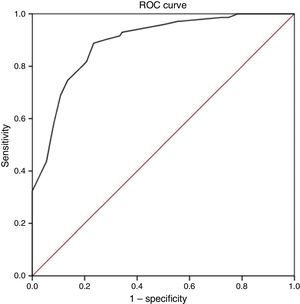
Discriminant validityThe ROC curve analysis showed that the optimal cut-off point, with the diagnosis established by the ID-Pain questionnaire as the criterion variable, was S-LANSS score ≥12. The analysis obtained a ROC value of 0.89 (95% CI, 0.85-0.94; P<.001; Fig. 1); while this denotes a moderate likelihood of correct diagnosis, it is very close to the threshold for high likelihood (>0.9). This cut-off point also showed the highest Youden index value, 0.65. This was the highest value found in the analysis and therefore reflects the highest values for sensitivity and specificity, at 88.7 and 76.6%, respectively. Finally, the level of agreement with diagnoses made using the ID-Pain scale, based on the cut-off point of S-LANSS score ≥12, was substantial, with a kappa index score of 0.63 (P<.001). Table 3 shows the values of measures of diagnostic accuracy for total S-LANSS scale score cut-off points with Youden index values >0.6.
Measures of diagnostic accuracy for the Spanish-language version of S-LANSS for cut-off points with Youden index values above 0.6.
| S-LANSS cut-off point | ||||
|---|---|---|---|---|
| ≥11 | ≥12 | ≥13 | ≥14 | |
| Youden index value | 0.62 | 0.65 | 0.61 | 0.61 |
| Sensitivity | 90.1 | 88.7 | 81.7 | 74.6 |
| Specificity | 72.1 | 76.6 | 79.3 | 86.5 |
| PPV | 0.674 | 0.708 | 0.716 | 0.779 |
| NPV | 0.919 | 0.914 | 0.871 | 0.842 |
| PLR | 3.23 | 3.79 | 3.95 | 5.53 |
| NLR | 0.14 | 0.15 | 0.23 | 0.29 |
| Kappa coefficient | 0.59* | 0.63* | 0.60* | 0.62* |
NLR, negative likelihood ratio; NPV, negative predictive value; PLR, positive likelihood ratio; PPV, positive predictive value.
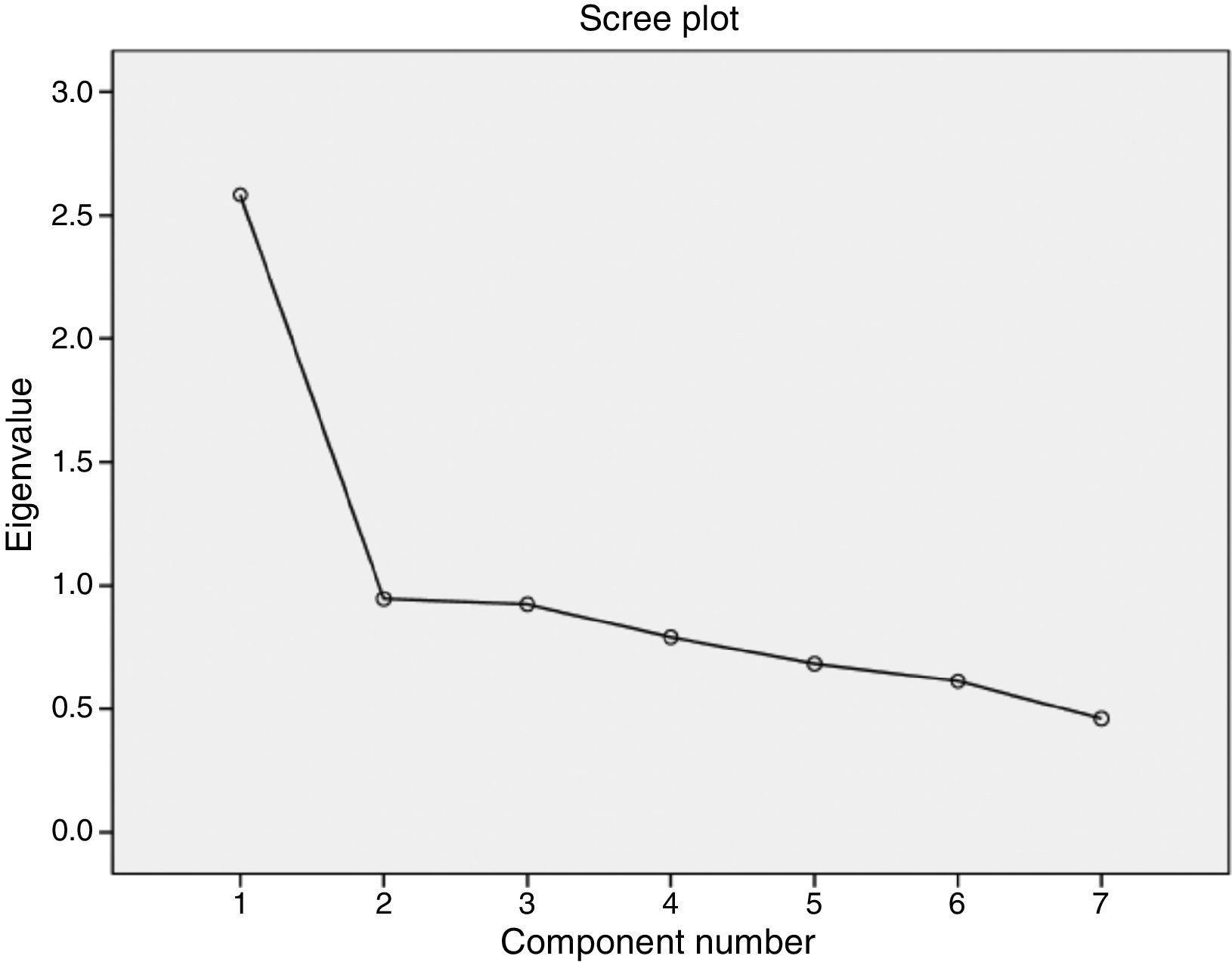
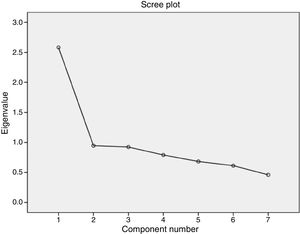
According to the exploratory factor analysis, the scale's factor structure comprised a single factor. The one-factor solution was established by means of a principal components analysis with an Oblimin rotation, which explained 36.9% of variance; however, this rotation was not applied as there was only one factor. The results of the Bartlett test of sphericity and the KMO test demonstrated the importance of factor analysis.34 Specifically, the Bartlett test result was highly significant (chi-square=337.881; P<.001), whereas the KMO test returned a value of 0.774, which denotes middling multicollinearity. Finally, the determination of a single factor was supported visually by the scree plot (Fig. 2). Table 4 shows the factor loadings of the different items, according to the exploratory factor analysis.
Factor loading of each item in the Spanish-language version of the S-LANSS according to an exploratory factor analysis based on principal component analysis.
| S-LANSS item | Single factor |
|---|---|
| 1. In the area where you have pain, do you also have “pins and needles,” tingling, or prickling sensations? | 0.591 |
| 2. Does the painful area change colour (perhaps look mottled or more red) when the pain is particularly bad? | 0.425 |
| 3. Does your pain make the affected skin abnormally sensitive to touch? Getting unpleasant sensations or pain when lightly stroking the skin might describe this | 0.610 |
| 4. Does your pain come on suddenly and in bursts for no apparent reason when you are completely still? Words like “electric shocks,” jumping, and bursting might describe this | 0.493 |
| 5. In the area where you have pain, does your skin feel unusually hot like a burning pain? | 0.655 |
| 6. Gently rub the painful area with your index finger and then rub a non-painful area (e.g., an area of skin further away or on the opposite side from the painful area). How does this rubbing feel in the painful area? | 0.756 |
| 7. Gently press on the painful area with your fingertip and then gently press in the same way onto a non-painful area (the same non-painful area that you chose in the last question). How does this feel in the painful area? | 0.661 |
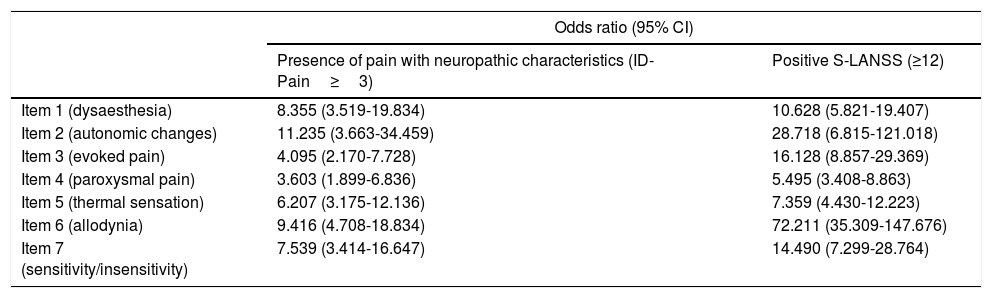
We independently evaluated each item's relationship with total S-LANSS score and with the criterion variable (ID-Pain). In order to calculate the odds ratio (95% CI) for detecting pain with neuropathic characteristics for each item with relation to total S-LANSS score, the total score was categorised as positive or negative according to the established cut-off point (≥12: positive; <12: negative). The results of this analysis confirmed each item's contribution to the total score for the scale, and construct and discriminant validity, as every item was significantly associated with positive total score for both S-LANSS and ID-Pain (Table 5).
Likelihood of detecting pain with neuropathic characteristics for each S-LANSS item with respect to total scale score and to the criterion variable.
| Odds ratio (95% CI) | ||
|---|---|---|
| Presence of pain with neuropathic characteristics (ID-Pain≥3) | Positive S-LANSS (≥12) | |
| Item 1 (dysaesthesia) | 8.355 (3.519-19.834) | 10.628 (5.821-19.407) |
| Item 2 (autonomic changes) | 11.235 (3.663-34.459) | 28.718 (6.815-121.018) |
| Item 3 (evoked pain) | 4.095 (2.170-7.728) | 16.128 (8.857-29.369) |
| Item 4 (paroxysmal pain) | 3.603 (1.899-6.836) | 5.495 (3.408-8.863) |
| Item 5 (thermal sensation) | 6.207 (3.175-12.136) | 7.359 (4.430-12.223) |
| Item 6 (allodynia) | 9.416 (4.708-18.834) | 72.211 (35.309-147.676) |
| Item 7 (sensitivity/insensitivity) | 7.539 (3.414-16.647) | 14.490 (7.299-28.764) |
CI, confidence interval.
The Pearson correlation coefficient for total S-LANSS (not considering cut-off points) and ID-Pain scores was 0.734 (P<.001). This indicates a strong relationship between the 2 questionnaires, reflecting an appropriate level of convergent validity.
ReliabilityThe internal consistency of the scale was acceptable, with a Cronbach alpha value of 0.71 for the relationship between each item and the total score (95% CI, 0.66-0.76). This result demonstrates that the S-LANSS scale is reliable for classifying patients with chronic pain.
DiscussionThe aims of the present study were to validate the Spanish-language version of the S-LANSS and to verify the scale's reliability. Specifically, we analysed the tool's discriminant, construct, and convergent validity. Our results show that the scale is valid and reliable for clinical use in Spanish-speaking adults with chronic pain.
Several diagnostic techniques, including such highly advanced methods as laser-evoked potentials, are available for identifying patients with neuropathic pain.35 Despite advances in this field, there is not yet an accepted gold standard technique for diagnosing the condition,35 with diagnosis by a specialist being the most widely accepted criterion. Therefore, diagnostic questionnaires may assist clinicians in diagnosis and decision-making.36 The Spanish-language version of the S-LANSS provides Spanish-speaking clinicians with a new tool in the difficult task of diagnosing chronic pain. As the questionnaire is self-administered, clinical examination can be based on the patient's results for the scale; this may result in treatment approaches being better suited to the characteristics of the patient's pain. However, it should be stressed that no screening test can replace a specialist's diagnosis, based on clinical history, a physical examination, and complementary testing.
In the analysis of discriminant validity, we determined an optimal cut-off point for diagnosis of pain with neuropathic characteristics of 12 points or higher, indicating a moderate-to-high likelihood of correct diagnosis. This optimal cut-off point coincides with that found for previously validated versions of the scale,17–19 with the sole exception of the interview format version,17 which had an optimal cut-off point of 10 points or higher. This exception can be disregarded for our purposes, as we aimed to validate the self-administered version of the scale. The sensitivity and specificity values of the Spanish-language version of the S-LANSS were 88.7 and 76.6%, respectively; these values are higher than those reported in a recent systematic review on the capacity of questionnaires to detect neuropathic pain.8 That review did not include the validation of the Greek-language version of the S-LANSS, which was published subsequently.20 However, if we compare our values for the main measures of diagnostic accuracy to those obtained for validations of other language versions,17,18,20 we can observe that the Spanish-language version presents the highest level of sensitivity, 88.7%. We suspect that this higher value may be explained by the use of the ID-Pain questionnaire as the criterion variable, given the strong association between the 2 questionnaires; other S-LANSS validation studies use diagnosis as the criterion variable. The Spanish-language version of the scale had a specificity of 76.6%, which is very similar to the values determined for previously validated versions (76% for the original English-language version17 and 80% for the Turkish-language version18), with the exception of the Greek-language version (95.2%).20 The authors are unable to explain why the Greek-language of the S-LANSS had such a high value for specificity.
The results of the factor analysis reflect the existence of one factor and show that each item contributes to total scale score, with similar discriminant validity to that observed in other language versions, especially the original English-language version.17,18 Therefore, the presence of such clinical characteristics as allodynia and autonomic changes appears to be the most important variable in identifying pain with neuropathic characteristics.
The Spanish-language version of the S-LANSS shows an acceptable level of internal consistency, with a Cronbach alpha value of 0.71. This is consistent with the results observed for previously validated versions of the scale (original English version: 0.76,17 Arabic version: 0.72,19 Turkish version: 0.74,18 Greek version: 0.6720). The present study used the largest sample for this test, with a total of 321 patients.
Pain intensity was greater in patients with pain with neuropathic characteristics than in those whose pain did not have a neuropathic component. Reports on this subject in the literature are controversial, with some studies confirming this finding37–40 and others reporting no difference.17–19 It is also important to stress that some studies use the NPRS, whereas others use the visual analogue scale; this issue may play a role in the conflicting results reported by different studies. However, despite the discrepancy in the results reported, these differences were not clinically significant.41–45
Our study was not free of limitations. For the validation, we used the Spanish-language version of the ID-Pain questionnaire as the criterion variable for diagnosis of pain with neuropathic characteristics. The Special Interest Group on Neuropathic Pain of the International Association for the Study of Pain recommends establishing a diagnosis of neuropathic pain on the basis of clinical history, thorough clinical examination, and/or appropriate diagnostic testing to confirm or rule out the presence of a somatosensory system lesion.46 Future validation studies for the Spanish-language version of the S-LANSS should therefore use diagnosis by a specialist as the criterion variable: while no universal consensus criteria have yet been agreed for the diagnosis of neuropathic pain, this diagnosis is used in clinical decision-making and for sampling in clinical trials.47 Another limitation is that we did not evaluate test–retest reliability, which prevents us from knowing the scale's behaviour over time. Furthermore, our results cannot be extrapolated to patients with acute/subacute pain or to paediatric or adolescent patients, as our sample only included patients aged over 18 years old with chronic pain. Finally, we believe that despite the efforts to discriminate between the 2 types of pain, many patients may present mixed pain, which may have influenced our findings. The latter limitation could be minimised by using a visual analogue scale on which the specialist would indicate his/her level of certainty about the diagnosis; other studies have adopted this approach.17,48 We should also note the marked heterogeneity of our sample in terms of disease; many patients had mixed pain, such as low back or neck pain. Therefore, we may expect to observe different results if we were to use a sample whose pain was more clearly defined as neuropathic or nociceptive.
In conclusion, our study results demonstrate that the Spanish-language version of the S-LANSS is valid and reliable for identifying patients with chronic pain with neuropathic characteristics; we therefore recommend its use in clinical practice. There is a need for future research to compare the likelihood of correct diagnosis associated with this scale and with diagnosis by a specialist, and to assess test–retest reliability.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors are grateful to Dr María Ángeles Mangas Guijarro, Gema Yagüe de Antonio, and Dr Manuel Lara Lara for participating in the expert panel and for assisting in recruiting patients. We would also like to thank Dr Michael Bennett for creating the S-LANSS and for authorising the translation and validation of a Spanish-language version.
Please cite this article as: López-de-Uralde-Villanueva I, Gil-Martínez A, Candelas-Fernández P, de Andrés-Ares J, Beltrán-Alacreu H, La Touche R. Validación y fiabilidad de la versión española de la escala autoadministrada de Evaluación de Signos y Síntomas Neuropáticos de Leeds (S-LANSS). Neurología. 2018;33:505–514.