The recent development of highly effective treatments for multiple sclerosis (MS) and the potential risk of infectious complications require the development of prevention and risk minimisation strategies. Vaccination is an essential element of the management of these patients. This consensus statement includes a series of recommendations and practical scenarios for the vaccination of adult patients with MS who are eligible for highly effective immunosuppressive treatments.
MethodologyA formal consensus procedure was followed. Having defined the scope of the statement, we conducted a literature search on recommendations for the vaccination of patients with MS and specific vaccination guidelines for immunosuppressed patients receiving biological therapy for other conditions. The modified nominal group technique methodology was used to formulate the recommendations.
DevelopmentVaccination should be considered in patients eligible for immunosuppressive therapy before the treatment is started, if the patient’s clinical situation allows it. We recommend administering the vaccines included in the adult vaccination calendar and some specific vaccines, depending on the patient’s pre-existing immune status. Live attenuated vaccines are contraindicated when immunosuppressive treatment has already started. For vaccines providing a correlate of protection, serological response should be monitored after an interval of 1–2 months after the last dose.
La reciente aparición de tratamientos de alta efectividad para el tratamiento de la Esclerosis Múltiple (EM) con potencial riesgo de complicaciones infecciosas obliga plantear estrategias de prevención y minimización de riesgos. La vacunación constituye una parte esencial del manejo de estos pacientes. Este consenso recoge una serie de pautas y escenarios prácticos de vacunación en pacientes adultos con EM candidatos a tratamiento inmunosupresor.
MetodologíaSe llevó a cabo un consenso de tipo formal. Tras definir el alcance del documento, se realizó una búsqueda bibliográfica de vacunación en pacientes con EM así como guías de vacunación específicas de pacientes inmunosuprimidos y en tratamiento biológico con otras patologías. Para la formulación de las recomendaciones se empleó la metodología de Modified Nominal Group Technique.
DesarrolloLa vacunación en pacientes candidatos a tratamiento inmunosupresor se debe plantear antes de iniciar un tratamiento inmunosupresor siempre que la situación clínica del paciente lo permita. Se recomendarán tanto aquellas indicadas en el calendario vacunal del adulto, como algunas específicas, en función de la inmunidad previa. Si ya está instaurado el tratamiento inmunosupresor las vacunas vivas atenuadas estarán contraindicadas. Para aquéllas vacunas que dispongan de un correlato de protección se recomienda monitorizar la respuesta serológica transcurridos 1–2 meses de la última dosis.
Multiple sclerosis (MS), an inflammatory demyelinating disease of the central nervous system, represents the leading cause of non-traumatic disability in young adults.1 Recent years have seen significant advances in the long-term prognosis of patients with MS, largely because regulators have approved a range of highly active immunotherapy medications with demonstrated effects on disease progression and the prevention of disability.2–4 These drugs include monoclonal antibodies (natalizumab, alemtuzumab, and ocrelizumab) and oral medications (fingolimod, dimethyl fumarate, and teriflunomide). The action mechanisms of these drugs, which include alteration of lymphocyte trafficking, lymphocyte depletion, and disruption of lymphocyte replication, are associated with a potential risk of reactivation of latent pathogens, worsening of chronic asymptomatic infections, and new infections.5 These infectious episodes may increase the risk of MS relapses,6–8 worsening of existing symptoms (pseudorelapses),9 and complications (hospitalisation and mortality).10
Therefore, to provide individualised treatment to these patients we must seek a balance between effectiveness and adverse events, and incorporate a series of preventive strategies into management algorithms to minimise risks. Many of these risks for patients with MS receiving highly active therapies can be prevented with vaccination. In recent years, various guidelines and reviews have recommended vaccination of patients with MS who are eligible for immunosuppressant therapy,11–14 based on previous experience with other autoimmune disorders.15–17 However, a certain resistance to vaccination persists, and vaccine coverage among these patients and their households remains low. The main reasons for this are concerns about the safety and effectiveness of vaccines, together with a lack of clear guidelines on how to approach vaccination. Several studies conducted over the last 2 decades have demonstrated that inactivated vaccines, such as those for influenza, tetanus, hepatitis B, and human papillomavirus, do not increase the risk of MS or relapses18–21; however, less evidence is available on the safety of live-attenuated vaccines.22–24 Theoretically, highly active therapies for MS may reduce the effectiveness of vaccines since they reduce the patient’s capacity to generate an immune response; however, this has not been systematically evaluated25,26 and the available studies on the subject report contradictory findings.27–31
While it is increasingly apparent that vaccination is an important part of risk management strategies for patients with MS receiving immunosuppressants,32–34 doubts remain in clinical practice about which specific vaccines to recommend, when they should be administered, and which specific drugs may influence patients’ response to the vaccine. A considerable amount of new evidence on the vaccination of patients with other autoimmune diseases has been published in the last decade, and can be extrapolated to patients with MS.26,35 In this context, there has been growing demand among neurologists and other professionals including primary care physicians for a national consensus document that may serve as a reference to assist professionals in deciding upon the best approach to vaccination for their patients. In this consensus statement, we propose a series of practical recommendations and scenarios for the vaccination of adult patients with MS. The document addresses relevant aspects of safety and immunogenicity, as well as the best time to administer vaccines in an optimal strategy.
MethodsThese recommendations were prepared in response to a general request by members of the Spanish Society of Neurology’s Study Group for Demyelinating Diseases. The document was drafted according to a formal consensus methodology including the steps summarised below.
- 1
Constitution of the working group: a multidisciplinary working group was created including experts on MS from across Spain, as well as several experts on preventive medicine and infectious diseases.
- 2
Determination of the scope of the recommendations: during a preparatory meeting held in person, members of the working group selected the issues to be addressed in the document, the specific methodology to be used for establishing consensus, and the general programme of work and distribution of tasks.
- 3
Literature search and synthesis of evidence: a literature search was conducted to gather evidence on the vaccination of patients with MS. We also took into account national and international recommendations on the vaccination of immunosuppressed patients36–42 and specific vaccination guidelines for patients receiving biological therapies for other autoimmune diseases.16,43–46 The preventive medicine experts in the group synthesised the evidence and proposed a set of recommendations to be submitted to the consensus process.
- 4
Formulation of the recommendations: consensus on the recommendations included in the document was established according to the modified nominal group technique,47 a highly structured procedure for reaching expert consensus on topics for which little evidence is available and expert opinions are important. The consensus process was performed in 2 phases, and a threshold of 80% agreement was established a priori for accepting a recommendation. In phase 1, each recommendation was submitted to a first round of individual voting via e-mail, in which all members of the working group used a 9-point Likert-type scale, scored as follows: 1-3: inappropriate strategy; 4-6: unclear; 7-9: appropriate strategy. During this phase, members of the working group were invited to add new recommendations or to modify the existing proposals in the event of disagreement. In phase 2, the new/amended recommendations were again forwarded to all members of the group for a final review. Individual voting was only repeated for recommendations with less than 80% consensus in the previous phase. The results of the consensus process can be consulted in the Supplementary Material (available online).
- 5
Complete review and approval of the manuscript by all members of the working group.
Vaccines are immunobiological products that can safely induce a sufficient antibody response to immunise an individual against specific infections. They can be classified by the type of antigen and the means of production (live-attenuated and inactivated vaccines). Live-attenuated vaccines are composed of live microorganisms whose virulence is removed through a range of procedures, and which are able to stimulate a cellular or humoural immune response similar to that induced by the natural infection. These vaccines are highly immunogenic and provide lasting protection. Inactivated vaccines are manufactured either from the complete microorganism or one of its parts (polysaccharides, proteins, toxoids, subunits, etc), which through various physical or chemical processes lose their virulence and ability to replicate, but not their immunogenicity.
Healthy adults are considered properly immunised if they have received the following vaccines37,40:
- 1
Five doses of DTaP/Td. Vaccination should be started or completed, as applicable, with up to 5 doses of a vaccine including a tetanus component. Primary vaccination in adults comprises 3 doses of Td (with a minimum of one month between the first and second doses and 6 months between the second and third), followed by 2 booster doses, with an interval of 1-10 years between primary vaccination and the first booster dose and between booster doses. A final booster dose should be administered at approximately 65 years of age. However, the vaccines committee of the Spanish Society of Preventive Medicine, Public Health, and Hygiene (SEMPSPH) and international bodies including the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices (ACIP) continue to recommend vaccination with Td or (preferably) Tdap every 10 years throughout adulthood.37,48
- 2
Three doses of poliomyelitis vaccine.
- 3
Two doses of measles vaccine (measles, mumps, and rubella [MMR] vaccine) or natural immunity demonstrated by serology findings.
- 4
Two doses of varicella vaccine, history of the infection, or natural immunity demonstrated by serology findings.
- 5
Annual doses of influenza vaccine from the age of 65 years. In some Spanish autonomous regions, the cut-off age for recommending influenza vaccination is 60 years or older. The SEMPSPH recommends vaccinating individuals aged 50 or older.
- 6
One dose of 23-valent pneumococcal vaccine after the age of 65 years. The SEMPSPH vaccines committee and national and international bodies including the ACIP49 and a working group of members of various Spanish scientific societies38 recommend systematic vaccination of individuals aged 65 and older, following a sequential schedule: initial vaccination with 13-valent conjugate vaccine, followed by 23-valent polysaccharide vaccine.
Both inactivated and live-attenuated vaccines are safe biological products and can be administered to patients with MS. These patients, and especially those who will need immunosuppressive therapy at some point during the progression of their disease, are eligible to receive vaccines due to the increased risk of infection and/or infectious complications derived both from the disease itself and from the treatment.32,34,50,51 They should receive both those indicated in the adult immunisation schedule (tetanus, MMR, varicella) and other specific vaccines.
While vaccines can be administered at any point during disease progression, it should be noted that in patients receiving immunosuppressants, live-attenuated vaccines are contraindicated, and response to inactivated vaccines may be reduced, as the integrity of the immune system influences the immunogenicity of vaccines.26,52 Therefore, whenever the patient’s clinical situation allows it, vaccines should be administered at the time of diagnosis, before immunosuppressive therapy is started. If the patient’s usual hospital has a preventive medicine department with a vaccination clinic, the patient should be referred to that unit.
Below, we present the key aspects of the vaccination of patients with MS, taking into account the moment of disease progression (before or after onset of immunosuppressive therapy).
Identifying the necessary vaccinesA dual strategy is recommended for determining a patient’s vaccination needs:
- 1
The patient’s vaccination history should be gathered, with special emphasis placed on immunisation against seasonal influenza, pneumococcus, hepatitis A and B viruses, tetanus/diphtheria, varicella, and measles.
- 2
Pre-vaccination serology studies should be performed for the following markers: anti–hepatitis A virus IgG antibodies (HAV IgG), hepatitis B virus surface antigen (HBsAg), anti–hepatitis B virus core (anti-HBc) antibodies, anti–hepatitis B virus surface antigen (anti-HBs) antibodies, anti–measles virus IgG antibodies, anti–rubella virus IgG antibodies (only needed in women of childbearing age), and anti–varicella zoster virus IgG antibodies.
This group includes patients eligible for immunosuppressive therapy who are not currently receiving any disease-modifying drug, or who are receiving immunomodulatory treatments (interferons or glatiramer acetate).
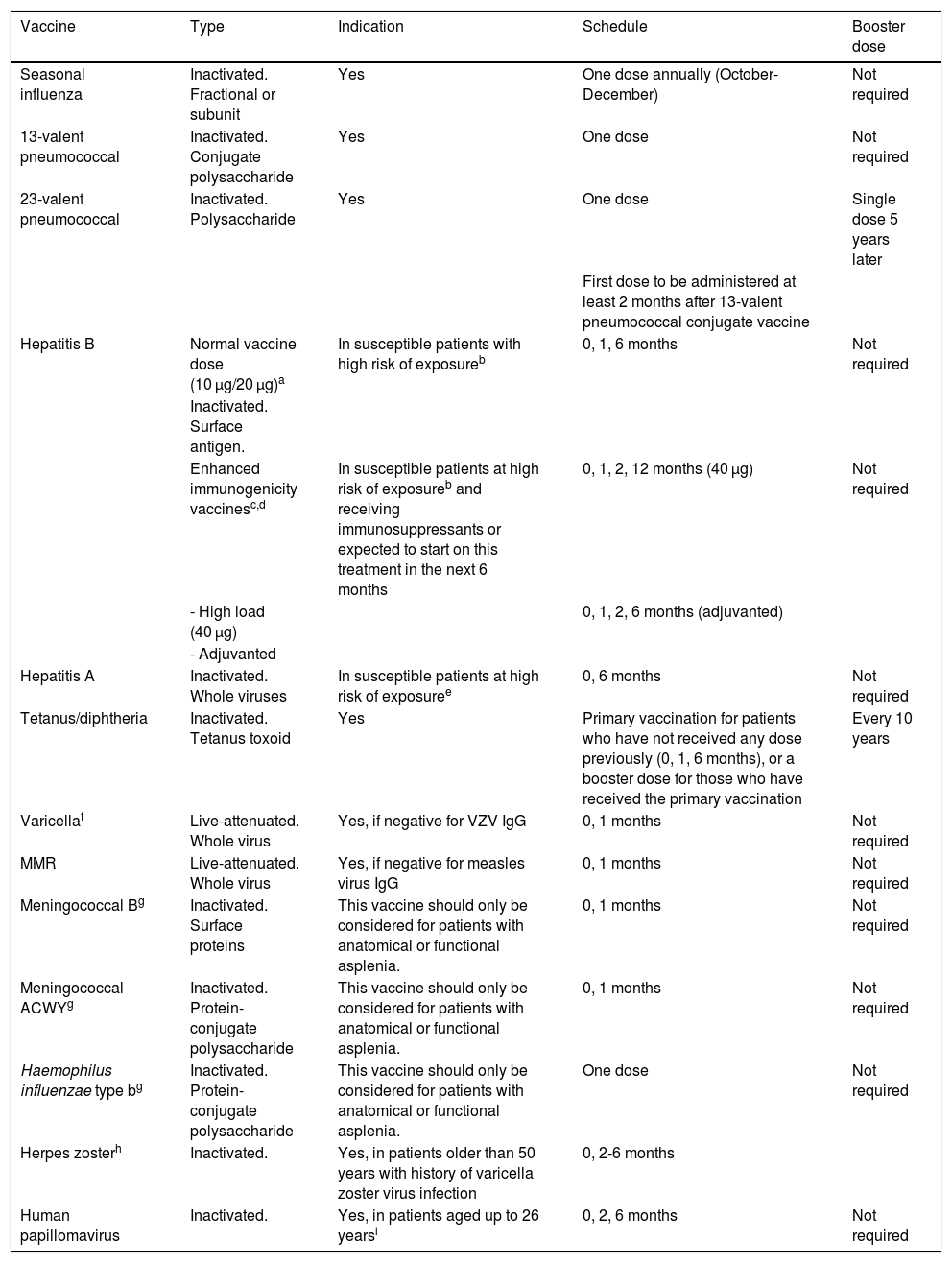
Table 1 summarises the characteristics, indications, and administration schedules of the vaccines recommended for patients with MS potentially needing immunosuppressive therapy:
- 1
Systematic vaccines on the adult immunisation schedule:
- -
Tetanus (one dose of Td or Tdap) if no booster dose was administered in the last 10 years and 3 primary vaccination doses were administered.
- -
Varicella and/or MMR (2 separate doses with a minimum interval of 4 weeks) if serology findings are negative.
- -
- 2
Hepatitis B: primary vaccination against hepatitis B virus (HBV) is recommended in patients negative for anti-HBs antibodies and at risk of exposure (sexual contact, close contact with an infected individual, patients receiving dialysis, intravenous drug users, men who have sex with men, individuals with multiple sexual partners, sex workers, healthcare workers presenting occupational risk, patients with specific comorbidities [HIV or hepatitis C virus infection, chronic liver disease, patients undergoing solid organ transplantation or haematopoietic stem cell transplantation], individuals receiving blood products).53
Vaccines to consider in patients with multiple sclerosis eligible for immunosuppressive therapy.
| Vaccine | Type | Indication | Schedule | Booster dose |
|---|---|---|---|---|
| Seasonal influenza | Inactivated. Fractional or subunit | Yes | One dose annually (October-December) | Not required |
| 13-valent pneumococcal | Inactivated. Conjugate polysaccharide | Yes | One dose | Not required |
| 23-valent pneumococcal | Inactivated. Polysaccharide | Yes | One dose | Single dose 5 years later |
| First dose to be administered at least 2 months after 13-valent pneumococcal conjugate vaccine | ||||
| Hepatitis B | Normal vaccine dose (10 μg/20 μg)a | In susceptible patients with high risk of exposureb | 0, 1, 6 months | Not required |
| Inactivated. Surface antigen. | ||||
| Enhanced immunogenicity vaccinesc,d | In susceptible patients at high risk of exposureb and receiving immunosuppressants or expected to start on this treatment in the next 6 months | 0, 1, 2, 12 months (40 μg) | Not required | |
| - High load (40 μg) | 0, 1, 2, 6 months (adjuvanted) | |||
| - Adjuvanted | ||||
| Hepatitis A | Inactivated. Whole viruses | In susceptible patients at high risk of exposuree | 0, 6 months | Not required |
| Tetanus/diphtheria | Inactivated. Tetanus toxoid | Yes | Primary vaccination for patients who have not received any dose previously (0, 1, 6 months), or a booster dose for those who have received the primary vaccination | Every 10 years |
| Varicellaf | Live-attenuated. Whole virus | Yes, if negative for VZV IgG | 0, 1 months | Not required |
| MMR | Live-attenuated. Whole virus | Yes, if negative for measles virus IgG | 0, 1 months | Not required |
| Meningococcal Bg | Inactivated. Surface proteins | This vaccine should only be considered for patients with anatomical or functional asplenia. | 0, 1 months | Not required |
| Meningococcal ACWYg | Inactivated. Protein-conjugate polysaccharide | This vaccine should only be considered for patients with anatomical or functional asplenia. | 0, 1 months | Not required |
| Haemophilus influenzae type bg | Inactivated. Protein-conjugate polysaccharide | This vaccine should only be considered for patients with anatomical or functional asplenia. | One dose | Not required |
| Herpes zosterh | Inactivated. | Yes, in patients older than 50 years with history of varicella zoster virus infection | 0, 2-6 months | |
| Human papillomavirus | Inactivated. | Yes, in patients aged up to 26 yearsi | 0, 2, 6 months | Not required |
The standard dose for adults (20 μg/10 μg, according to the specific product) may be used if immunosuppressive treatment is not scheduled in the next 6 months.
HBs IgG < 10 IU/mL and exposure risk (sexual contact, close contact with an infected individual, patients receiving dialysis, intravenous drug users, men who have sex with men, individuals with multiple sexual partners, sex workers, healthcare workers presenting occupational risk, patients with specific comorbidities [HIV or hepatitis C virus infection, chronic liver disease, patients undergoing solid organ transplantation or haematopoietic stem cell transplantation], individuals receiving blood products).53
Immunosuppressed patients and those starting on immunosuppressants in the next 6 months. Enhanced immunogenicity vaccines include high-dose (HBVaxpro® 40 μg) and adjuvanted formulations (Fendrix®).
In the event of non-response to primary vaccination, revaccination may be considered with vaccines adjuvanted with AS04C (0, 1, 2, 6 months) or double dose of HBV (40 μg; 0, 1, 6 months).53
HAV IgG < 20 IU/mL and high risk of exposure due to risky sexual behaviour or substance abuse (men who have sex with men, individuals with multiple sexual partners, sex workers, alcohol abuse, intravenous drug use), specific comorbidities (chronic liver disease, liver transplantation), occupational risk, and/or travel to endemic regions.55
In patients not scheduled to receive immunosuppressants in the next 6 months, HBV vaccination can be administered at the normal adult dosage (20 µg/10 µg, depending on the product), in 3 doses (0, 1, and 6 months). Patients who are scheduled to receive immunosuppressants in the next 6 months should receive other products that present enhanced immunogenicity (Table 1): adjuvanted HBV vaccines (20 µg) or double-dose versions (40 µg), which are administered in 4 doses, as established in the summary of product characteristics (0, 1, 2, and 12 months for HBV 40 µg and 0, 1, 2, and 6 months for adjuvanted HBV 20 µg). While this is an off-label indication, it is endorsed by official guidelines.53 The same vaccine preparation should be used until the course is completed.
In all cases, vaccine response should be confirmed via serology testing, which may be performed from one month after the last dose. In the event of negative serology results after vaccination, revaccination with either of these enhanced immunogenicity vaccines is recommended. In patients presenting no response after 2 courses of vaccination, we recommend prophylaxis with a specific immunoglobulin (0 and 1 month) in the event of exposure.
- 3
Pneumococcus: a sequential schedule should be administered, with an initial dose of 13-valent pneumococcal conjugate vaccine and a subsequent dose of 23-valent pneumococcal polysaccharide vaccine at least 2 months afterwards. A single booster dose of 23-valent pneumococcal polysaccharide vaccine should be administered at least 5 years later.26 Anti-pneumococcal vaccination is particularly important in patients receiving treatments that cause B-cell depletion, as severe respiratory infections have been reported in phase III trials.54,55
- 4
Influenza: one dose of influenza vaccine should be administered annually, at any time during the influenza season (October-March), preferably at the beginning of this period. If available, the quadrivalent vaccine (H1N1, H3N2, B/Victoria, and B/Yamagata) is preferable in immunosuppressed patients and the adjuvanted vaccine is recommended for those older than 65 years.53,56
- 5
Hepatitis A: primary vaccination against hepatitis A virus is recommended for susceptible patients with high risk of exposure due to risky sexual behaviour or substance abuse (men who have sex with men, individuals with multiple sexual partners, sex workers, alcohol abuse, intravenous drug use), specific comorbidities (chronic liver disease, liver transplantation), occupational risk, and/or travel to endemic regions.57
- 6
Human papillomavirus: vaccination against human papillomavirus is performed routinely in Spain. It is recommended for women aged up to 26 years; the nonavalent vaccine is recommended. Women with MS who are scheduled to receive treatment with alemtuzumab or fingolimod may benefit from this vaccination independently of their age, as post-marketing surveillance data have demonstrated that these drugs are associated with increased risk of genital warts and cervical dysplasia. Furthermore, women indicated for cervical conisation due to moderate or severe cervical intraepithelial neoplasia (CIN 2+) or endocervical adenocarcinoma in situ should be vaccinated regardless of age. The available evidence does not support human papillomavirus vaccination for men with MS aged under 26 who are eligible for immunosuppressive treatment; however, these patients may benefit from the vaccination due to protection against anal, penile, or oropharyngeal cancer.39
- 7
Herpes zoster: the inactivated herpes zoster virus vaccine is recommended for patients aged 50 or older with history of infection with the virus.58 Patients receiving drugs associated with increased risk of infection (eg, fingolimod, cladribine, natalizumab, and alemtuzumab) may benefit from this vaccine. The herpes zoster virus vaccine was approved by the European Medicines Agency on 21 April 2018, and therefore can be distributed to all member states of the European Union. However, it is not expected to be marketed in Spain until 2020.53
- 8
Haemophilus influenzae and meningiococcal vaccines are only recommended in cases of asplenia or suspected functional hyposplenism.59
For vaccines for which a correlate of protection is available, serological response should be monitored one to 2 months after the last dose. A safety interval should be observed between the last dose and the onset of immunosuppressive therapy, as follows:
- -
For inactivated vaccines: no safety interval is needed, although it is preferable to administer the vaccine at least 2 weeks prior to onset of immunosuppression to ensure an optimal immune response.
- -
For live-attenuated vaccines, a safety period of at least 4 weeks should be observed. A period of 6 weeks is specified in the summaries of product characteristics for alemtuzumab and ocrelizumab.
This group includes patients receiving any of the following immunosuppressive disease-modifying drugs: rituximab, alemtuzumab, ocrelizumab, mitoxantrone, azathioprine, cladribine, cyclophosphamide, natalizumab, fingolimod, teriflunomide, or dimethyl fumarate. It should be noted that the potential risk of infection varies according to the therapeutic target and action mechanism of each drug60; therefore, recommendations about vaccination must be made on an individual basis.
Furthermore, corticosteroid treatment is considered to be immunosuppressive at doses equal to or greater than 20 mg/day of prednisone or equivalent doses of other corticosteroids, if the treatment course is equal to or longer than 15 days. Treatment with high-dose boluses of corticosteroids (1 g/day) for 3 or 5 days seems not to have an immunosuppressive effect,61 although no clear recommendations have been made on this subject due to a lack of evidence.
In patients already receiving potentially immunosuppressive drugs, the same vaccines specified for the previous group should be evaluated, taking into account the following points:
- -
No safety problems are associated with the administration of inactivated vaccines during immunosuppressive therapy; however, immunogenicity may be reduced, particularly in patients using drugs with a stronger immunosuppressive effect (rituximab, ocrelizumab, cladribine, and alemtuzumab).62 We therefore recommend the use of enhanced immunogenicity vaccines (for vaccination against HBV) and monitoring of serological response one to 2 months after the last dose for vaccines for which a correlate of protection is available.
- -
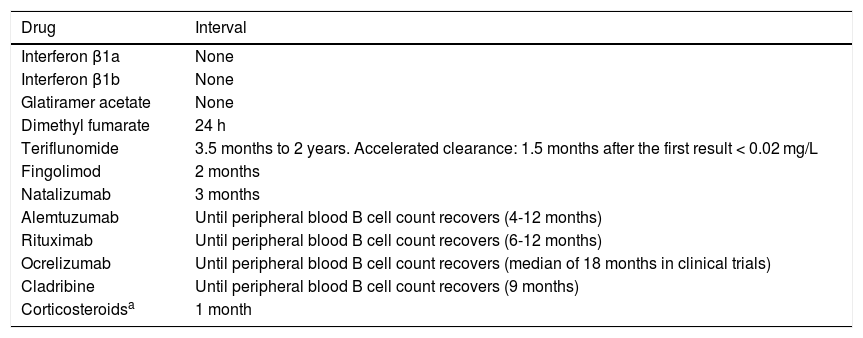
Live-attenuated vaccines are contraindicated. Patients susceptible to varicella or measles should be treated with post-exposure prophylaxis in the event of potential exposure, within 5 days of exposure for varicella and 6 days for measles.42 Immunisation should also be considered in the future in the event that immunosuppressive therapy is suspended for a sufficient period to enable clearance of the drug and its immunosuppressive effect, ensuring the safety of the vaccine. While there are no specific indicators that guarantee immune restoration, some authors suggest that determining basic immunological parameters (such as the subpopulations of CD4, CD8, and CD19 cells) and total immunoglobulin levels, as well as waiting at least 5 half-lives of the biological agent, may be useful approaches.63 Based on this approach and the summaries of product characteristics for the different drugs, we recommend observing the safety periods specified in Table 2.
Table 2.Intervals to be observed between the end of immunosuppressive therapy and the administration of live-attenuated vaccines.
Drug Interval Interferon β1a None Interferon β1b None Glatiramer acetate None Dimethyl fumarate 24 h Teriflunomide 3.5 months to 2 years. Accelerated clearance: 1.5 months after the first result < 0.02 mg/L Fingolimod 2 months Natalizumab 3 months Alemtuzumab Until peripheral blood B cell count recovers (4-12 months) Rituximab Until peripheral blood B cell count recovers (6-12 months) Ocrelizumab Until peripheral blood B cell count recovers (median of 18 months in clinical trials) Cladribine Until peripheral blood B cell count recovers (9 months) Corticosteroidsa 1 month Based on: summary of product characteristics for each drug; Vacunación en grupos de riesgo de todas las edades y en determinadas situaciones, Comisión de Salud Pública del Consejo Interterritorial del Sistema Nacional de Salud. Ministerio de Sanidad, Consumo y Bienestar Social, July 2018; Rubin, et al.42
The vaccines recommended for international travel vary according to the destination, the type of trip, and patient characteristics.64 They include vaccinations against hepatitis A, typhoid fever (oral or intramuscular), rabies, meningococcus (quadrivalent conjugate vaccine), yellow fever, Japanese encephalitis, and tick-borne encephalitis. All these vaccines are inactivated, with the exception of the yellow fever and oral anti-typhoid vaccines, which are live-attenuated and are therefore contraindicated in immunosuppressed patients. Patients planning trips to tropical or subtropical regions should be evaluated at specialised clinics offering travel advice, and vaccines should be indicated on an individual basis, with the risks and benefits of each vaccine to be taken into account.
PregnancyThe vaccine indications for pregnant patients with MS are the same as those for pregnant women in the general population. Only live-attenuated vaccines are contraindicated during pregnancy due to the theoretical risk of infection of the fetus. The 2 most strongly recommended vaccines during pregnancy are65:
- -
Influenza vaccination early in the influenza season (October-November), in any trimester of pregnancy.
- -
Vaccination against diphtheria, tetanus, and whooping cough (Tdap) from week 27 of pregnancy, and preferably before week 36, to ensure optimal transfer of anti-pertussis antibodies to the fetus. All pregnant women should receive this vaccine, regardless of whether the Td vaccine has been administered previously.
Households and close contacts of patients with MS receiving immunosuppressants should be vaccinated annually against influenza. Members of the household who are not immune to measles and/or varicella (through vaccination or natural immunity) should also be administered the MMR and/or varicella vaccines if the patient is not properly immunised against these infections.
ConclusionsThe recent paradigm shift in the treatment of patients with MS has made it essential to adopt a correct approach to prevention. As live-attenuated vaccines cannot be administered after the onset of immunosuppressive therapy, and inactivated vaccines are potentially less immunogenic, vaccination should be considered in the early stages of the disease in patients eligible for immunosuppressive therapy, wherever the patient’s clinical situation permits this. Given the lack of evidence on the effectiveness of vaccination in combination with the different drugs used to treat MS, response to the vaccine should be tested wherever valid measures are available.
These consensus recommendations are based on the current evidence and on clinical experience from other fields on the vaccination of immunosuppressed patients. The recommendations are subject to modification as further evidence becomes available on the long-term impact on the risk of infection associated with the new highly active therapies for MS. Furthermore, some new vaccines in advanced stages of development may be indicated for these patients.66–68 Therefore, the working group intends to publish updated recommendations in 3 years’ time, or sooner if there are substantial changes in the evidence.
Conflicts of interestJ. Ara has received consultancy fees from Novartis and Merck Serono and lecture honoraria, travel expenses for scientific conferences, and research funding from Bayer, Biogen Idec, Genzyme, Merck Serono, Novartis, and Sanofi-Aventis.
Y. Blanco has received consultancy fees and lecture honoraria from Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, and Teva Pharmaceuticals.
O. Carmona has received consultancy fees and lecture honoraria from Merck, Novartis, Roche, Genzyme, and Biogen.
S. Eichau has received consultancy fees and lecture honoraria from Roche, Novartis, Merck, Sanofi-Genzyme, Biogen, and Almirall.
C. García-Vidal was awarded a Strategic plan for research and innovation in health-PERIS 2016–2020 grant and is a member of the FungiCLINIC Research Group (AGAUR-Project 2017SGR1432, Catalan Health Service). She has received lecture honoraria from Gilead Science, MSD, Novartis, Pfizer, and Jannsen, and a grant from Gilead Science.
J.A. García-Merino has received lecture honoraria, consultancy fees, and travel expenses from Genzyme, Merck, Novartis, Roche, Biogen, and Teva, and research funding from Novartis, Genzyme, and Biogen.
M. González Platas has received lecture honoraria from Novartis, Genzyme, Sanofi Aventis, Roche, and Merck.
J.E. Meca-Lallana has received subsidies, lecture honoraria, and consultancy fees from Almirall, Biogen, Celgene, Genzyme, Merck, Novartis, Roche, and Teva.
E. Moral has received lecture honoraria and consultancy fees from Actelion, Almirall, Bayer, Merck, Biogen, Sanofi-Genzyme, Roche, and Teva.
S. Otero-Romero has received lecture honoraria and consultancy fees from Genzyme, Biogen-Idec, Novartis, Excemed, and MSD, and research funding from Novartis, Bayer HealthCare Pharmaceuticals, and Biogen.
J.M. Prieto has received consultancy fees from Idec Inc., Genzyme Corporation, Merck Serono, Novartis Pharmaceuticals Corporation, Sanofi-Aventis, Teva Pharmaceuticals, Roche Pharma, and Almirall Prodesfarma S.A; lecture honoraria from Almirall Prodesfarma S.A., Bayer HealthCare Pharmaceuticals, Biogen Idec Inc, Genzyme Corporation, Merck Serono, Novartis Pharmaceuticals Corporation, Sanofi-Aventis, Teva Pharmaceuticals, and Roche; and research funding from Almirall Prodesfarma S.A., Biogen Idec, Novartis Pharmaceuticals Corporation, and Sanofi Genzyme S.A.
A. Rodríguez-Antigüedad has received lecture honoraria, consultancy fees, and travel expenses from Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva.
J. Rodríguez has received lecture honoraria and consultancy fees from Pfizer, GSK, Sanofi Aventis, and Biogen.
M. Tintoré has received lecture honoraria and consultancy fees from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, and Teva Pharmaceuticals. She is co-editor of the journal Multiple Sclerosis Journal-Experimental, Translational and Clinical.
A. Vilella has participated in vaccine trials sponsored by Pfizer, MSD, and GSK.
M. Calles, B. Casanova, L. Costa-Frossard, M. Llaneza, J. Meca, E. Moral, and M. Martínez-Ginés have no conflicts of interest to declare.
The authors would like to thank Azalia Martín Almazán for her technical assistance.
Please cite this article as: Otero-Romero S, Rodríguez-García J, Vilella A, Ara JR, Brieva L, Calles C, et al. Recomendaciones para la vacunación en pacientes con esclerosis múltiple candidatos a terapias inmunosupresoras: documento de consenso español. Neurología. 2021;36:50–60.