To study the clinico-radiological paradox in multiple sclerosis (MS) relapse by analyzing the number and location of gadolinium-enhanced (Gd+) lesions on brain MRI before methylprednisolone (MP) treatment.
MethodsWe analyzed brain MRI from 90 relapsed MS patients in two Phase IV multicenter double-blind randomized clinical trials that showed the noninferiority of different routes and doses of MP administration. A 1.5- or 3-T brain MRI was performed at baseline before MP treatment and within 15 days of symptom onset. The number and location of Gd+ lesions were analyzed. Associations were studied using univariate analysis.
ResultsSixty-two percent of patients had at least 1 Gd+ brain lesion; the median number was 1 (interquartile range 0–4), and 41% of patients had 2 or more lesions. The most frequent location of Gd+ lesions was subcortical (41.4%). Gd+ brain lesions were found in 71.4% of patients with brainstem-cerebellum symptoms, 57.1% with spinal cord symptoms and 55.5% with optic neuritis (ON). Thirty percent of patients with brain symptoms did not have Gd+ lesions, and only 43.6% of patients had symptomatic Gd+ lesions. The univariate analysis showed a negative correlation between age and the number of Gd+ lesions (p=0.002).
ConclusionMost patients with relapse showed several Gd+ lesions on brain MRI, even when the clinical manifestation was outside of the brain. Our findings illustrate the clinico-radiological paradox in MS relapse and support the value of brain MRI in this scenario.
Estudiar la paradoja clínico-radiológica en el brote de la esclerosis múltiple (EM) mediante el análisis de lesiones captantes de gadolinio (Gd+) en la RM cerebral antes del tratamiento con metilprednisolona (MP).
MétodosAnalizamos la RM cerebral basal de 90 pacientes con EM en brote de 2 ensayos clínicos aleatorizados multicéntricos fase IV que demostraron la no inferioridad de diferentes vías y dosis de MP, realizadas antes del tratamiento con MP y en los 15 días siguientes a la aparición de los síntomas. Se analizaron el número y la localización de las lesiones Gd+. Se estudiaron las asociaciones mediante análisis univariado.
ResultadosEl 62% de los pacientes tenía al menos una lesión Gd+cerebral y el 41% de los pacientes tenía 2 o más lesiones. La localización más frecuente fue la subcortical (41,4%). Se encontraron lesiones Gd+cerebrales en el 71,4% de los pacientes con síntomas de tronco cerebral o cerebelo, en el 57,1% con síntomas medulares y en el 55,5% con neuritis óptica. El 30% de los pacientes con síntomas cerebrales no tenían lesiones Gd+ y sólo el 4,.6% de los pacientes tenían lesiones Gd+sintomáticas. El análisis univariante mostró una correlación negativa entre la edad y el número de lesiones Gd+(p=0,002).
ConclusionesLa mayoría de los pacientes en brote mostraron varias lesiones Gd+en la RM cerebral, incluso cuando la manifestación clínica fue medular u óptica. Nuestros hallazgos ilustran la paradoja clínico-radiológica en el brote de la EM y apoyan el valor de la RM cerebral en este escenario.
Brain MRI is the most useful tool for multiple sclerosis (MS) diagnosis, helping to determine its dissemination in time and space.1 Gadolinium enhancement (Gd+) is a sensitive technique for detecting active MS lesions and confirms the inflammatory activity of the disease. The correlation between Gd+ lesions and clinical disease activity is not well known.2,3
Previous studies have shown that Gd+ lesions are not a good surrogate marker for relapses4 because of the poor correlation between MRI lesions and the clinical disease course.5 This “clinico-radiological paradox” was described by Barkhof in 2002.6 Nevertheless, brain MRI has value in monitoring treatment effects and disease activity, serving as an important secondary outcome measure in Phase III clinical trials and often being used as the primary outcome in exploratory Phase II studies.7,8
There are few published studies that evaluate the role of Gd+ lesions in brain MRI at the time of relapse. We found only two retrospective studies3,9; the first study showed that 69% of relapsed patients had Gd+ lesions, and almost 50% of them had two or more Gd+ lesions.3 The second study found Gd+ lesions in only 36.5% of relapsed patients and showed that Gd+ enhancement depended on age and was correlated with disease duration and Expanded Disability Status Scale (EDSS) scores.9
With the aim of evaluating the existence of the clinico-radiological paradox in MS relapse, we analyzed the number and location of Gd+ lesions on brain MRI before methylprednisolone (MP) treatment.
MethodsStudy populationAn analysis was performed on the basal data of a subset of 90 relapsed patients from two different Phase IV multicenter double-blind randomized clinical trials led by our research team that showed noninferiority of different routes and doses of MP administration (ClinicalTrials.gov NTC 007537 and NTC 01986998). The clinical trial NTC 007537 showed the noninferiority of the administration of 1g of intravenous MP compared to 1250mg of oral MP.10 The recruitment period for this study was from 2008 to 2011. The second clinical trial NTC 01986998 showed the noninferiority of 1250mg of oral MP compared to 675mg of oral MP11; the recruitment period for this clinical trial was from 2013 to 2015. The study included adults who had experienced a relapse and met the 2005 McDonald criteria for RRMS in the first clinical trial and the 2010 McDonald criteria for RRMS in the second clinical trial. Both studies were approved by the Ethics Committees of the participating centers, and all patients gave written informed consent to participate.
The inclusion criteria for the two studies were as follows: (a) an age of 18–59 years; (b) an EDSS score of 0–5.0 prior to relapse; (c) recent onset (<15 days) of relapse without fever; and (d) one month of clinical stability prior to relapse. The exclusion criteria were as follows: (a) clinically isolated syndrome or progressive MS; (b) use of steroids during the previous 3 months; (c) ongoing pregnancy or breastfeeding; and (d) any concomitant disease that could contraindicate treatment with steroids.
Demographic and clinical variablesDemographic characteristics (age and sex) and clinical characteristics (disease duration and use of disease-modifying therapy (DMT)) were analyzed. Clinical relapse manifestations were classified as brainstem-cerebellum, spinal cord, optic neuritis (ON) and others. Baseline EDSS scores were assessed before starting MP treatment, and previous EDSS scores were collected.
A relapse was defined as a new symptom that lasted more than 24h and that represented an increase of more than 1 point in the total EDSS score. Relapses were classified as mild (EDSS score increased<1 point), moderate (EDSS score increased 1–2.5 points) or severe (EDSS score increased≥3 points).10–12 For mild relapses with an increase of <1 point, the involvement of two or more functional systems was mandatory. All patients were evaluated by neurologists specializing in MS.
Radiological variables and MRI protocolBrain MRI was assessed at the time of relapse diagnosis, within 15 days of symptom onset and before MP treatment (baseline MRI). The brain MRIs were acquired on a 1.5- or 3-T MRI scanner. We obtained an axial proton density (PD) and T2-weighted turbo spin-echo sequence and an axial T1-weighted spin-echo sequence before and after the injection of 0.1mmol/kg of body weight of macrocyclic gadolinium-based contrast agents (GBCAs) (gadoterate meglumine; 5-min delay). For each sequence, 44 contiguous, 3-mm-thick slices were obtained. The number of Gd+ lesions was analyzed. The location of the Gd+ lesions on the baseline MRI scan (periventricular, subcortical, juxtacortical, brainstem and cerebellum) and the presence of symptomatic Gd+ lesions were analyzed retrospectively. A Gd+ lesion was considered symptomatic when its location matched the clinical topography of the relapse. All brain MRIs were evaluated centrally by two neuroradiologists with expertise in MS (AR and JC).
Statistical analysisDescriptive statistics are shown as the mean (± standard deviation) or median (interquartile range (IQR)) for continuous variables or as frequencies (percentages) for categorical variables. Associations were studied using univariate analysis. Comparisons were performed by Student's t-test for parametric continuous variables, the Mann–Whitney test for nonparametric continuous variables and the chi-squared test for categorical variables. The Kruskal–Wallis test was used to compare groups. We used Spearman's coefficient to assess the correlation between variables. p values were two-sided, with values less than 0.05 considered statistically significant. All statistical analyses were performed with the statistical software IBM SPSS Statistics 23 (Chicago, IL, USA).
Data availabilityAnonymized data not published within the article are available upon request.
ResultsNinety patients were included in the study (Fig. 1). The mean age was 38 years (± 8.8), and 78% of patients were women. The median score of the baseline EDSS was 2.0 (IQR 1–2.5), and the median disease duration was 9 (IQR 6–16) years. Forty-four patients (50.6%) did not receive DMT, and approximately the other 50% received injectable platform therapies. Regarding the topography of the relapse, 42 patients (45.6%) were myelitis, 28 (31.1%) were classified as brainstem-cerebellum, 9 (10%) were ON and 11 (12.2%) were classified as others. Moderate relapse severity (n=59, 65.6%) was the most common. The baseline demographic, clinical, and imaging data are provided in Table 1.
Baseline characteristics.
| Baseline characteristics (n=90) | |
| Age (years; mean ±SD) | 38.1±8.8 |
| Sex (n, % women) | 70 (77.8%) |
| Baseline EDSS score (median, IQR) | 2 [1–2.5] |
| Disease duration (years; median, IQR) | 9 [6–16] |
| Disease-modifying therapy (n; %) (n=87) | |
| No treatment | 44 (50.6%) |
| Interferon | 32 (36.8%) |
| Glatiramer acetate | 10 (11.5%) |
| Dimethyl fumarate | 1 (1.1%) |
| Relapse characteristics | |
| Baseline relapse EDSS score (median, IQR) (n=87) | 3 [3–4] |
| Severity (n, %) (n=87) | |
| Mild | 24 (27.6%) |
| Moderate | 59 (67.8%) |
| Severe | 4 (4.6%) |
| Topography (n, %) (n=90) | |
| Optic neuritis (ON) | 9 (10%) |
| Brainstem-cerebellum | 28 (31.1%) |
| Spinal cord | 42 (46.7%) |
| Others | 11 (12.2%) |
| MRI characteristics (n=90) | |
| Patients with Gd+ lesions (n, %) | 56 (62.2%) |
| Number of Gd+ lesions (median, IQR) | 1 [0–4] |
| 1 lesion (n, %) | 19 (21.1%) |
| 2 lesions (n, %) | 11 (12.2%) |
| 3 or more lesions (n, %) | 26 (28.9%) |
| Symptomatic Gd+ lesions | |
| Yes | 17 (18.9%) |
| No | 22 (24.4%) |
| n/a (ON and myelitis without spinal cord MRI) | 51 (56.7%) |
SD: standard deviation; EDSS: Expanded Disability Status Scale; IQR: interquartile range; ON: optic neuritis; Gd+ lesions: gadolinium-enhanced lesions; n/a: not available.
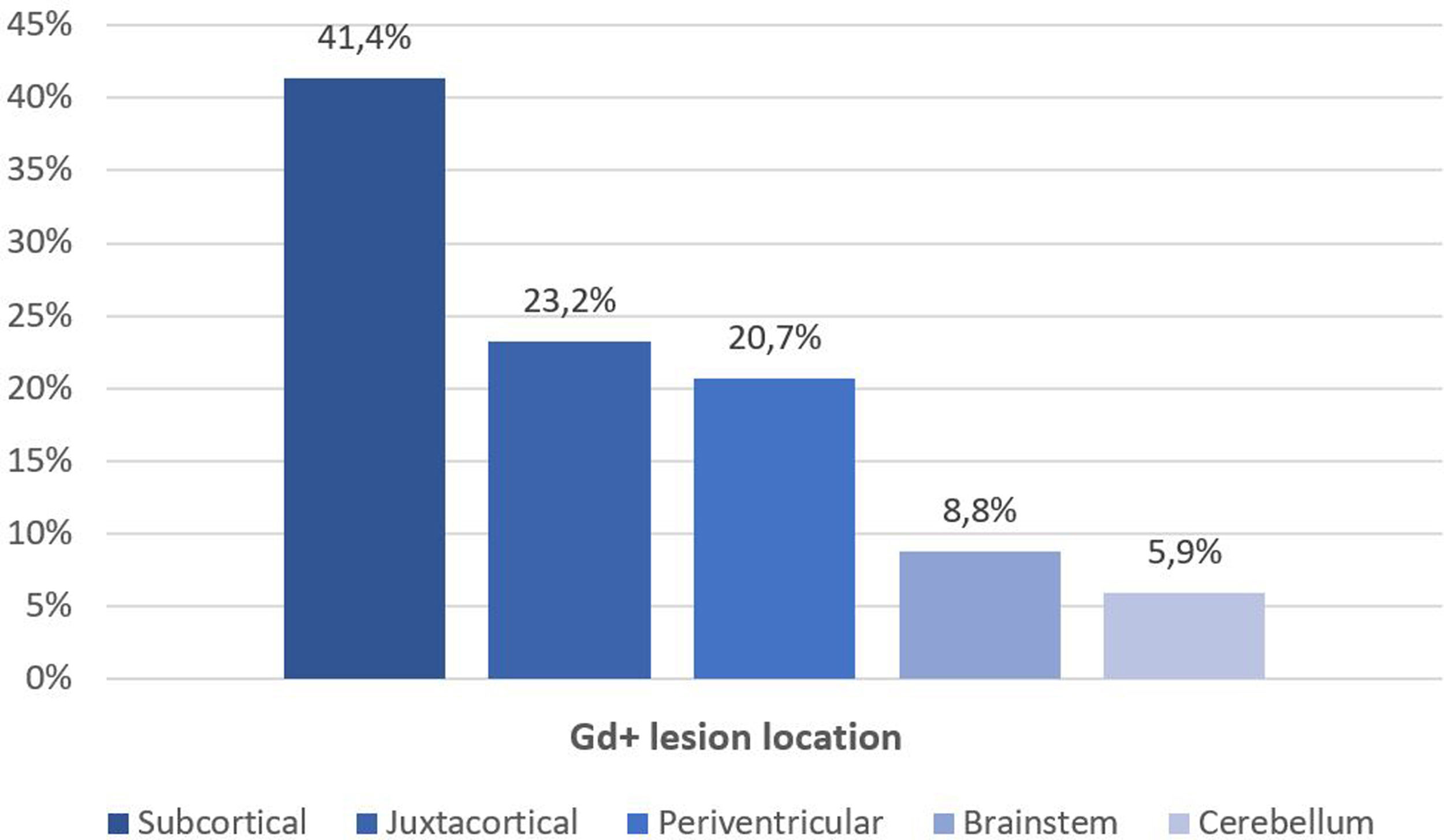
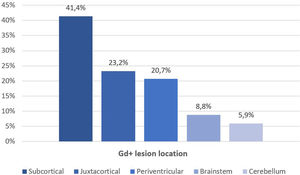
Fifty-six patients (62.2%) had Gd+ brain lesions, the median number of Gd+ lesions was 1 (IQR 0–4), and 41% of patients had 2 or more Gd+ lesions. The most frequent location of Gd+ lesions was the subcortical region (41.4%), followed by the juxtacortical region (23.2%), periventricular region (20.7%), brainstem (8.8%) and cerebellum (5.9%) (Fig. 2). We found a relationship between age and the presence of Gd+ lesions (36.2 vs 41.1 years, p=0.013), with more Gd+ lesions in patients who were younger (p=0.002, Table 2). The disease duration and baseline EDSS were lower in patients with Gd+ lesions (8 vs 13 years; p=0.26 and 2.0 vs 4.0, p=0.32), but these differences were not statistically significant. No differences were observed in baseline EDSS (Table 2).
Relationship between Gd+ lesions and clinical characteristics.
| Presence of Gd+ lesions | p | Number of Gd+ lesions (n=90) | p | ||
|---|---|---|---|---|---|
| Gd+ (n=56) | Gd- (n=34) | ||||
| Age (years; mean±SD) | 41±9.1 | 36±8.1 | 0.013 | – | 0.002 |
| Baseline EDSS (median, IQR) | 2 (1.5–2.5) | 2 (1–3) | 0.41 | – | 0.98 |
| Disease duration (years; median, IQR) | 8 (6–13) | 13 (6–18) | 0.26 | – | 0.32 |
| Baseline relapse EDSS (median, IQR) | 2 (1.5–2.5) | 4 (3–4) | 0.32 | – | 0.92 |
| Severity (n, %) (n=87) | |||||
| Mild (n=24) | 13 (23.2%) | 11 (32.4%) | 0.65 | 1 (0–3) | 0.36 |
| Moderate (n=59) | 37 (66.1%) | 22 (64.7%) | 1 (0–4) | ||
| Severe (n=4) | 3 (5.4%) | 1 (2.9%) | 5 (0–48) | ||
| Topography (n, %) | |||||
| Optic neuritis (ON) (n=9) | 5 (8.9%) | 4 (11.8%) | 1 (0–2) | ||
| Brainstem-cerebellum (n=28) | 20 (35.7%) | 8 (23.5%) | 0.58 | 1 (0–5) | 0.61 |
| Spinal cord (n=42) | 24 (42.9%) | 18 (52.9%) | 1 (0–3) | ||
| Others (n=11) | 7 (12.5%) | 4 (11.8%) | 1 (0–5) | ||
| Disease-modifying therapy (n, %) (n=87) | |||||
| Yes (n=43) | 25 (44.6%) | 18 (52.9%) | 0.38 | 1 (0–4) | 0.63 |
| No (n=44) | 29 (51.8%) | 15 (44.1%) | 1 (0–4) | ||
Gd+ lesion: gadolinium-enhanced lesion; SD: standard deviation; EDSS: Expanded Disability Status Scale; IQR: interquartile range; ON: optic neuritis.
Twenty patients out of 28 with brainstem-cerebellum relapse (71.4%), 24 out of 42 patients with myelitis (57.1%) and five out of 9 patients with ON (55.6%) had Gd+ lesions on brain MRI. Among patients with brain symptoms (n=39), only 17 (43.6%) had a symptomatic Gd+ lesion, and 12 (30.8%) did not have brain Gd+ lesions. The univariate analysis did not show relationships between clinical topography, relapse severity or disease-modifying treatment and the presence or number of Gd+ brain lesions (Table 2). For the analysis of symptomatic Gd+ lesions, we excluded patients with clinical myelitis and no spinal cord MRI, and we also excluded patients with optic neuritis because axial brain sequences are not adequate for the study of the optic nerve.
DiscussionOur study shows that 62% of relapsed MS patients have Gd+ brain lesions, and it provides valuable data regarding the number and location of Gd+ lesions. Most relapsed patients showed several Gd+ lesions on brain MRI, even when the clinical manifestation was in the spinal cord or in the optic nerve, as more than 50% of patients with spinal cord symptoms or ON had silent Gd+ brain lesions.
The percentage of patients with brain Gd+ lesions is similar to that previously reported in one of the retrospective studies (69%)3 but differs from the percentage reported by the other study (36.5%).9 This difference may have arisen because we did not include patients with progressive MS and because the time from symptom onset to the performance of baseline MRI was shorter (<15 days vs. more than 1 month). Furthermore, we found a negative correlation between age and the presence of Gd+ lesions. This relationship has been previously described,9,13,14 and it is well known that the frequency and number of Gd+ lesions were higher in younger MS patients.15,16 Otherwise, although disease duration and baseline EDSS were lower in patients with Gd+ lesions, we have not found statistically significant associations, unlike previously published studies.9
In our study, patients who had a higher presence of Gd+ lesions were those with brainstem-cerebellum relapse (70%), but we did not find a significant difference regarding clinical topography because approximately half of our patients had spinal cord symptoms, and we did not include spinal cord MRI in our clinical trial protocols. When we analyzed only patients with brain symptoms, less than 50% of patients had a symptomatic Gd+ lesion that could explain the symptoms of relapse, and approximately 30% of patients with MS relapse did not have brain Gd+ lesions. These findings support the use of brain MRI in MS relapse to avoid misdiagnosis, especially in those cases in which relapse involves a therapeutic change.
The natural history of Gd+ lesions is highly variable and unpredictable; axonal loss and degeneration are thought to contribute to clinical worsening and disability,17,18 but we found that most Gd+ lesions are clinically silent. In our study, more than 50% of patients with spinal cord symptoms or with optic neuritis had silent Gd+ lesions on brain MRI. These findings highlighted the mismatch between clinical and radiological measures in MS. Thus, performing a brain MRI in all patients who relapse, even in those patients with spinal cord symptoms, might increase sensitivity in detecting radiological activity and would help optimize therapeutic decisions.
Given the evidence regarding the deposition of gadolinium in deep gray matter structures, particularly in the dentate nucleus and globus pallidus, which is orders of magnitude more evident in patients receiving linear GBCAs than macrocyclic GBCAs,19–23 the European Commission decided to suspend the use of linear chelates for CNS MRI examinations and recommended that Gd should be used only if essential and in the lowest dose needed.24 Indications to use GBCAs in clinical practice should be limited, and the policy of reducing their use in MS patients is reasonable. Even so, MS patients need serial MRI to detect silent activity, monitor disease activity and detect treatment-related complications.25 Our study suggests that it could be informative to obtain a postcontrast T1 sequence on brain MRI in patients with a clinical suspicion of relapse, as a marker of blood-brain barrier disruption, to predict future disease activity and especially in those patients in which the demonstration of Gd+ lesions is a requirement to initiate or change a specific disease-modifying treatment immunomodulatory treatment.26
A major limitation of our study is the lack of spinal cord and optic nerve MRI at the moment of relapse. Considering that most of our patients had spinal symptoms, the percentage of patients with symptomatic Gd+ lesions would be higher if a spinal MRI was performed. Another point to study would be the formation of new T2 lesions, but most of our patients did not have a recent MRI to compare, and the baseline MRI was performed in the first 15 days from symptom onset. Gd+ enhancement usually persists for 2–6 weeks27; thus, the possibility of having new T2 lesions without Gd+ enhancement is minor. Another point that should be highlighted in our study is the low proportion of patients under disease modifying treatment (DMT). Our clinical trials were conducted between 2008 and 2015, when many of patients were not treated because of the lack of highly effective and well-tolerated treatments. This can be a reason why we could not find a difference in the percentage or the number of Gd+ lesions between patients that were receiving or not a DMT.
In summary, our results show that most patients with relapse demonstrate several Gd+ lesions on brain MRI, even when the clinical manifestation is in the spinal cord or the optic nerve. Otherwise, thirty percent of patients with MS relapse did not have brain Gd+ lesions. These results highlight the clinico-radiological paradox in MS relapse and support the value of brain MRI in therapeutic decision making. Further radiological studies in larger cohorts are needed to better understand the pathophysiology of MS relapse.
EthicsNTC 007537 and NTC 01986998 studies were approved by the research committee of the institution.
Funding sourcesThis work was supported in part by the Ministry of Health of Spain (grant numbers EC07/90278 and EC11/132) and personal grant Rio Hortega CM19/00042 to LMA.
Conflicts of interestLorena Martín-Aguilar has received speaking honoraria from Roche. Silvia Presas-Rodríguez has received speaking honoraria from Novartis and Merck. Àlex Rovira serves/served on scientific advisory boards for Bayer, Biogen, Novartis, OLEA Medical, Sanofi-Genzyme, and Synthetic MR; has received speaker honoraria from Bayer, Biogen, Bracco, Merck-Serono, Novartis, Roche, Sanofi-Genzyme, and Teva Pharmaceutical Industries Ltd.; and has research agreements with Siemens AG. Mar Tintore has received compensation for consulting services and speaking honoraria from Almirall, Bayer Schering Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis, Roche, Sanofi-Aventis, Viela Bio and Teva Pharmaceuticals and is a coeditor of Multiple Sclerosis Journal-ETC. Luis Brieva has received payment as a consultant, as a speaker at scientific meetings, or travel expenses or research grants from Carlos III Institute of Health and Pharmaceutical Industry: Merck, Biogen, Sanofi, Biogen, Roche, Bayer, Teva, Mylan, Almirall and Novartis. Antonio Cano has received speaking honoraria from Novartis, Teva, Sanofi-Genzyme, Merck, Serono and Roche. José Vicente Hervás-García has received speaking honoraria from Roche and Sanofi. Cristina Ramo-Tello has received compensation for being a member of Scientific Advisory Boards as well as travel and research support from Almirall Pharma, Biogen-Idec, Genzyme, Merck-Serono, Novartis and Roche. The other authors report no conflicts.