Patients with history of stroke or transient ischaemic attack present considerable risk of future vascular events. Reducing levels of low-density lipoprotein (LDL) cholesterol decreases the incidence of new vascular events, although in a substantial number of patients, the currently available lipid-lowering therapies fail to achieve the therapeutic goals recommended in clinical guidelines. The aim of this consensus statement is to provide updated information on the role of the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors alirocumab and evolocumab in the secondary prevention of vascular events in patients with history of ischaemic stroke.
MethodsA literature review was performed to identify the main evidence on the use of PCSK9 inhibitors in these patients and the recommended therapeutic targets of LDL cholesterol. The results were discussed in 2 consensus meetings that constituted the basis for the drafting of the document.
ConclusionsPCSK9 inhibitors are effective in reducing vascular risk in secondary prevention; evolocumab specifically has achieved this reduction in patients with history of ischaemic stroke. Moreover, both alirocumab and evolocumab present good safety profiles, even in patients achieving LDL cholesterol levels < 20 mg/dL, and no signs of cognitive impairment have been observed in patients treated with evolocumab who achieved very low levels of LDL cholesterol. In the light of this evidence, we provide practical recommendations about the use of PCSK9 inhibitors in secondary prevention of vascular events in patients with history of ischaemic stroke and follow-up of these patients.
Los pacientes, tras un ictus o un ataque isquémico transitorio, presentan un riesgo muy elevado de sufrir nuevos episodios vasculares. La reducción del nivel de colesterol unido a lipoproteínas de baja densidad (cLDL) reduce la incidencia de nuevos episodios, si bien una proporción importante de pacientes no alcanza los objetivos terapéuticos recomendados con los tratamientos hipolipemiantes actuales. El objetivo de este documento de consenso es actualizar el papel de los inhibidores de la proproteína convertasa subtilisina/kexina tipo 9 (iPCSK9; alirocumab y evolocumab) en la prevención secundaria de episodios vasculares en pacientes con ictus isquémico previo.
MétodosSe realizó una revisión bibliográfica para identificar las principales evidencias sobre el uso de iPCSK9 en estos pacientes y los objetivos terapéuticos recomendados de cLDL. Los resultados se discutieron en 2 reuniones de consenso, que constituyeron la base para la elaboración del documento.
ConclusionesLos iPSCSK9 son eficaces en la reducción del riesgo vascular en prevención secundaria y, específicamente, evolocumab ha demostrado esta reducción en pacientes con ictus isquémico previo. Ambos fármacos han demostrado un buen perfil de seguridad, incluso en pacientes que alcanzaron un nivel de cLDL <20 mg/dL. En este sentido, en el subestudio de episodios neurocognitivos con evolocumab no se observó ninguna señal de empeoramiento de la función cognitiva en pacientes con nivel muy bajo de cLDL. Con base en estas evidencias, en el documento se presentan recomendaciones prácticas sobre el uso de iPCSK9 para la prevención secundaria y seguimiento de episodios vasculares en pacientes con ictus isquémico previo.
Cerebrovascular disease is one of the leading causes of morbidity, disability, and mortality in Europe.1,2 In Spain, it constitutes the second leading cause of death.3 Patients with history of stroke present an elevated risk of further cardiovascular events, including recurrent stroke.2,4,5 A meta-analysis of 13 studies including a total of 9115 patients who survived a first stroke reported cumulative risk of recurrent stroke at 30 days and 1, 5, and 10 years of 3%, 11%, 26%, and 39%, respectively.5 Recurrent stroke is also associated with poorer prognosis, greater incidence of disability and functional dependence, and a high mortality rate.4,6
Low-density lipoprotein cholesterol (LDL-C) is a widely-recognised risk factor for atherosclerotic cardiovascular disease.7,8 In the light of the fact that this form of cardiovascular disease is one of the main risk factors for ischaemic stroke, control of dyslipidaemia, with particular focus on LDL-C, constitutes a key modifiable risk factor to be targeted in primary and secondary stroke prevention.1,4,9 Reducing LDL-C levels through treatment with various lipid-lowering agents has been shown to decrease the incidence of cardiovascular events, including ischaemic stroke.8,10–12 With respect to secondary prevention, reduction of LDL-C levels is also associated with a decrease in the risk of cardiovascular events in patients with history of stroke.13–15 Furthermore, the available evidence suggests that greater reductions in LDL-C levels are associated with a greater decrease in the risk of cardiovascular events, including stroke.4,10,12,16–18 A recent study randomly allocated patients with stroke or transient ischaemic attack (TIA) and signs of atherosclerotic disease to receive treatment with a target LDL-C level of either < 70 mg/dL or 90-100 mg/dL.17 The composite primary endpoint of major cardiovascular events (ischaemic stroke, myocardial infarction, new symptoms requiring urgent carotid or coronary revascularisation, or death from cardiovascular causes) occurred in significantly fewer patients from the first group (8.5% vs 10.9%, hazard ratio: 0.78; 95% confidence interval, 0.61-0.98; P = 0.04). Similarly, a meta-analysis of 23 randomised trials of lipid-lowering treatments reported a 23.5% reduction in the relative risk of stroke for every 1 mmol/L (39 mg/dL) reduction in LDL-C.18 This association between LDL-C level and stroke risk is linear, and is consistent across studies of both primary and secondary prevention.
Despite this, the available data on the degree of control of dyslipidaemia in secondary cardiovascular prevention in Spanish and European patients with history of stroke show that a considerable percentage of these patients present poor control of LDL-C.4,19 Data from the stroke module of the EUROASPIRE III study show that, of 881 patients with history of stroke who were interviewed 550 days after the episode, only 34.4% had reached their target LDL-C levels, despite the fact that 56.8% were receiving treatment with statins.19 Likewise, the recent EUROASPIRE V study confirmed that the majority of European patients in secondary cardiovascular prevention did not reach target LDL-C levels.20 In Spain, several studies have reported poor control of vascular risk factors, including dyslipidaemia, in secondary stroke prevention.4,21–23 According to the latest health survey conducted in Spain on cardiovascular risk factors, 83.0% of patients with cerebrovascular disease had LDL-C levels below 130 mg/dL, while only 56.5% had levels below 100 mg/dL.24
Historically, statins have been the fundamental pillar of lipid-lowering treatment for cardiovascular prevention.25–27 Nonetheless, patients who do not achieve therapeutic objectives despite receiving optimal statin therapy, or who are intolerant to these drugs, require additional lipid-lowering treatments.25–28 Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors are lipid-lowering drugs that achieve significant reductions in LDL-C levels in patients receiving standard lipid-lowering agents, and present a good safety profile.8,28,29 Two of these drugs (the monoclonal antibodies evolocumab and alirocumab) have been marketed in Spain; both are indicated for the treatment of primary hypercholesterolaemia, mixed dyslipidaemia, and established atherosclerotic cardiovascular disease.25,30,31 Data from the FOURIER study show that the addition of evolocumab to statin treatment in patients with established atherosclerotic cardiovascular disease significantly reduces the risk of further cardiovascular events.8 The ODYSSEY Outcomes trial found that alirocumab reduces the risk of recurrent ischaemic events in patients with history of acute coronary syndrome.32 Furthermore, a subanalysis from the FOURIER trial, including 5337 patients with history of stroke, confirmed that evolocumab reduces vascular risk in this patient group.15 As a consequence of these findings, indications for PCSK9 inhibitors have recently been expanded to include the reduction of vascular risk in adults with established cardiovascular disease.27,28
Results on the safety and efficacy of these drugs,8,32 together with new evidence on the benefits and safety of low LDL-C levels,7,8,12,28,32,33 have facilitated the publication of numerous clinical practice guidelines for reducing vascular risk and controlling dyslipidaemia, positioning PCSK9 inhibitors in the treatment arsenal and reviewing treatment objectives in patients at high risk or with established cardiovascular disease.1,25,26,34–41 Therefore, we considered it beneficial to prepare a consensus statement focusing on the field of neurology, aiming to contribute practical recommendations on the use of PCSK9 inhibitors for the secondary prevention of cardiovascular events in patients with history of ischaemic stroke, reflecting both the available evidence and the authors’ clinical experience.
MethodsThis consensus statement presents the work of an expert group of neurologists at a participative session held in Madrid on 28 November 2018, with the objectives, methodology, and working dynamic being defined in advance. During the meeting, we held a structured debate with participation of all members of the group, establishing what information to include in each section of the document, the profiles of patients with stroke who may benefit the most from treatment with PCSK9 inhibitors, and treatment objectives for each patient profile.
After the session, we prepared a draft document with the support of a professional medical writer. A literature review of the MEDLINE database was conducted between November 2018 and January 2019 to gather the data needed to draft the document, and relevant studies published in English or Spanish were selected. After 2 reviews of the manuscript by the authors, a second meeting was held (Madrid, 3 December 2019) to discuss and incorporate new evidence that emerged during the drafting of the document and to definitively approve the group’s consensus regarding patient profiles and treatment objectives. After the meeting, these changes were incorporated into a final version of the document, which was sent to all authors for final approval.
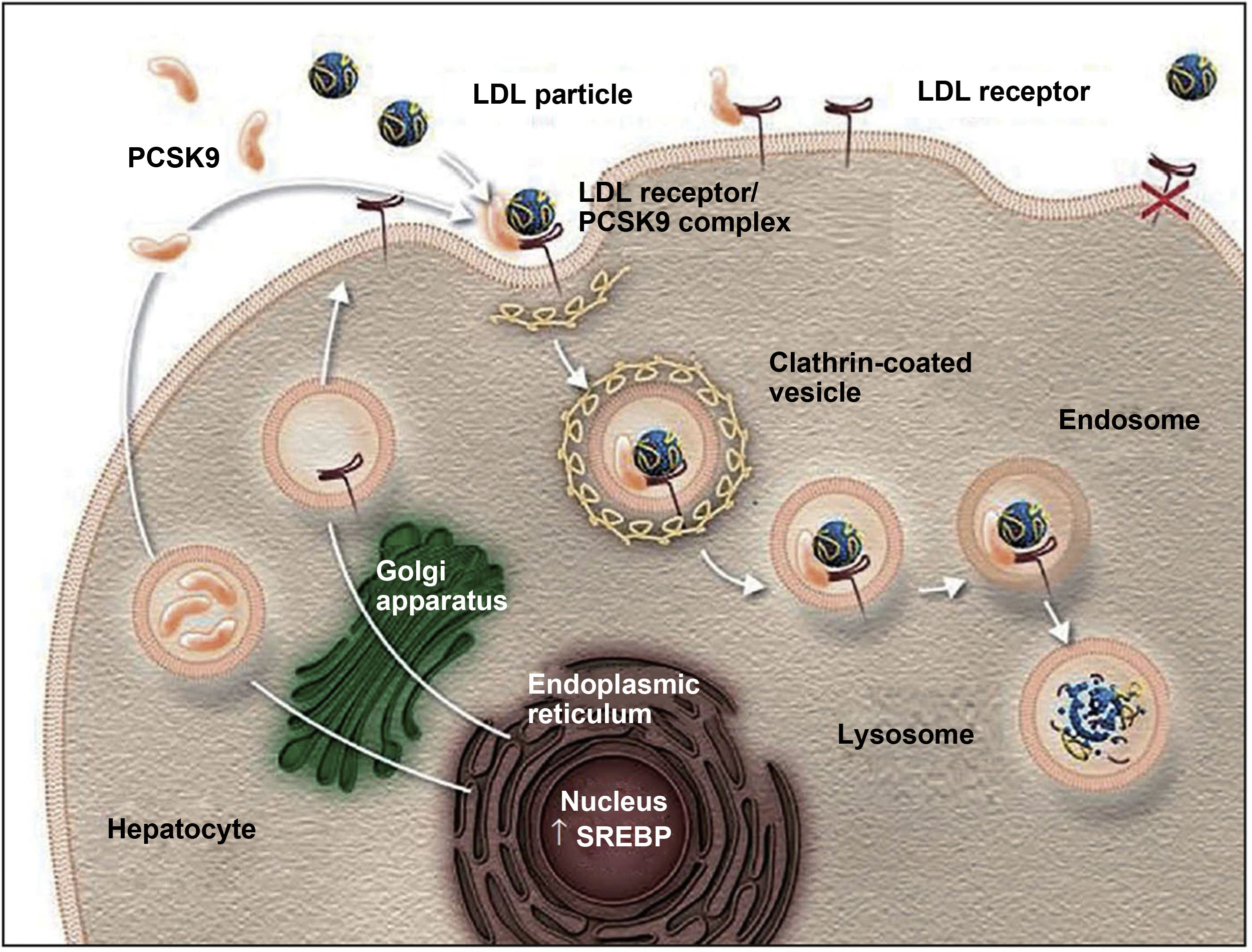
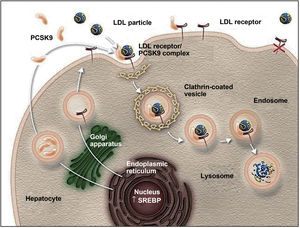
Action mechanism and indications of PCSK9 inhibitorsThe PCSK9 protein is mainly synthesised in the liver and participates in regulating LDL-C metabolism.25,27 In normal conditions, LDL-C receptors on the surface of hepatocytes capture circulating LDL-C and are internalised into the cytoplasm by endocytosis. In the endosome, LDL-C is separated from the receptor and degraded, and the receptor is recycled to the cell surface.25,27,42,43 When PCSK9 binds to an LDL-C receptor, the protein-receptor complex undergoes the same process of endocytosis but PCSK9 prevents recycling of the receptor to the surface of the hepatocyte, reducing the uptake of circulating LDL-C (Fig. 1).26,27,42,43 PCSK9 also seems to increase intracellular degradation of LDL-C receptors, even before they are secreted.27 Inhibition of PCSK9 by monoclonal antibodies blocks the action of the protein, preventing it from binding to the LDL-C receptor, thus enabling recycling of the receptors and consequently reducing circulating LDL-C levels.27,42 PCSK9 inhibitors have a rapid, potent effect on LDL-C levels, with maximum suppression of circulating PCSK9 occurring 4-8 hours after administration of the antibody; LDL-C levels decrease by approximately 60% in patients with hypercholesterolaemia.8,27 Compared to placebo and conventional lipid-lowering treatments, PCSK9 inhibitors have also been associated with increased levels of high-density lipoprotein cholesterol (HDL-C) and reduced levels of total cholesterol, triglycerides, and lipoprotein (a).42,44,45 PCSK9 inhibitors have also been shown to have a synergistic effect when combined with statins, as the latter not only increase the expression of LDL-C receptors but also increase PCSK9 expression in hepatocytes.43,46,47
The role of PCSK9 inhibitors in the degradation of LDL cholesterol receptors.79
Authorised by Amgen Inc.
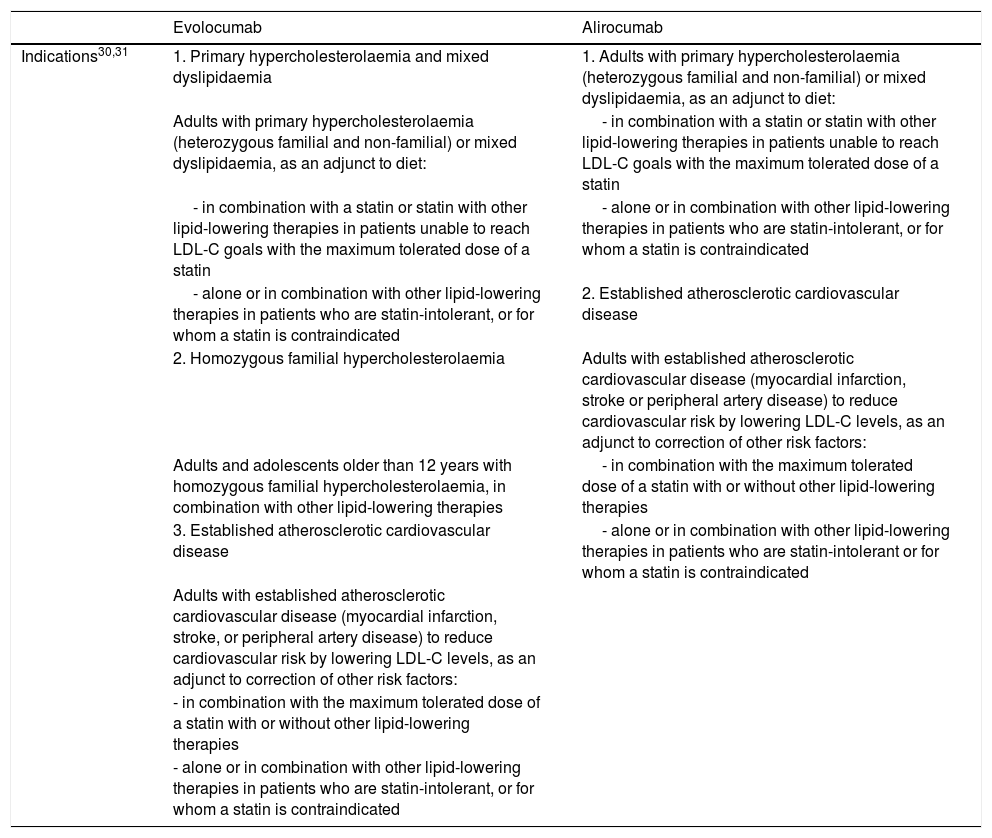
The approved indications for evolocumab and alirocumab are summarised in Table 1.30,31 Both drugs are administered subcutaneously.30,31
Indications for PCSK9 inhibitors approved by the European Medicines Agency and funded by the Spanish national healthcare system.
| Evolocumab | Alirocumab | |
|---|---|---|
| Indications30,31 | 1. Primary hypercholesterolaemia and mixed dyslipidaemia | 1. Adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet: |
| Adults with primary hypercholesterolaemia (heterozygous familial and non-familial) or mixed dyslipidaemia, as an adjunct to diet: | - in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin | |
| - in combination with a statin or statin with other lipid-lowering therapies in patients unable to reach LDL-C goals with the maximum tolerated dose of a statin | - alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated | |
| - alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated | 2. Established atherosclerotic cardiovascular disease | |
| 2. Homozygous familial hypercholesterolaemia | Adults with established atherosclerotic cardiovascular disease (myocardial infarction, stroke or peripheral artery disease) to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors: | |
| Adults and adolescents older than 12 years with homozygous familial hypercholesterolaemia, in combination with other lipid-lowering therapies | - in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies | |
| 3. Established atherosclerotic cardiovascular disease | - alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant or for whom a statin is contraindicated | |
| Adults with established atherosclerotic cardiovascular disease (myocardial infarction, stroke, or peripheral artery disease) to reduce cardiovascular risk by lowering LDL-C levels, as an adjunct to correction of other risk factors: | ||
| - in combination with the maximum tolerated dose of a statin with or without other lipid-lowering therapies | ||
| - alone or in combination with other lipid-lowering therapies in patients who are statin-intolerant, or for whom a statin is contraindicated |
LDL-C: low-density lipoprotein cholesterol; PCSK9: proprotein convertase subtilisin/kexin type 9.
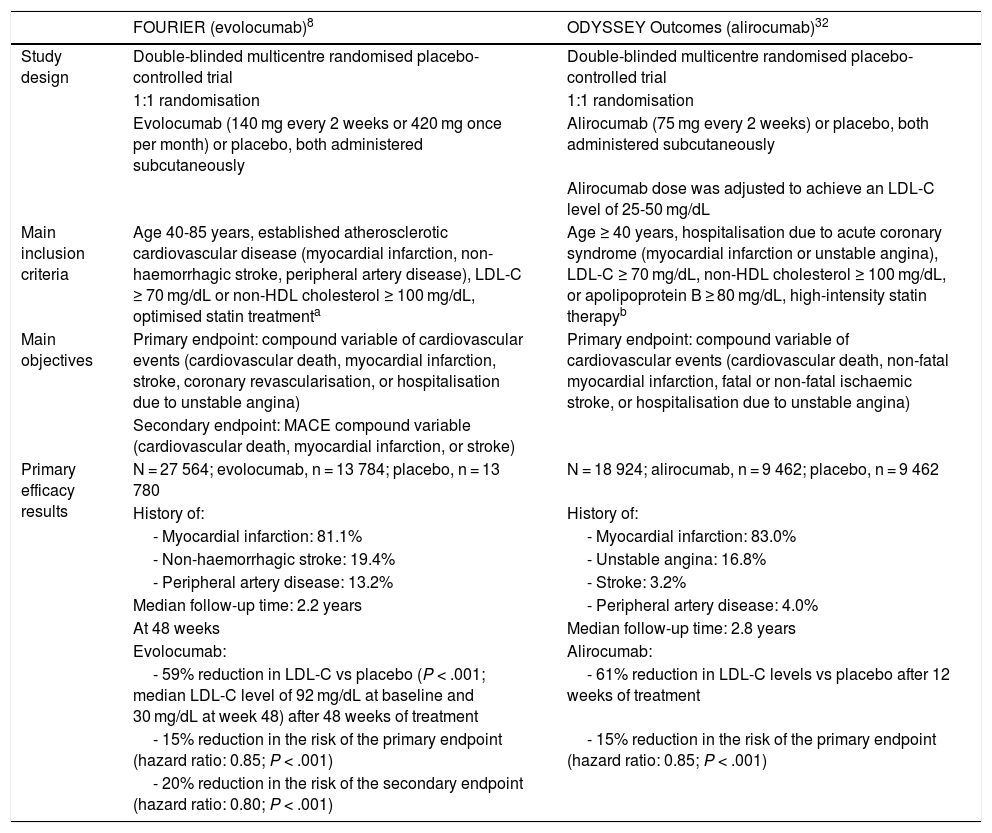
The effect of PCSK9 inhibitors in reducing vascular risk has been evaluated in 2 large randomised clinical trials, the FOURIER trial (evolocumab) and the ODYSSEY Outcomes trial (alirocumab); the main characteristics and results of these trials are summarised in Table 2.8,29,32,41 These trials assessed the efficacy and safety of PCSK9 inhibitors in patients with cardiovascular disease and high levels of LDL-C or non-HDL cholesterol despite moderate- or high-intensity statin therapy. Evolocumab and alirocumab reduced the baseline LDL-C level by approximately 60%, compared to placebo. Both trials achieved the primary efficacy endpoint, with significant reductions of 15% vs placebo in the risk of cardiovascular events (including death from cardiovascular causes, myocardial infarction, stroke, coronary revascularisation, and hospitalisation due to unstable angina, in the FOURIER trial; and death due to coronary heart disease, non-fatal myocardial infarction, fatal or non-fatal ischaemic stroke, and hospitalisation due to unstable angina, in the ODYSSEY Outcomes trial).8,32
Main characteristics and efficacy results from the FOURIER and ODYSSEY Outcomes randomised clinical trials.
| FOURIER (evolocumab)8 | ODYSSEY Outcomes (alirocumab)32 | |
|---|---|---|
| Study design | Double-blinded multicentre randomised placebo-controlled trial | Double-blinded multicentre randomised placebo-controlled trial |
| 1:1 randomisation | 1:1 randomisation | |
| Evolocumab (140 mg every 2 weeks or 420 mg once per month) or placebo, both administered subcutaneously | Alirocumab (75 mg every 2 weeks) or placebo, both administered subcutaneously | |
| Alirocumab dose was adjusted to achieve an LDL-C level of 25-50 mg/dL | ||
| Main inclusion criteria | Age 40-85 years, established atherosclerotic cardiovascular disease (myocardial infarction, non-haemorrhagic stroke, peripheral artery disease), LDL-C ≥ 70 mg/dL or non-HDL cholesterol ≥ 100 mg/dL, optimised statin treatmenta | Age ≥ 40 years, hospitalisation due to acute coronary syndrome (myocardial infarction or unstable angina), LDL-C ≥ 70 mg/dL, non-HDL cholesterol ≥ 100 mg/dL, or apolipoprotein B ≥ 80 mg/dL, high-intensity statin therapyb |
| Main objectives | Primary endpoint: compound variable of cardiovascular events (cardiovascular death, myocardial infarction, stroke, coronary revascularisation, or hospitalisation due to unstable angina) | Primary endpoint: compound variable of cardiovascular events (cardiovascular death, non-fatal myocardial infarction, fatal or non-fatal ischaemic stroke, or hospitalisation due to unstable angina) |
| Secondary endpoint: MACE compound variable (cardiovascular death, myocardial infarction, or stroke) | ||
| Primary efficacy results | N = 27 564; evolocumab, n = 13 784; placebo, n = 13 780 | N = 18 924; alirocumab, n = 9 462; placebo, n = 9 462 |
| History of: | History of: | |
| - Myocardial infarction: 81.1% | - Myocardial infarction: 83.0% | |
| - Non-haemorrhagic stroke: 19.4% | - Unstable angina: 16.8% | |
| - Peripheral artery disease: 13.2% | - Stroke: 3.2% | |
| Median follow-up time: 2.2 years | - Peripheral artery disease: 4.0% | |
| At 48 weeks | Median follow-up time: 2.8 years | |
| Evolocumab: | Alirocumab: | |
| - 59% reduction in LDL-C vs placebo (P < .001; median LDL-C level of 92 mg/dL at baseline and 30 mg/dL at week 48) after 48 weeks of treatment | - 61% reduction in LDL-C levels vs placebo after 12 weeks of treatment | |
| - 15% reduction in the risk of the primary endpoint (hazard ratio: 0.85; P < .001) | - 15% reduction in the risk of the primary endpoint (hazard ratio: 0.85; P < .001) | |
| - 20% reduction in the risk of the secondary endpoint (hazard ratio: 0.80; P < .001) |
HDL: high-density lipoprotein; LDL-C: low-density lipoprotein cholesterol; MACE: major adverse cardiac events.
All patients in the ODYSSEY Outcomes trial had been hospitalised due to acute coronary syndrome in the year prior to study inclusion; inclusion criteria for the FOURIER trial included established atherosclerotic cardiovascular disease, such as myocardial infarction, non-haemorrhagic stroke, and peripheral artery disease.8,32 In the ODYSSEY Outcomes trial, 3.2% of patients in each group had history of stroke, although no specific analysis was conducted for this subgroup of 611 patients.32 In the FOURIER trial, 19.4% of patients (n = 5337) had history of non-haemorrhagic stroke; this enabled the authors to evaluate the effect of evolocumab treatment on vascular risk in this subgroup.8,15 Baseline LDL-C and HDL-C levels in this patient group were 97.5 and 47.8 mg/dL, respectively. In this subgroup, evolocumab significantly reduced LDL-C levels by 56% vs placebo (similar to the effect observed in the general population), with a total reduction of 53 mg/dL, achieving a median LDL-C level of 29 mg/dL (vs 89 mg/dL in the placebo group; P < .001), with no safety issues.15 The primary efficacy endpoint for reducing the incidence of cardiovascular events was also achieved in the subgroup of patients with stroke, with a significant reduction of 15% in the risk of presenting a new cardiovascular event, including death from cardiovascular causes, myocardial infarction, stroke, coronary revascularisation, and hospitalisation due to unstable angina (P = .047). Therefore, treatment with evolocumab seems to present similar benefits and safety in patients with history of stroke and those with history of other cardiovascular diseases.15 As mentioned above, the results of the FOURIER trial led to the indication of evolocumab for reducing vascular risk in adults with established atherosclerotic disease, including those with history of stroke.31
In the light of the risk of recurrent stroke, which persists in the medium to long term after a first stroke,5 lipid-lowering treatments used in secondary prevention should be efficacious in the long term. In this sense, clinical trials have shown that treatment with both alirocumab and evolocumab leads to persistent reductions in LDL-C levels8,32; however, more long-term data are needed on both drugs. Evolocumab has been shown to achieve a sustained 58% reduction vs baseline levels after 5 years of treatment, with no new safety issues.48
Treatment adherence is another fundamental aspect of any secondary preventive therapy, as poor adherence is associated with poorer clinical outcomes.49,50 Adherence to PCSK9 inhibitors in the clinical trials was high49,51,52: adherence to evolocumab treatment was 79% after 44 months of treatment, higher than the values reported for other lipid-lowering treatments, including statins.52 While limited real-life information is available, the data published to date suggest satisfactory adherence to treatment with PCSK9 inhibitors.50
Safety of low LDL-C levelsA direct relationship has been established between LDL-C level and the risk of cardiovascular events.41,53 Published data on treatment with statins,11 ezetimibe plus statins,12 and PCSK9 inhibitors7,8,32,52 have confirmed that the risk of cardiovascular events decreases in line with LDL-C levels; this concept has been referred to as “the lower the better,” or the “zero LDL hypothesis.”28,33,53 This theory is particularly relevant since the marketing of PCSK9 inhibitors and the publication of the results of the FOURIER and ODYSSEY Outcomes trials, which reported even lower levels of LDL-C than those achieved with statins or statins plus ezetimibe, with the added benefit of even further reducing the risk of further cardiovascular events.7,8,32,53,54
Physicians’ main concern in the management of patients achieving low or very low LDL-C values is the safety of these low levels. Initial studies with statins indicated a possible relationship between low LDL-C levels and increased risk of haemorrhagic stroke.13 A single prospective study reported a greater risk of intracerebral haemorrhage in patients with LDL-C levels below 70 mg/dL than in those with levels between 70 and 99 mg/dL.55 However, the methodological limitations of that study hinder extrapolation of the results to other populations: data were collected from a cohort of patients with vascular risk factors from a specific region in China (the risk of intracerebral haemorrhage is greater in Asian than in white populations),56 and no data are reported on the level of control of arterial hypertension or neuroimaging studies that may have enabled classification of patients according to haemorrhagic risk.55
On the other hand, other studies, as well as the 2019 European Society of Cardiology and European Atherosclerosis Society (ESC/EAS) guidelines for the management of dyslipidaemias, question the association between low LDL-C levels and increased risk of intracerebral haemorrhage.9,11,17,18,28,57 The IMPROVE-IT trial found no significant association between risk of intracerebral haemorrhage and the most intensive lipid-lowering treatment with ezetimibe plus statins.12 The previously mentioned study of patients with stroke or TIA who were allocated for treatment with target LDL-C values of < 70 mg/dL or 90-110 mg/dL found no differences between groups in the incidence of intracranial haemorrhage.17 The recent meta-analysis of randomised studies of lipid-lowering treatments (> 222 000 patients) also reported no association between lower levels of LDL-C and increased risk of haemorrhagic stroke.18 Similarly, the recently published studies of PCSK9 inhibitors have not observed an increase in the risk of intracerebral haemorrhage, even in patients achieving very low LDL-C levels.7,8,28,32,54 In the FOURIER trial, no new safety issues were observed in the 25% of patients receiving evolocumab who achieved LDL-C < 20 mg/dL31 or in the 10% of patients who achieved LDL-C < 10 mg/dL.7,58 Similarly, pooled analysis of the trials of alirocumab found that 37% and 9% of patients achieved < 25 and < 15 mg/dL LDL-C, respectively, with no new safety issues.41,59 Despite the need for continued monitoring of the safety of these drugs,36 the long-term safety profile of PCSK9 inhibitors shows no new findings with follow-up periods of up to 5 years, in the case of evolocumab.32,60
There is some evidence linking the use of statins to the development of neurocognitive deficits, although these results have not been consistent, and it has recently been suggested that no such association exists.61 Earlier studies suggested an association between PCSK9 inhibitors and cognitive adverse events41,62; however, the FOURIER and ODYSSEY Outcomes trials, which included larger patient samples, as well as the findings of meta-analyses, have not demonstrated such an association.7,32,41,58,62–64 The EBBINGHAUS study, which included a subgroup of 1204 FOURIER trial participants and specifically evaluated cognitive performance, found no association between treatment with evolocumab and cognitive performance, even in patients with lower LDL-C levels.7,62
Some data suggest that statin treatment increases the risk of developing diabetes, particularly in patients with prediabetes.65 However, neither the IMPROVE-IT trial, with ezetimibe,12,28 nor clinical trials of PCSK9 inhibitors28,32,58,59,65 have demonstrated any association between the use of these medications and low LDL-C levels and the development of diabetes. LDL-C levels < 25 mg/dL were not associated with risk of developing diabetes in patients treated with alirocumab.59 In a subanalysis of the FOURIER trial, evolocumab did not increase the risk of new-onset diabetes or alter blood glucose levels, even in patients with prediabetes, and showed consistent efficacy and safety in patients with and without diabetes.65 Furthermore, patients achieving very low levels of LDL-C (< 20 mg/dL) do not present increased risk of new-onset diabetes.7,52
Finally, neither the IMPROVE-IT trial nor the trials of PCSK9 inhibitors have reported muscular or hepatobiliary adverse reactions.7,8,12,32,58 Therefore, there is no need to adjust the dose of PCSK9 inhibitors in patients with mild (evolocumab) or mild to moderate (alirocumab) liver failure or with mild to moderate kidney failure.30,31
Recommendations on the safety of low LDL-C levels| Excessively low LDL-C levels are considered not to exist. |
| No relevant adverse reactions associated with low LDL-C levels are considered to exist. |
| We do not recommend modifying lipid-lowering treatment schedules in patients achieving the target LDL-C level, unless the patient presents an adverse reaction. |
| Lipid-lowering therapy should not be modified after onset of treatment with PCSK9 inhibitors, and efficacy should be re-evaluated after the next determination of LDL-C level. |
Based on the available evidence, clinical practice guidelines,1,37–39,57,66 and the authors’ clinical experience, we established the following profiles of patients with ischaemic stroke who may benefit more from secondary prevention with PCSK9 inhibitors, and the different treatment objectives (LDL-C levels) for these profiles.
Recommendations on the use of PCSK9 inhibitors for secondary prevention in patients with history of ischaemic stroke| Secondary prevention of cardiovascular events with PCSK9 inhibitors is indicated for all patients with ischaemic stroke of any aetiology (TOAST classification)67 and LDL-C levels > 100 mg/dL, receiving maximally tolerated statin therapy or with intolerance to or contraindications for statins (based on the guidelines of the therapeutic position statement).68,69 |
In line with the general recommendations of the European guidelines on dyslipidaemia,57 we recommend adding a PCSK9 inhibitor if the target level of LDL-C is not reached after 4-6 weeks (depending on clinical practice) of maximally tolerated statin therapy.
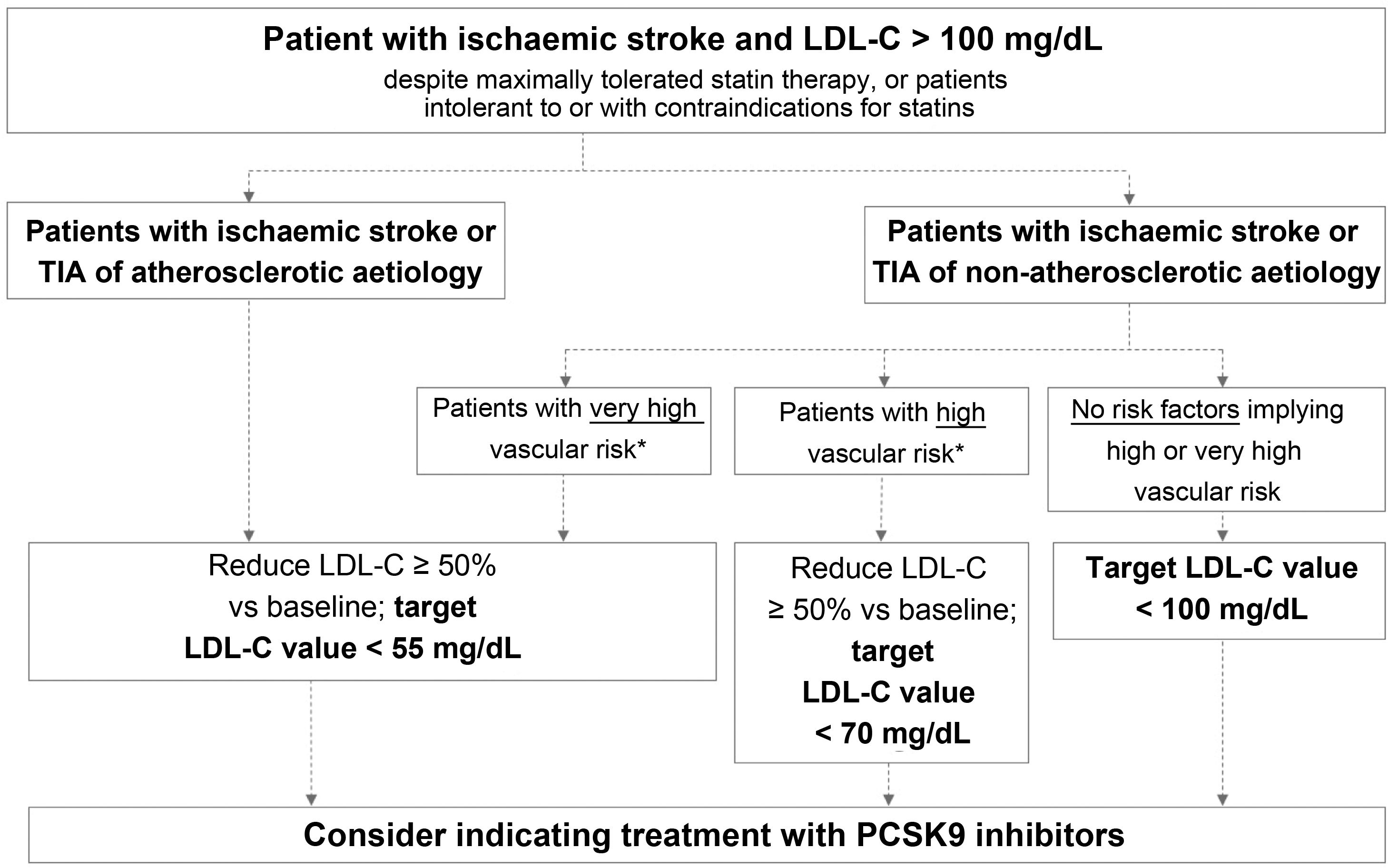
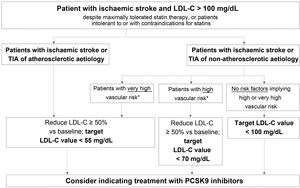
Fig. 2 presents the target LDL-C values recommended for different profiles of patients with history of ischaemic stroke or TIA, according to aetiology (atherosclerotic or non-atherosclerotic) and the presence of factors indicating high or very high vascular risk. In the case of stroke of unusual aetiology not associated with endothelial dysfunction or atheromatosis, such as cardiac myxoma, arterial dissection, or embolic sources of unknown risk, intensive lipid-lowering therapy is not necessary.
Target LDL cholesterol values recommended for patients with ischaemic stroke (see Table 3).
LDL-C: low-density lipoprotein cholesterol; PCSK9: proprotein convertase subtilisin/kexin type 9; TIA: transient ischaemic attack.
This group includes patients diagnosed with ischaemic stroke or TIA of atherosclerotic aetiology who continue to present LDL-C levels > 100 mg/dL despite optimal lipid-lowering therapy, as well as those patients meeting these characteristics but who present intolerance to or contraindications for statins. These patients present very high risk of further atherosclerotic events, including recurrent stroke.38 The guidelines of the American College of Cardiology establish a target LDL-C level of < 70 mg/dL for patients with established atherosclerotic cardiovascular disease.39 The American Association of Clinical Endocrinologists recommends a more ambitious target value, < 55 mg/dL, for patients with extreme cardiovascular risk, including the following patient groups: 1) progressive atherosclerotic cardiovascular disease including unstable angina in patients after achieving an LDL-C level < 70 mg/dL; 2) established clinical cardiovascular disease in patients with diabetes mellitus, grade 3/4 chronic kidney disease, or heterozygous familial hypercholesterolaemia; and 3) history of premature atherosclerotic cardiovascular disease (< 55 years in men and < 65 years in women).37 The 2019 ESC/EAS guidelines classify vascular risk into 4 categories (very high, high, moderate, low) according to the presence of certain clinical entities and risk factors (Table 3).57 Thus, patients with ischaemic stroke or TIA of atherosclerotic aetiology are considered to present very high risk; the guidelines recommend reducing LDL-C by at least 50% vs baseline, with a target value of < 55 mg/dL.57 All the guidelines mentioned above recommend adding PCSK9 inhibitors to oral lipid-lowering therapy if statins (alone or in combination with ezetimibe) do not achieve target values or in the event of intolerance to or contraindications for statins.37,39,57 The European stroke action plan for 2018-2030 also recommends the use of additional lipid-lowering agents, including PCSK9 inhibitors, in patients with history of stroke who present poor lipid control with standard lipid-lowering therapies.1
Vascular risk categories of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS).
| Risk category | |
|---|---|
| Very high risk | Documented ASCVD: ACS (MI or unstable angina), stroke or TIA, stable angina, peripheral artery disease, coronary revascularisation (PCI, CABG, and other arterial revascularisation procedures) |
| DM with target organ damage (eg, proteinuria) or at least 3 major risk factors (smoking, dyslipidaemia, or hypertension), or DM type 1 of long duration (> 20 years) | |
| Severe CKD (eGFR < 30 mL/min/1.73 m2) | |
| Calculated SCORE ≥ 10% | |
| FH with ASCVD or another major risk factor | |
| High risk | Markedly elevated single risk factors, in particular total cholesterol > 8 mmol/L (> 310 mg/dL), LDL-C > 4.9 mmol/L (> 190 mg/dL), or BP > 180/110 mm Hg |
| DM with duration > 10 years or another additional risk factor | |
| Moderate CKD (eGFR 30-59 mL/min/1.73 m2) | |
| Calculated SCORE ≥ 5% and < 10% | |
| FH without other major risk factors | |
| Moderate | Calculated SCORE ≥ 1% and < 5% |
| Low | Calculated SCORE < 1% |
ACS: acute coronary syndrome; ASCVD: atherosclerotic cardiovascular disease; BP: blood pressure; CABG: coronary artery bypass graft surgery; CKD: chronic kidney disease; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; FH: familial hypercholesterolaemia; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; MI: myocardial infarction; PCI: percutaneous coronary intervention; SCORE: Systematic Coronary Risk Estimation; TIA: transient ischaemic attack.
Adapted from Mach et al.57
According to the evidence discussed above, we recommend aiming to reduce LDL-C by at least 50% vs baseline, with a target value of < 55 mg/dL.
Patients with ischaemic stroke or transient ischaemic attack of non-atherosclerotic aetiology and presenting very high vascular riskThis group includes patients diagnosed with ischaemic stroke or TIA of non-atherosclerotic origin who present at least one factor indicating very high vascular risk and continue to present LDL-C levels > 100 mg/dL despite optimal lipid-lowering therapy, or who present intolerance to or contraindications for statin treatment.
Table 3 presents the factors used to establish very high vascular risk, according to the ESC/EAS guidelines.57 Despite the non-atherosclerotic origin of stroke,67 these patients present very high risk of a further cardiovascular event; therefore, the same treatment objectives are recommended for them as in patients with ischaemic stroke or TIA of atherosclerotic origin.57
We recommend aiming to reduce LDL-C levels by at least 50% vs baseline, with a target value of < 55 mg/dL.
Recommended treatment objectives for this patient groupIf this objective is not reached with maximally tolerated statin therapy, we recommend adding other lipid-lowering agents, including PCSK9 inhibitors. Alirocumab and evolocumab have been associated with a decrease in vascular risk in patients with one or more factors indicating very high vascular risk, as well as in patients with ischaemic heart disease.8,32 The efficacy of evolocumab in reducing vascular risk has also been demonstrated in patients with history of multiple myocardial infarctions or multivessel coronary artery disease.70 Evolocumab has been shown to promote plaque regression after 78 weeks of treatment in patients with coronary artery disease, even those with baseline LDL-C levels < 70 mg/dL.71 Furthermore, the drug has been shown to reduce the risk of major cardiovascular events and major adverse limb events in a subgroup of patients from the FOURIER trial with symptomatic peripheral artery disease, who presented incremental benefits until reaching LDL-C levels < 10 mg/dL.72 As mentioned above, evolocumab has also been found to reduce vascular risk in patients with history of ischaemic stroke.15 Diabetes is one of the most prevalent vascular risk factors, and both evolocumab and alirocumab are safe and efficacious for reducing vascular risk in this patient group.65,73
Patients with ischaemic stroke or transient ischaemic attack of non-atherosclerotic aetiology and presenting high vascular riskThis group includes patients with ischaemic stroke or TIA of non-atherosclerotic origin who present at least one factor indicating high vascular risk and continue to present LDL-C levels > 100 mg/dL despite optimal lipid-lowering therapy with statins, or who present intolerance to or contraindications for these drugs.
Table 3 lists the risk factors indicating high vascular risk. For this patient group, the ESC/EAS guidelines recommend reducing LDL-C levels by at least half with respect to baseline, with a target level of < 70 mg/dL.
We recommend aiming to reduce LDL-C by at least 50% vs baseline, with a target value of < 70 mg/dL.
Recommended treatment objectives for this patient groupPatients with ischaemic stroke or transient ischaemic attack of non-atherosclerotic aetiology and not presenting high or very high vascular riskThis subgroup includes the remaining patients with history of ischaemic stroke or TIA of non-atherosclerotic origin who present none of the factors associated with high or very high vascular risk listed in Table 3, and therefore not included in the groups addressed above.
We recommend a target LDL-C level of < 100 mg/dL for these patients.
Recommended treatment objectives for this patient groupAdministration of PCSK9 inhibitors to reduce vascular risk is indicated for patients fitting this profile who present LDL-C level < 100 mg/dL despite maximally tolerated statin therapy or who present intolerance to or contraindications for these drugs.
Secondary prevention of cardiovascular events in patients with ischaemic stroke: the role of lipid specialistsSecondary prevention constitutes one of the pillars of the European stroke action plan for 2018-2030.1 It is applicable in practically all patients with stroke or TIA, and can reduce stroke recurrence rates by up to 80%. Secondary prevention encompasses reducing the risk of further strokes, TIAs, or other cardiovascular diseases, as well as such complications as cognitive impairment and dementia, anxiety or mood disorders, fatigue, and poor quality of life.1 Despite this, in general terms, secondary prevention represents an area for improvement in the management of patients with stroke; this is also the case in Spain.4,21–23
Recommendations on the role of specialists in secondary prevention in patients with stroke| It would be advisable for stroke units to have greater involvement in early follow-up and secondary prevention in patients with history of stroke or TIA, until the target LDL-C level is reached. |
| We should continue raising awareness among neurologists of the consequences of high LDL-C levels and the importance of good lipid control in these patients. |
| Specialists should work with the management of each hospital centre, and in multidisciplinary teams with primary care physicians, to progressively incorporate PCSK9 inhibitors into secondary prevention protocols for patients with history of ischaemic stroke and high vascular risk. |
Table 4 presents a list of key points to be addressed in the secondary prevention of patients with history of ischaemic stroke or TIA.1,4,9
Secondary prevention checklist for patients with history of ischaemic stroke or transient ischaemic attack.
| Secondary prevention measures to control modifiable risk factors: |
| Diet: promote healthy diets rich in fruits and vegetables |
| Alcohol consumption: consume alcohol in moderation |
| Smoking: recommend smoking cessation |
| Drug use: cessation of drug use |
| Obesity: recommend a weight control plan and increase physical exercise |
| Sedentary lifestyle: recommend increasing physical exercise |
| Hypertension: address according to clinical practice guidelines |
| Dyslipidaemia: address according to clinical practice guidelines |
| LDL-C is recommended as the main treatment objective. |
| Specialists should continue following up the patients until the target LDL-C value is reached. |
| Follow-up laboratory analyses should be conducted every 8 (± 4) weeks66 until the treatment objective is achieved. |
| Minimum lipid profile parameters: total cholesterol, LDL-C, HDL-C, triglycerides, lipoprotein (a) |
| Atrial fibrillation: address according to clinical practice guidelines on the use of anticoagulants. |
| Diabetes mellitus: address according to clinical practice guidelines. |
| Diagnosis and specific management of concomitant diseases: kidney disease, heart disease, peripheral artery disease, etc. |
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TIA: transient ischaemic attack.
Given the approved indications for evolocumab and alirocumab, up to 80% of patients with atherosclerotic cardiovascular disease in Europe are eligible for secondary prevention with PCSK9 inhibitors.35 To date, 2 cost-effectiveness studies of evolocumab have been conducted in the context of the Spanish National Health System, with diverging results.74,75
Villa et al.74 used a Markov model to evaluate the cost-effectiveness of evolocumab plus statins vs statins alone, assuming lifetime treatment in 2 groups of patients with LDL-C > 100 mg/dL: 1) familial hypercholesterolaemia with or without history of cardiovascular events, and 2) history of cardiovascular events (secondary prevention). The authors report that despite the greater costs associated with evolocumab, the greater reductions in LDL-C levels and in lifetime vascular risk had a greater influence on the incremental cost-effectiveness ratio for patients in both groups who continued to present high vascular risk despite receiving maximally tolerated statin therapy.74
The second study used a decision tree model and a 10-year simulation using a Markov model to analyse the budget impact for the Spanish public healthcare system, based on data from the FOURIER trial.75 The authors conclude that evolocumab treatment is associated with a reduction in the frequency of cardiovascular events, but that it is inefficient from a pharmacoeconomic perspective.75 However, the conclusions of this study have been questioned on account of the methodology used and some of the assumptions made.76 Therefore, it appears necessary to continue studying pharmacoeconomic aspects of PCSK9 inhibitor treatment in clinical practice and to contextualise these aspects as a function of clinical outcomes, including absolute vascular risk and absolute LDL-C levels; these factors determine the number needed to treat to prevent a cardiovascular event.36 It is also important to consider the lifelong impact of these treatments.36 Similarly, in the field of secondary prevention, it is essential to evaluate the cost of PCSK9 inhibitors from the perspective of the costs associated with recurrent stroke, which, compared to a first stroke, typically causes higher levels of disability, functional dependence, morbidity, and mortality, and consequently greater healthcare and social costs.77
The CONOCES study analysed the real annual costs per patient of stroke treated at stroke units in Spain, establishing the cost at €27 711, more than two-thirds of which corresponds to direct costs unrelated to the healthcare system, associated with informal care.78
Future directionsFurther research into the use of PCSK9 inhibitors in secondary vascular prevention in patients with stroke in Spain would deepen our understanding of the effect of these drugs on the different subtypes of ischaemic stroke, as well as their long-term safety and efficacy and their impact on disability and death from cardiovascular causes.
To date, the use of PCSK9 inhibitors in secondary prevention of stroke has been limited to the chronic stage; however, these drugs reduce the level of LDL-C very rapidly (within 1-2 weeks after the first dose)8,32; therefore, it would be interesting to specifically evaluate their use in the acute stage of ischaemic stroke in selected high-risk patients.
ConclusionsThis consensus statement presents a practical review of the available evidence on the use of PCSK9 inhibitors in the secondary prevention of cardiovascular events in patients with history of ischaemic stroke, with a view to informing neurologists about the patient profiles that may benefit the most from this treatment, as well as recommending target LDL-C levels. Our recommendations are based on expert opinion and experience; while these are based on the available evidence, it is important to highlight the fact that only a small part of this evidence is from specifically designed clinical trials including patients with history of stroke; this constitutes the main limitation of this consensus statement. For this reason, we consider it essential to conduct further clinical trials including patients with stroke of clearly defined aetiology, in order to obtain scientific evidence on the stroke subtypes that may benefit the most from these drugs. Table 5 presents the key points made in the article.
Key conclusions of this consensus statement on the use of PCSK9 inhibitors in the secondary prevention of patients with ischaemic stroke or transient ischaemic attack.
| Secondary prevention is fundamental in all patients with stroke or TIA. |
| Based on the recommendations made in different clinical practice guidelines (ESC/EAS, AACE), we recommend a target LDL-C level of < 50 mg/dL for patients with ischaemic stroke or TIA of atherosclerotic origin and for patients with ischaemic stroke or TIA of non-atherosclerotic origin but who present very high cardiovascular risk. A target LDL-C level of < 70 mg/dL is recommended for patients with ischaemic stroke or TIA of non-atherosclerotic origin and high cardiovascular risk. For patients with ischaemic stroke or TIA of non-atherosclerotic origin and no factors indicating high or very high cardiovascular risk, we recommend a target LDL-C level of < 100 mg/dL. |
| Clinical trials of both PCSK9 inhibitors, evolocumab and alirocumab (the FOURIER and ODYSSEY Outcomes trials, respectively), found that both drugs decrease the LDL-C level by approximately 60% in patients receiving high- or moderate-intensity statin therapy at baseline. |
| PCSK9 inhibitors have been shown to significantly reduce the risk of cardiovascular events in patients with cardiovascular disease and elevated LDL-C or non-HDL cholesterol levels despite moderate- or high-intensity statin therapy. |
| PCSK9 inhibitors are indicated in secondary prevention to reduce cardiovascular risk in patients with established atherosclerotic cardiovascular disease. |
| In the light of the currently available evidence, we consider that excessively low LDL-C levels do not exist, and that no relevant safety issues exist in association with low LDL-C levels; therefore, we do not recommend modifying lipid-lowering therapy in patients achieving the target LDL-C levels, unless they present adverse reactions. |
| Secondary prevention of cardiovascular events with PCSK9 inhibitors is indicated for all patients with ischaemic stroke of any aetiology and LDL-C levels > 100 mg/dL, receiving maximally tolerated statin therapy or with intolerance to or contraindications for statins (based on the guidelines of the therapeutic position statement). |
| PCSK9 inhibitors present a favourable safety profile, even in patients with low LDL-C and diabetes mellitus. They have not been associated with increased risk of muscle or hepatobiliary issues, intracranial haemorrhage, or cognitive alterations. |
| Neurologists should continue following up lipid levels in patients under secondary prevention following stroke until the target LDL-C level is reached. |
| It is essential to conduct further clinical trials including patients with stroke with clearly defined aetiologies, in order to obtain scientific evidence on the stroke subtypes that may benefit the most from these drugs. |
HDL: high-density lipoprotein; LDL-C: low-density lipoprotein cholesterol; PCSK9: proprotein convertase subtilisin/kexin type 9; TIA: transient ischaemic attack.
To conduct this project, we received logistical support from Amgen SA. The opinions, interpretations, and conclusions expressed are solely the authors’ own.
Conflicts of interestAGN has received consulting fees and lecture honoraria from AGA Medical Corporation, Almirall, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Esteve, Ferrer, MSD, Novartis, Pfizer, Sanofi-Aventis, Servier, Solvay, and Uriach.
JM, MC, JFA, and FP have received consulting fees from Amgen.
TS has received consulting fees and lecture honoraria from Amgen, AstraZeneca, Boehringer Ingelheim, and Bayer.
EP has received consulting fees and lecture honoraria from Esteve, Rovi, MSD, and Amgen.
PC has received honoraria from Amgen, Boheringer Ingelheim, Daiichi Sankyo, and Ferrer.
JT has received consulting fees and lecture honoraria from Amgen, Allergan, and Zambon Pharma.
MC has received consulting fees and lecture honoraria from Amgen, Boehringer Ingelheim, Daiichi Sankyo, and Allergan.
The authors are grateful to Amgen SA for supporting this project and to Ogilvy Health and Paula Martín Vaquero, PhD for the methodological support and assistance with editing and drafting this consensus statement.
Please cite this article as: Gil-Núñez A, Masjuan J, Montaner J, Castellanos M, Segura T, Cardona P, et al. Inhibidores de la proproteína convertasa subtilisina/kexina tipo 9 (iPCSK9) en la prevención secundaria de episodios vasculares en pacientes con ictus isquémico: Documento de consenso y aplicaciones prácticas. Neurología. 2022;37:136–150.