Brain microbleeds (BMB) are haemosiderin deposits contained within macrophages, which are displayed as hypointense images in some T2-weighted magnetic resonance imaging sequences. There are still many questions to be answered about the pathophysiology and clinical relevance of BMB.
DevelopmentWe conducted a literature review of the main epidemiological, clinical, and anatomical pathology studies of BMB performed in the general population, in patients at risk of or already suffering from a vascular disease, and in patients with cognitive impairment. We analysed the prevalence of BMB, risk factors, and potential pathophysiological mechanisms and clinical implications.
ConclusionsThe prevalence of BMB is highly variable (3%-27% in the general population, 6%-80% in patients with vascular risk factors or vascular disease, and 16%-45% in patients with cognitive impairment). BMB are associated with ageing, Alzheimer disease (AD), and in particular haemorrhagic or ischaemic cerebrovascular disease. The pathological substrate of BMB is either lipohyalinosis (subcortical BMB) or cerebral amyloid angiopathy (lobar BMB). BMB exacerbate cognitive impairment, possibly through cortical–subcortical and intracortical disconnection, and increase the risk of death, mostly due to vascular causes. BMB also increase the risk of cerebral haemorrhage, particularly in patients with multiple lobar BMB (probable cerebral amyloid angiopathy). Therefore, anticoagulant treatment may be contraindicated in these patients. In patients with lower risk of bleeding, the new oral anticoagulants and the combination of clinical and magnetic resonance imaging follow-up could be helpful in the decision-making process.
Las microhemorragias cerebrales (MHC) son depósitos de hemosiderina, fagocitados por macrófagos, que se visualizan como imágenes hipointensas en determinadas secuencias de adquisición T2 de resonancia magnética cerebral. Existen muchas incógnitas acerca de su fisiopatología y significado clínico.
DesarrolloRevisión bibliográfica de los principales estudios epidemiológicos, clínicos y anatomopatológicos de MHC en la población general, en pacientes con enfermedad o riesgo vascular y en pacientes con deterioro cognitivo. Descripción de la prevalencia, factores de riesgo, mecanismos fisiopatológicos y posibles implicaciones clínicas de las MHC.
ConclusionesLa prevalencia de las MHC es muy variable (3-27% en la población general, 6-80% en pacientes con enfermedad o riesgo vascular, 16-45% en pacientes con deterioro cognitivo). Las MHC se asocian a la edad, a la enfermedad de Alzheimer y, en particular, a la enfermedad vascular (hemorrágica o isquémica) cerebral. El sustrato patológico es la lipohialinosis (MHC subcorticales) o la angiopatía amiloide cerebral (MHC lobulares). Las MHC contribuyen al deterioro cognitivo, posiblemente a través de una desconexión córtico-subcortical e intracortical, y se asocian a una mayor mortalidad, especialmente de causa vascular. Las MHC aumentan el riesgo de sufrir hemorragia cerebral, especialmente en pacientes con múltiples MHC lobulares (probable angiopatía amiloide cerebral), por lo que el tratamiento anticoagulante podría estar contraindicado en estos pacientes. En pacientes con menor riesgo de sangrado, los nuevos anticoagulantes orales y la realización de un seguimiento combinado—clínico y mediante resonancia magnética—podrían ser útiles en la toma de decisiones.
Few neuroradiological phenomena have drawn so much attention as cerebral microbleeds (CMB), which were first described barely 2 decades ago. A frequent finding in brain magnetic resonance imaging (MRI) studies, CMBs cause alarm in patients and confusion in healthcare professionals. Are CMBs merely “innocent bystanders” in cerebrovascular disease (CVD), or do they play a role in symptom pathogenesis, prognosis, and treatment? On the basis of some common pathogenic mechanisms, CMBs are thought to constitute the pathophysiological connection or “missing link” between CVD and Alzheimer disease (AD) that may explain the frequent co-occurrence of these 2 entities.1
We conducted a literature review to gather data on the prevalence, localisation, and risk factors of CMBs, with a view to furthering our knowledge of the pathophysiology and the clinical and therapeutic implications of CMBs.
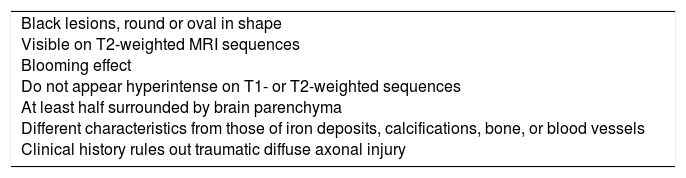
Definition and pathological substrateCMBs are foci of haemosiderin-laden macrophages which appear on T2-weighted MRI sequences as black lesions, round or oval in shape, measuring less than 10mm in diameter. The sequences used to detect CMBs, from most to least sensitive, are susceptibility weighted imaging (SWI), gradient-recalled echo-planar imaging (GRE-EPI), gradient-recalled echo (GRE), gradient echo (GE), and spin echo (SE). Sensitivity increases with accelerated high-spatial-resolution 3D imaging,2 greater magnetic field strength, greater section thickness, and reduced distance between sections.3 Diagnostic criteria for CMBs have changed over time; the criteria currently used were established by an expert group in 2009 (Table 1).4 Precisely defining CMB size has no impact on detection, although microbleed size is standardised at 5-10mm on T2-weighted GRE sequences.5
Diagnostic criteria for cerebral microbleeds.
| Black lesions, round or oval in shape Visible on T2-weighted MRI sequences Blooming effect Do not appear hyperintense on T1- or T2-weighted sequences At least half surrounded by brain parenchyma Different characteristics from those of iron deposits, calcifications, bone, or blood vessels Clinical history rules out traumatic diffuse axonal injury |
MRI: magnetic resonance imaging.
The first autopsy studies of CMBs were performed in patients who died due to intracerebral haemorrhage (ICH). According to these studies, vessels located near CMBs showed 2 distinct histopathological patterns: lipohyalinosis and cerebral amyloid angiopathy.6
Lipohyalinosis (also known as hypertensive microangiopathy or arteriolosclerosis) is a focal, segmental condition affecting vessel walls and associated with the traditional vascular risk factors, especially arterial hypertension (AHT). It is usually observed in small perforating arteries of the basal ganglia and deep white matter, probably due to the high blood pressure in these vessels. The basement membrane thickens, the blood-brain barrier (BBB) breaks down, and proteinaceous fibrinoid material accumulates under the tunica intima. More advanced stages are associated with vessel wall thickening, fibroblast proliferation, collagen deposition (with collagen replacing muscle cells, giving the appearance of a transparent vessel wall), and a progressive decrease in lumen size. Intramural deposition of lipids may increase; occasionally, false aneurysms (microaneurysms) appear due to lesions to the tunica media (Charcot-Bouchard miliary aneurysms).7,8
Cerebral amyloid angiopathy (CAA) is mainly associated with older age, but also with AD, and can follow an autosomal dominant inheritance pattern. The condition is characterised by β-amyloid (Aβ) deposition in the tunica media and adventitia of the small arteries and capillaries of the leptomeninges, cortex, and area between the grey and white matter. Aβ deposition reduces lumen size and vascular reactivity, and may lead to bleeding due to rupture of the vessel wall or microaneurysm formation.9 Brains with CAA and lobar ICH usually display haemosiderin-containing macrophages, or multiple small cortical haemorrhages.10 A recent prospective study of patients with CAA showed new CMBs in the areas with greater amyloid deposition.11
CMBs result from small blood extravasations secondary to rupture of the walls of small arteries, arterioles, or capillaries, whether the cause is lipohyalinosis, microaneurysm, or CAA. Lipohyalinosis causes CMBs in the basal ganglia, thalamus, brainstem, and cerebellum, whereas CAA causes CMBs in the cortex, periventricular and deep white matter, and cerebellum. Lipohyalinosis and CAA may co-occur, particularly in elderly patients; in these cases, CMBs usually present mixed lobar and deep distribution. CMBs and ICHs probably have a shared pathogenic mechanism. As is the case with CMBs, ICHs occur due to rupture of one or 2 small arteries, followed by secondary rupture of other arteries.7,12
CMBs and cerebral ischaemia are closely related. Autopsy studies show that microbleeds are usually associated with small areas of tissue necrosis combined with lipohyalinosis6,13,14 or CAA.15 Patients with Binswanger disease display CMBs inside ischaemic lesions, as well as ischaemic lesions surrounding CMBs.16
These findings, combined with a deeper knowledge of the mechanisms involved in BBB disruption, have led to a broader classification of CMBs17,18:
Primary CMBs result from the rupture of the walls of small arteries or arterioles (secondary to lipohyalinosis or CAA) or from alterations in components of the BBB in capillaries (tight junctions, basement membrane, pericytes, astrocytes).
Secondary CMBs result from haemorrhagic transformation of small ischaemic strokes.
EpidemiologyEpidemiological studies have shed light on the prevalence, localisation, and risk factors for CMBs, and deepened our knowledge on the pathophysiological mechanisms of the condition. The results of the main epidemiological studies into CMBs are summarised below.
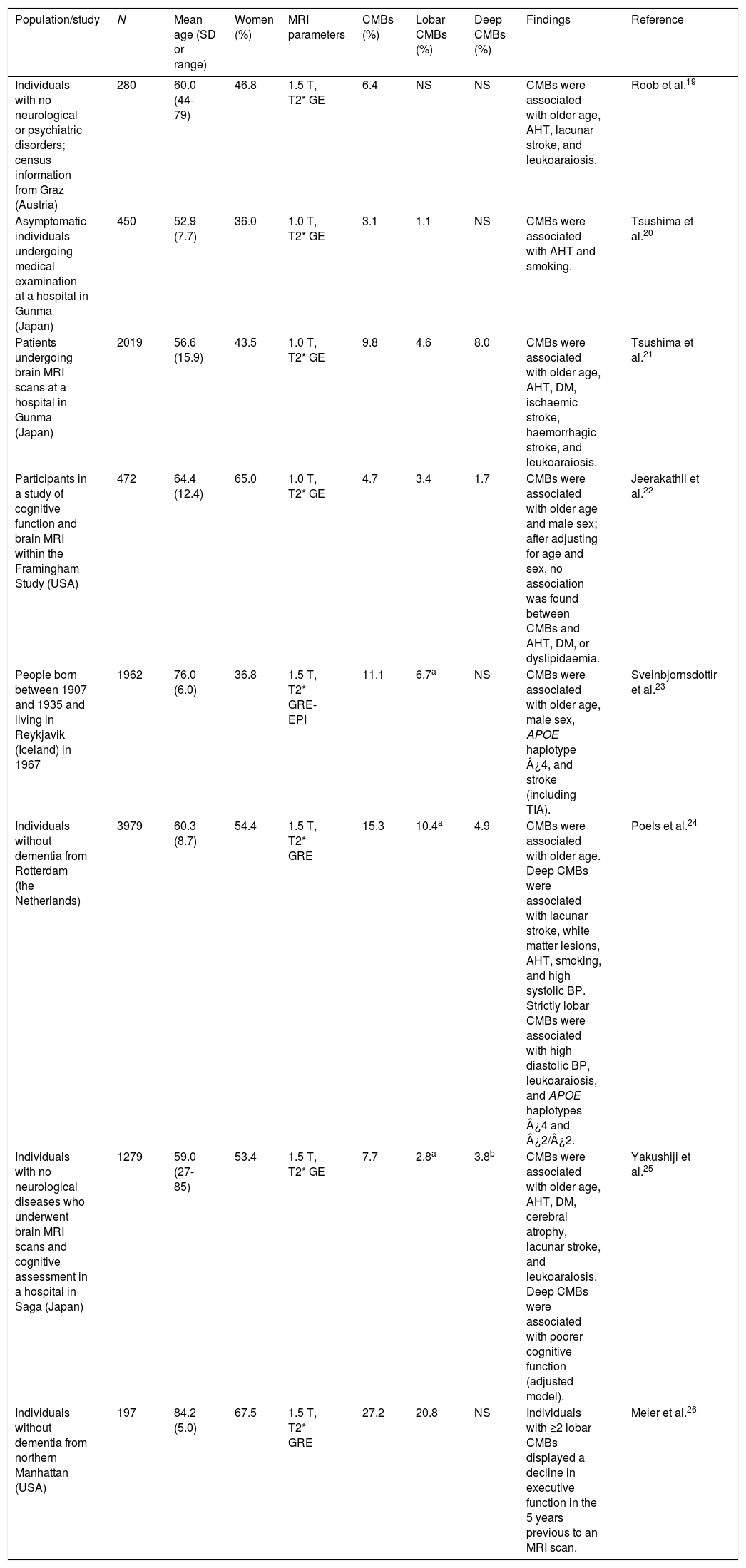
Population studiesPopulation studies and studies including healthy individuals report that the prevalence of CMBs ranges between 3% and 27%; this variability is explained by demographic heterogeneity and methodological differences in MR image acquisition and processing.19–26 CMBs have consistently been associated with ageing, AHT, and white matter lesions (leukoaraiosis). Weaker associations have been established between CMBs and such factors as male sex, smoking, diabetes mellitus, cognitive impairment, and haplotypes ¿4 and ¿2 of the apolipoprotein E gene (APOE) (Table 2).
Studies of cerebral microhaemorrhages in the general population or in healthy individuals.
| Population/study | N | Mean age (SD or range) | Women (%) | MRI parameters | CMBs (%) | Lobar CMBs (%) | Deep CMBs (%) | Findings | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Individuals with no neurological or psychiatric disorders; census information from Graz (Austria) | 280 | 60.0 (44-79) | 46.8 | 1.5 T, T2* GE | 6.4 | NS | NS | CMBs were associated with older age, AHT, lacunar stroke, and leukoaraiosis. | Roob et al.19 |
| Asymptomatic individuals undergoing medical examination at a hospital in Gunma (Japan) | 450 | 52.9 (7.7) | 36.0 | 1.0 T, T2* GE | 3.1 | 1.1 | NS | CMBs were associated with AHT and smoking. | Tsushima et al.20 |
| Patients undergoing brain MRI scans at a hospital in Gunma (Japan) | 2019 | 56.6 (15.9) | 43.5 | 1.0 T, T2* GE | 9.8 | 4.6 | 8.0 | CMBs were associated with older age, AHT, DM, ischaemic stroke, haemorrhagic stroke, and leukoaraiosis. | Tsushima et al.21 |
| Participants in a study of cognitive function and brain MRI within the Framingham Study (USA) | 472 | 64.4 (12.4) | 65.0 | 1.0 T, T2* GE | 4.7 | 3.4 | 1.7 | CMBs were associated with older age and male sex; after adjusting for age and sex, no association was found between CMBs and AHT, DM, or dyslipidaemia. | Jeerakathil et al.22 |
| People born between 1907 and 1935 and living in Reykjavik (Iceland) in 1967 | 1962 | 76.0 (6.0) | 36.8 | 1.5 T, T2* GRE-EPI | 11.1 | 6.7a | NS | CMBs were associated with older age, male sex, APOE haplotype ¿4, and stroke (including TIA). | Sveinbjornsdottir et al.23 |
| Individuals without dementia from Rotterdam (the Netherlands) | 3979 | 60.3 (8.7) | 54.4 | 1.5 T, T2* GRE | 15.3 | 10.4a | 4.9 | CMBs were associated with older age. Deep CMBs were associated with lacunar stroke, white matter lesions, AHT, smoking, and high systolic BP. Strictly lobar CMBs were associated with high diastolic BP, leukoaraiosis, and APOE haplotypes ¿4 and ¿2/¿2. | Poels et al.24 |
| Individuals with no neurological diseases who underwent brain MRI scans and cognitive assessment in a hospital in Saga (Japan) | 1279 | 59.0 (27-85) | 53.4 | 1.5 T, T2* GE | 7.7 | 2.8a | 3.8b | CMBs were associated with older age, AHT, DM, cerebral atrophy, lacunar stroke, and leukoaraiosis. Deep CMBs were associated with poorer cognitive function (adjusted model). | Yakushiji et al.25 |
| Individuals without dementia from northern Manhattan (USA) | 197 | 84.2 (5.0) | 67.5 | 1.5 T, T2* GRE | 27.2 | 20.8 | NS | Individuals with ≥2 lobar CMBs displayed a decline in executive function in the 5 years previous to an MRI scan. | Meier et al.26 |
AHT: arterial hypertension; APOE: apolipoprotein E gene; BP: blood pressure; CMB: cerebral microbleed; DM: diabetes mellitus; GE: gradient echo; GRE: gradient-recalled echo; GRE-EPI: gradient-recalled echo-planar imaging; MRI: magnetic resonance imaging; N: sample size; NS: not specified; SD: standard deviation.
The study with the largest sample (the Rotterdam scan study) differentiates between “strictly lobar microbleeds” (no associated deep CMBs) and “deep or infratentorial microbleeds”, confirming that the pathophysiological mechanism of CMBs depends on microbleed location. Lobar microbleeds were associated with high diastolic blood pressure and APOE haplotypes ¿4 and ¿2, whereas subcortical microbleeds were associated with lacunar strokes, smoking, and high systolic blood pressure.24
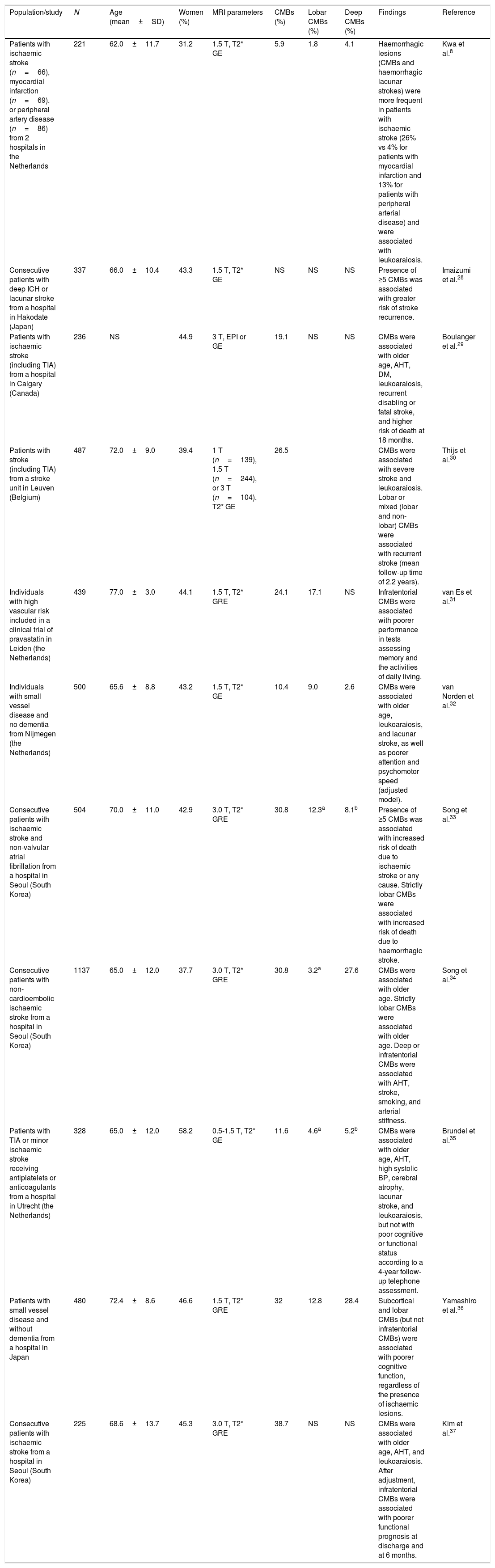
Studies of patients with vascular diseases or vascular riskThese studies have mainly included patients with CVD and report highly variable prevalence rates (6%-80%), normally higher than those obtained in population studies (Table 2). The highest prevalence rates were reported by a study including 15 elderly patients with lobar ICHs (80%)27 and another study including elderly patients with Binswanger disease (77%).16 The lowest prevalence rate (6%) was found in a study of patients with ischaemic stroke, myocardial infarction, and peripheral artery disease.8Table 3 summarises the characteristics and results of the studies with the largest samples.8,28–37 CMBs are usually more frequent in patients with ICHs than in those with ischaemic CVD,38 regardless of whether the ICH is lobar27,39 or subcortical.14 When CMBs and ICHs co-occur, they are usually located in the same regions,14,27,39 although deep CMBs are not rare among patients with lobar ICHs.40
Studies of cerebral microbleeds including patients with vascular diseases or vascular risk.
| Population/study | N | Age (mean±SD) | Women (%) | MRI parameters | CMBs (%) | Lobar CMBs (%) | Deep CMBs (%) | Findings | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Patients with ischaemic stroke (n=66), myocardial infarction (n=69), or peripheral artery disease (n=86) from 2 hospitals in the Netherlands | 221 | 62.0±11.7 | 31.2 | 1.5 T, T2* GE | 5.9 | 1.8 | 4.1 | Haemorrhagic lesions (CMBs and haemorrhagic lacunar strokes) were more frequent in patients with ischaemic stroke (26% vs 4% for patients with myocardial infarction and 13% for patients with peripheral arterial disease) and were associated with leukoaraiosis. | Kwa et al.8 |
| Consecutive patients with deep ICH or lacunar stroke from a hospital in Hakodate (Japan) | 337 | 66.0±10.4 | 43.3 | 1.5 T, T2* GE | NS | NS | NS | Presence of ≥5 CMBs was associated with greater risk of stroke recurrence. | Imaizumi et al.28 |
| Patients with ischaemic stroke (including TIA) from a hospital in Calgary (Canada) | 236 | NS | 44.9 | 3 T, EPI or GE | 19.1 | NS | NS | CMBs were associated with older age, AHT, DM, leukoaraiosis, recurrent disabling or fatal stroke, and higher risk of death at 18 months. | Boulanger et al.29 |
| Patients with stroke (including TIA) from a stroke unit in Leuven (Belgium) | 487 | 72.0±9.0 | 39.4 | 1 T (n=139), 1.5 T (n=244), or 3 T (n=104), T2* GE | 26.5 | CMBs were associated with severe stroke and leukoaraiosis. Lobar or mixed (lobar and non-lobar) CMBs were associated with recurrent stroke (mean follow-up time of 2.2 years). | Thijs et al.30 | ||
| Individuals with high vascular risk included in a clinical trial of pravastatin in Leiden (the Netherlands) | 439 | 77.0±3.0 | 44.1 | 1.5 T, T2* GRE | 24.1 | 17.1 | NS | Infratentorial CMBs were associated with poorer performance in tests assessing memory and the activities of daily living. | van Es et al.31 |
| Individuals with small vessel disease and no dementia from Nijmegen (the Netherlands) | 500 | 65.6±8.8 | 43.2 | 1.5 T, T2* GE | 10.4 | 9.0 | 2.6 | CMBs were associated with older age, leukoaraiosis, and lacunar stroke, as well as poorer attention and psychomotor speed (adjusted model). | van Norden et al.32 |
| Consecutive patients with ischaemic stroke and non-valvular atrial fibrillation from a hospital in Seoul (South Korea) | 504 | 70.0±11.0 | 42.9 | 3.0 T, T2* GRE | 30.8 | 12.3a | 8.1b | Presence of ≥5 CMBs was associated with increased risk of death due to ischaemic stroke or any cause. Strictly lobar CMBs were associated with increased risk of death due to haemorrhagic stroke. | Song et al.33 |
| Consecutive patients with non-cardioembolic ischaemic stroke from a hospital in Seoul (South Korea) | 1137 | 65.0±12.0 | 37.7 | 3.0 T, T2* GRE | 30.8 | 3.2a | 27.6 | CMBs were associated with older age. Strictly lobar CMBs were associated with older age. Deep or infratentorial CMBs were associated with AHT, stroke, smoking, and arterial stiffness. | Song et al.34 |
| Patients with TIA or minor ischaemic stroke receiving antiplatelets or anticoagulants from a hospital in Utrecht (the Netherlands) | 328 | 65.0±12.0 | 58.2 | 0.5-1.5 T, T2* GE | 11.6 | 4.6a | 5.2b | CMBs were associated with older age, AHT, high systolic BP, cerebral atrophy, lacunar stroke, and leukoaraiosis, but not with poor cognitive or functional status according to a 4-year follow-up telephone assessment. | Brundel et al.35 |
| Patients with small vessel disease and without dementia from a hospital in Japan | 480 | 72.4±8.6 | 46.6 | 1.5 T, T2* GRE | 32 | 12.8 | 28.4 | Subcortical and lobar CMBs (but not infratentorial CMBs) were associated with poorer cognitive function, regardless of the presence of ischaemic lesions. | Yamashiro et al.36 |
| Consecutive patients with ischaemic stroke from a hospital in Seoul (South Korea) | 225 | 68.6±13.7 | 45.3 | 3.0 T, T2* GRE | 38.7 | NS | NS | CMBs were associated with older age, AHT, and leukoaraiosis. After adjustment, infratentorial CMBs were associated with poorer functional prognosis at discharge and at 6 months. | Kim et al.37 |
AHT: arterial hypertension; APOE: apolipoprotein E gene; BP: blood pressure; CMB: cerebral microbleed; DM: diabetes mellitus; EPI: echo-planar imaging; GE: gradient echo; GRE: gradient-recalled echo; ICH: intracerebral haemorrhage; MRI: magnetic resonance imaging; N: sample size; NS: not specified; SD: standard deviation; SE: spin echo; TIA: transient ischaemic attack.
There is a strong relationship between presence of CMBs and poor cognitive function.31,32,36 CMBs have also been associated with poorer baseline31,37 and 6-month functional status in patients admitted to hospital for ischaemic stroke who also had infratentorial CMBs.37 Some researchers have also reported increases in stroke recurrence (ischaemic and haemorrhagic) in patients with CMBs.28,30 A study of patients with CAA found a greater risk of recurrence of lobar ICH in patients receiving antiplatelet drugs; this effect was more pronounced in patients with greater numbers of CMBs.39
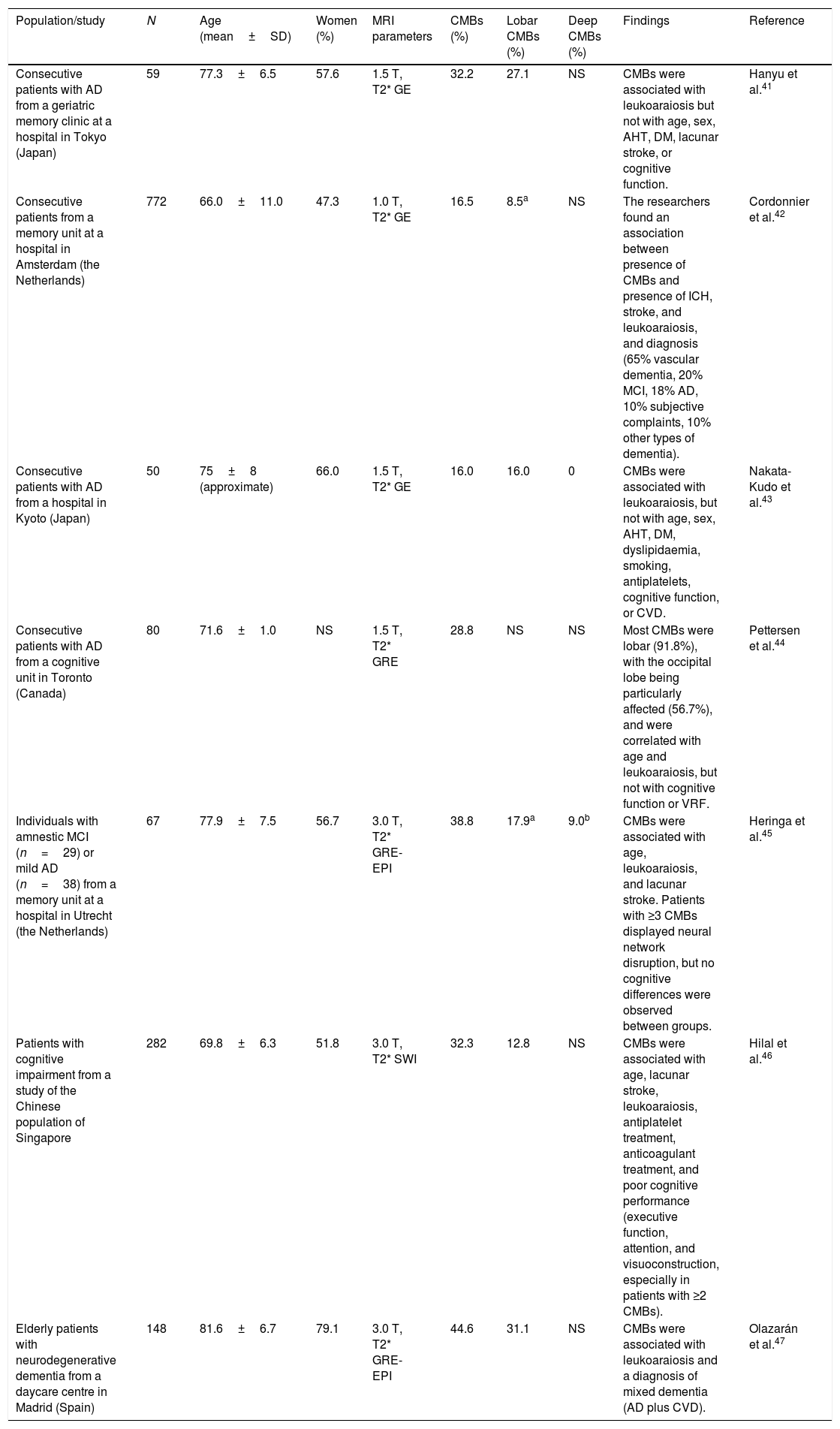
Studies of patients with cognitive impairmentThe prevalence of CMBs in patients with cognitive impairment ranges from 16% to 45%41–47 (Table 4); these rates fall between those observed in the general population (Table 2) and those of patients with vascular diseases or vascular risk (Table 3). The prevalence of CMBs in patients with AD ranges from 16% to 39%; these rates are higher than those reported in patients with other neurodegenerative dementias.42 In patients with AD, CMBs are predominantly lobar (involving the grey or white matter); no association has been established with vascular risk factors, which supports the role of CAA as a pathological substrate.16,43,44 No clear association has been observed between CMBs and cognitive impairment in patients with AD.44,45 A study using SWI demonstrated an association between CMBs and cognitive function, although the sample was not sufficiently well defined from an aetiological viewpoint.46
Studies of cerebral microbleeds including patients with cognitive impairment.
| Population/study | N | Age (mean±SD) | Women (%) | MRI parameters | CMBs (%) | Lobar CMBs (%) | Deep CMBs (%) | Findings | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Consecutive patients with AD from a geriatric memory clinic at a hospital in Tokyo (Japan) | 59 | 77.3±6.5 | 57.6 | 1.5 T, T2* GE | 32.2 | 27.1 | NS | CMBs were associated with leukoaraiosis but not with age, sex, AHT, DM, lacunar stroke, or cognitive function. | Hanyu et al.41 |
| Consecutive patients from a memory unit at a hospital in Amsterdam (the Netherlands) | 772 | 66.0±11.0 | 47.3 | 1.0 T, T2* GE | 16.5 | 8.5a | NS | The researchers found an association between presence of CMBs and presence of ICH, stroke, and leukoaraiosis, and diagnosis (65% vascular dementia, 20% MCI, 18% AD, 10% subjective complaints, 10% other types of dementia). | Cordonnier et al.42 |
| Consecutive patients with AD from a hospital in Kyoto (Japan) | 50 | 75±8 (approximate) | 66.0 | 1.5 T, T2* GE | 16.0 | 16.0 | 0 | CMBs were associated with leukoaraiosis, but not with age, sex, AHT, DM, dyslipidaemia, smoking, antiplatelets, cognitive function, or CVD. | Nakata-Kudo et al.43 |
| Consecutive patients with AD from a cognitive unit in Toronto (Canada) | 80 | 71.6±1.0 | NS | 1.5 T, T2* GRE | 28.8 | NS | NS | Most CMBs were lobar (91.8%), with the occipital lobe being particularly affected (56.7%), and were correlated with age and leukoaraiosis, but not with cognitive function or VRF. | Pettersen et al.44 |
| Individuals with amnestic MCI (n=29) or mild AD (n=38) from a memory unit at a hospital in Utrecht (the Netherlands) | 67 | 77.9±7.5 | 56.7 | 3.0 T, T2* GRE-EPI | 38.8 | 17.9a | 9.0b | CMBs were associated with age, leukoaraiosis, and lacunar stroke. Patients with ≥3 CMBs displayed neural network disruption, but no cognitive differences were observed between groups. | Heringa et al.45 |
| Patients with cognitive impairment from a study of the Chinese population of Singapore | 282 | 69.8±6.3 | 51.8 | 3.0 T, T2* SWI | 32.3 | 12.8 | NS | CMBs were associated with age, lacunar stroke, leukoaraiosis, antiplatelet treatment, anticoagulant treatment, and poor cognitive performance (executive function, attention, and visuoconstruction, especially in patients with ≥2 CMBs). | Hilal et al.46 |
| Elderly patients with neurodegenerative dementia from a daycare centre in Madrid (Spain) | 148 | 81.6±6.7 | 79.1 | 3.0 T, T2* GRE-EPI | 44.6 | 31.1 | NS | CMBs were associated with leukoaraiosis and a diagnosis of mixed dementia (AD plus CVD). | Olazarán et al.47 |
AD: Alzheimer disease; AHT: arterial hypertension; APOE: apolipoprotein E gene; BP: blood pressure; CMB: cerebral microbleed; CVD: cerebrovascular disease; DM: diabetes mellitus; EPI: echo-planar imaging; GE: gradient echo; GRE: gradient-recalled echo; MCI: mild cognitive impairment; MRI: magnetic resonance imaging; N: sample size; NS: not specified; SD: standard deviation; VRF: vascular risk factor.
To analyse the clinical impact of CMBs, we must first define the role of ischaemic CVD, which is closely associated with CMBs. Three population studies have observed an association between CMBs and poorer function in cognitive domains other than memory; this association persists after controlling for the effect of ischaemic lesions.32,48,49 The magnitude of this effect is small, however (regression coefficients of 0.20-0.25).33 A recent retrospective, longitudinal study found that patients with ≥2 lobar CMBs displayed a decline in executive function in the 5 years prior to a brain MRI scan; however, the effect of ischaemic lesions was not controlled for (Table 2).26
Other researchers have observed an association between CMBs and poorer cognitive function in patients with CVD37,50 and with subcortical vascular dementia.51 A recent meta-analysis of 15 case-control studies (10 of which included Asian populations) found a clear association between presence of CMBs in any location (except for the brainstem) and poorer performance in attention and executive function tasks. Cognitive function worsens as CMB count increases; the results of some studies support the need to control for concomitant ischaemic lesions secondary to ischaemic stroke or leukoaraiosis.52
The potential symptomatic role of CMBs in AD has received little attention. No differences in cognitive function were found in a study comparing AD patients with and without CMBs44 or in another study comparing patients with and without ≥3 CMBs.45 However, a group of researchers compared patients with ≥8 CMBs and patients with no CMBs and found significant differences in cognitive function (Mini-Mental State Examination score 17 vs 22, P<.05) and in the presence of biological markers of AD in the CSF (Aβ42 and p-tau), despite similar disease duration in both groups. After controlling for the effect of leukoaraiosis and mesial temporal atrophy, the CMB group continued to perform worse on language and executive function tasks.53 A study using SWI observed greater risk of dementia in patients with mild cognitive impairment who also displayed CMBs.54
Very few studies have addressed the pathophysiological mechanisms potentially responsible for the clinical expression of CMBs. A study conducted in mice showed transient astrocyte and neural dysfunction in nearby laser-induced microbleeds.55 In a study of patients with mild cognitive impairment or early AD examined with diffusion tensor imaging, patients with ≥3 CMBs displayed white matter alterations.45 In the light of the above, we may hypothesise that CMBs disrupt the connections between frontal and subcortical regions, or between different cortical regions, leading to cognitive impairment. This mechanism has previously been proposed for traumatic CMBs.56
Presence of CMBs is associated with higher mortality rates at 2-3 years. In a sample of 1138 consecutive patients from a memory clinic, CMBs were associated with greater mortality risk, especially in patients with ≥3 CMBs; in the latter patient group, the mortality risk due to CMBs was greater than that associated with ischaemic lesions.57 In a study including patients with ischaemic stroke and non-valvular atrial fibrillation, presence of ≥5 CMBs was associated with increased mortality due to ischaemic stroke or any cause, and presence of strictly lobar CMBs was associated with increased mortality due to haemorrhagic stroke.34 Other studies report that deep or infratentorial CMBs are associated with higher mortality due to cardiovascular disease.58,59
Therapeutic implicationsNumerous studies have demonstrated the role of CMBs as markers of vascular damage and as a risk factor for ICH or haemorrhagic transformation of ischaemic stroke.28,60–62 CMBs have also been associated with higher risk of ischaemic stroke, although this association is somewhat weaker.28,30,63,64 Presence of lobar CMBs increases sensitivity for detecting CAA, especially in patients with a history of stroke.65 Patients with CMBs should be closely monitored for vascular risk factors (especially AHT), ischaemic or haemorrhagic strokes, and haemorrhagic transformation in the case of ischaemic stroke.
Due to their role as markers of both ischaemic and haemorrhagic risk, CMBs place physicians in a difficult position when deciding whether to administer antiplatelet or anticoagulant treatment. Cohort studies have shown that antiplatelet treatment, and to a greater extent treatment with dicoumarol anticoagulants, increases the risk of ICH in patients with CMBs.66 Patients with lobar ICHs who also display numerous lobar CMBs should not receive anticoagulants due to the possibility of CAA and the associated high risk of rebleeding.38,65,67 In more ambiguous cases (few CMBs, non-lobar location), treatment should be tailored to the patient's needs, accounting for the risk of ischaemic and/or haemorrhagic stroke.68 If we opt for anticoagulant treatment (for example, in patients with prosthetic valves), we should administer new-generation anticoagulants (dabigatran, rivaroxaban, apixaban), which involve a lower risk of ICH. The Clinical Relevance of Microbleeds in Stroke study will provide valuable information on the usefulness of CMBs for predicting ICH in patients with atrial fibrillation and receiving anticoagulants.69
The increasing sensitivity and availability of MRI for detecting CMBs offers promising clinical possibilities. In patients receiving anticoagulants, the clinical worsening associated with an increase in the number of CMBs may constitute a practical criterion for discontinuing anticoagulation.70 Presence of CMBs is associated with a slight increase in the risk of ICH in patients with ischaemic stroke receiving thrombolytics, but does not constitute a formal contraindication for treatment.71–73
ConclusionsThe incidence of CMBs increases with age, presence of vascular risk factors (mainly AHT), AD, and especially CVD. Lipohyalinosis (mainly subcortical and infratentorial) and CAA (preferentially lobar and cerebellar) constitute the pathological substrate of CMBs. CMBs and cerebral ischaemia share some pathophysiological mechanisms, and the association between the 2 is probably bidirectional. It is yet to be understood why this type of vascular disease can cause ischaemia, haemorrhage, or both in a single patient.
CMBs increase the risk of stroke (both ischaemic and haemorrhagic) and mortality, both in asymptomatic individuals and in patients with a history of CVD; the risk increases in line with the number of CMBs. CMBs are also associated with increased risk of cognitive impairment, both in the general population and in stroke patients; however, the extent to which this effect is due to CVD or to probable AD is unclear. Although few studies have addressed this question, the available evidence suggests that CMBs are associated with poorer functional prognosis. The impact of CMBs on clinical domains other than cognitive function (functional capacity, affectivity, behaviour, motor function) requires further study.
The detection of CMBs in patients with CVD has a significant diagnostic and therapeutic impact. As markers of vascular damage, CMBs compel clinicians to strengthen preventive measures against ischaemic and haemorrhagic stroke (especially treatment for AHT) and discourage invasive diagnostic (cerebral angiography) and treatment techniques (brain surgery), especially when CMBs are numerous or lobar in location. The decision to administer anticoagulants to patients with CMBs should be clearly justified, as this treatment increases the risk of ICH. Antiplatelets may also be contraindicated for patients with lobar CMBs, especially when these are numerous or if CAA is suspected (prior ICH or cortical haemosiderosis).
According to epidemiological, clinical, and anatomical pathology findings, CMBs do not play a major pathophysiological role in CVD or AD; rather, they constitute a residual or collateral effect, indicating capillary fragility. This idea is consistent with observations of vasogenic oedema and new CMBs in patients with existing CMBs who had received drugs acting on cerebral amyloid (human immunoglobulin, monoclonal antibodies, vaccines). In the light of the above, it seems reasonable to continue excluding patients with CMBs from clinical trials of these agents.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Boyano I, Bravo N, Miranda J, Gil-Gregorio P, Olazarán J. Microhemorragias cerebrales: epidemiología e implicaciones clínicas. Neurología. 2018;33:515–525.