The gate control theory of pain was the starting point of the development of spinal cord stimulation (SCS). We describe the indications for the treatment in pain management and other uses not related to pain.
DevelopmentThere are currently several paradigms for SCS: tonic, burst, and high frequency. The main difference lies in the presence of paraesthesias. SCS is most beneficial for treating neuropathic pain. Patients with failed back surgery syndrome show the best response rates, although a considerable reduction in pain is also observed in patients with complex regional pain syndrome, diabetic neuropathy, radiculopathy, and low back pain without previous surgery. Phantom pain or pain related to cardiovascular or peripheral vascular disease may improve, although there is a lack of robust evidence supporting generalisation of its use. SCS also improves cancer-related pain, although research on this issue is scarce. Non-pain-related indications for SCS are movement disorders, spasticity, and sequelae of spinal cord injury. The main limiting factors for the use of SCS are mechanical complications and the cost of the treatment.
ConclusionIn its 50-year history, SCS has progressed enormously. The perfection of hardware and software may improve its effectiveness and reduce the rate of complications. Indications for SCS could include other diseases, and its use could be expanded, if the costs of the technology are reduced.
La descripción de la teoría de la compuerta del dolor fue el punto de partida para el desarrollo de la estimulación de la médula espinal (EME). El presente artículo describe las indicaciones para el tratamiento del dolor y otros usos no relacionados con este.
DesarrolloEn la actualidad existen diversos paradigmas de EME: tónica, de alta frecuencia en ráfaga o simplemente de alta frecuencia. Estas se distinguen por la presencia de parestesias. La EME ha mostrado beneficio sobretodo en dolor de tipo neuropático. El síndrome de cirugía espinal fallida muestra las mejores tasas de respuesta, aunque en casos de síndrome doloroso regional complejo y neuropatía diabética, así como de radiculopatía y lumbago sin cirugía espinal previa también se logra una reducción importante de dolor. Y aunque pareciera útil su aplicación en el dolor de tipo fantasma y en aquel asociado a enfermedad vascular periférica o cardiovascular, no existe una evidencia sólida para generalizar su empleo. Por otra parte, la EME también reduce el dolor neuropático secundario a tumor, a pesar de que esta línea ha sido poco explorada. Otras indicaciones no relacionadas con dolor son trastornos de movimiento, espasticidad y secuelas de trauma medular. Sin embargo, el uso de la EME se ve limitado por dos factores principales: las complicaciones mecánicas y el costo de la terapia.
ConclusiónDurante los 50 años de aplicación, la EME ha mostrado grandes avances. Asimismo, a medida que se perfeccione el hardware y el software relacionados con esta, se podrá mejorar la efectividad y reducir el rango de complicaciones. La indicación para la EME se podría extender a otras enfermedades y su uso se expandiría, si, además, se lograra diseñar una tecnología asequible.
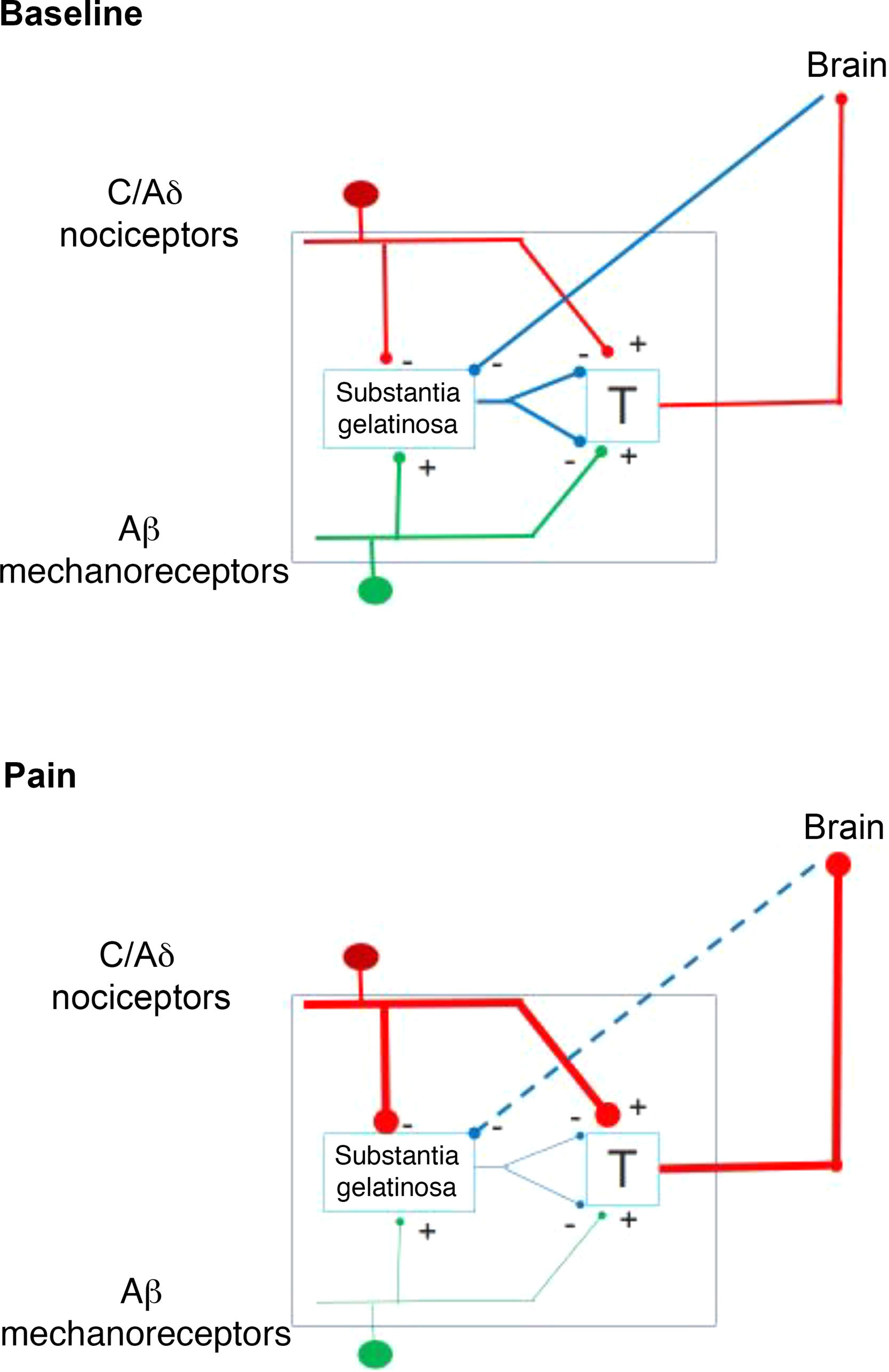
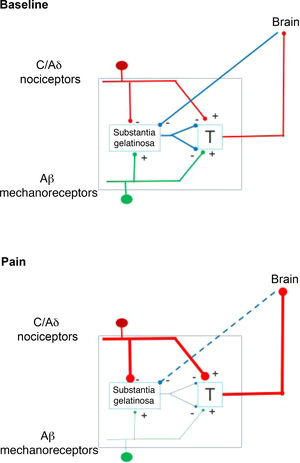
In 1959, the identification of 2 types of fibres for the transfer of sensory stimuli led the way to a new understanding of pain perception mechanisms. This is how the interaction between the large-diameter fibres that transport tactile, pressure, and vibration signals, and the small-diameter fibres associated with pain, merge in the gate control theory of pain (Fig. 1). This theory, developed by Melzack and Wall, postulates that stimulation of large-diameter fibres inhibits the response to painful stimuli carried by small-diameter fibres of dorsal column neurons.1 Based on this principle, the first device for spinal cord stimulation (SCS) was introduced in 1968.2 Implantable pulse generators (Itrel Medtronic®) and programmable percutaneous electrodes were introduced in 1984. Devices with rechargeable batteries (eg, Restore Medtronic®) have been available since 2004. The use of SCS has expanded over the last 2 decades, and approximately 30 000 devices are estimated to be implanted every year.3
Action mechanisms and stimulation paradigmsThe first available stimulation paradigm was that now known as the conventional or tonic paradigm. Electrodes, placed epidurally, are used to apply stimulation with frequencies of 35-80 Hz, pulse width of 200-450 μs, and an average amplitude of 5-6 mA. This type of stimulation generates paraesthesia in the target area.4 The action of SCS depends on the distribution of electric fields in the spinal structures (dura mater, cerebrospinal fluid), as well as on the relative effect of the position and distance of cathodes and anodes in the activation of the dorsal columns.5,6 White matter anisotropy also shows different degrees of conductivity that influence the effect of electrical stimulation.7 In the 1990s, Barolat8 published maps of sensory responses and contributed information on the correlation between anatomical and electrical properties and clinical effect.
From a simplified perspective, the effect of the stimulation was considered to be limited to the dorsal columns.9 However, there are clear differences in the stimulation threshold of afferent fibres of the nerve roots entering the spinal cord and that of the nerve fibres of the dorsal columns.10 A segmental effect is also supported by the fact that stimulation is more effective in more rostral locations with regard to the injured area.11 Likewise, SCS activates brain areas that send pain inhibitory stimuli in lower segments.12
At a molecular level, the effect is explained by the modulation of amino acids associated with the local inhibition system of the GABA neurotransmitter (gamma-aminobutyric acid).13,14 GABA B receptors seem to be particularly influenced by electrical stimulation.15 However, other substances, such as substance P and serotonin (5-HT), which have an inhibitory effect on the transmission of pain signals, are also involved.16 Furthermore, some data suggest the participation of a descending noradrenergic system. The excitatory neurotransmitters glutamate and aspartate have a more attenuating function, as they decrease with SCS.17 Whereas adenosine and acetylcholine influence the effect of SCS, the endogenous endorphin system seems not to play a role.18–20
High-frequency burst stimulation, or simply high-frequency stimulation, are 2 new available variants. Burst stimulation yields a signal similar to that observed in the propagation of central nervous system activity.21 The stimulus is therefore applied at low frequencies (40 Hz) with 5 closely spaced pulses (1 ms) at 500 Hz per burst, or 3 pulses at 100 Hz, followed by a repolarisation phase. This type of stimulation is also known as paraesthesia-free stimulation. Compared to tonic forms, this variant provides a lower charge per pulse and, at the same time, a higher charge per second.4 The higher charge per second modulates the neurons involved in pain transmission. Burst stimulation also activates some brain areas, including the dorsal anterior cingulate and the dorsolateral precentral cortex.22 High-frequency stimulation uses frequencies of 10 000 Hz, with a pulse width of 30 ms, and low amplitude (approximately 2-3 A). The advantages over tonic stimulation are still controversial, although it is clear that the absence of paraesthesia may make it more comfortable.23,24
Indications for pain managementFailed back surgery syndrome, radiculopathy, and lumbagoFailed back surgery syndrome is a well-recognised clinical entity that is defined by the persistence of pain despite successful spinal surgery, or recurrent pain after a surgery primarily intended to treat that pain.25 In initial trials, SCS showed a long-term effectiveness of 50% in reducing pain and of 41%-84% in reducing analgesic use.26–28 This pain reduction was more recently confirmed by 2 controlled clinical trials, which were compared against repeated operation29 or conventional medical management (PROCESS-Trial).30 Conventional medical management refers to the use of analgesics, nerve block, epidural steroids, and physical and psychological therapy. Improvements have only been documented in cases of radicular pain.29,30 A meta-analysis including 74 studies reported an average improvement of 58% after a 24-month follow-up period.31 The results of the PROMISE study (NCT01697358), which was completed in June 2017, suggest an additive effect of the use of SCS and conventional management.32,33 New SCS modalities, such as high-frequency stimulation, seem not to offer advantages in management.34 However, the application of multicolumn leads may be more effective in controlling radicular and axial pain.35
An equivalent to this syndrome involves the cervical region (failed neck surgery syndrome). The evidence on the use of SCS for managing this syndrome is limited. In a series of 15 patients, Hunter et al.36 demonstrated the usefulness of this type of therapy after a 12-month follow-up. Another study reported improved quality of life.37
Although chronic lumbar and/or radicular pain can be managed with SCS, its impact on patients without previous spinal surgery is unclear, as the great majority of studies include all types of patients with chronic pain. For example, in the study by Kapural et al.38 only 13% of patients in both study groups had no history of surgery. Approximately 36% of patients in the SCS-LUMINA study were considered to present failed spinal surgery syndromes.39 This study analysed a subgroup of patients who exclusively presented lumbar pain with no radicular component. Although radicular pain usually responds better than lower back pain, new technologies (eg, 3D neural targeting) seem to be able to overcome this problem.39 Another factor influencing the results of SCS is the co-presence of other conditions, such as peripheral neuropathy, joint replacement, and fractures. Granville et al.40 suggest that SCS may be useful in controlling pain, even when several causes of pain co-exist.
Complex regional pain syndromeComplex regional pain syndrome (CRPS) is characterised by the presence of persistent, disproportionate pain accompanied by sensory, vasomotor, and sweating abnormalities, as well as motor and trophic changes in the affected area.41 SCS may be effective in selected patients with a successful test phase.41,42 A systematic review including 30 studies concluded that SCS is effective in reducing perceived pain and increasing quality of life.43 However, the effect on functional status, sleep quality, and symptom resolution is unclear.
Diabetic neuropathy and other neuropathiesDiabetic neuropathy is a common complication that is frequently difficult to treat pharmacologically. SCS may be useful in cases in which conventional therapy has not substantially reduced pain. A randomised controlled trial including 60 patients found SCS to be effective in reducing pain intensity by more than 50% and improving quality of life after 6 months of treatment.44 Another study reported an improvement of up to 77% in the test phase and 59% with 6 months of permanent stimulation.45 An analysis of the effect after 5 years revealed treatment response in 55% and suspension of stimulation in 20% of cases. Severity of the neuropathy directly impacts the probability of long-term success of SCS.46
Successful use of the technique is reported in other neuropathies, for example in neuropathies associated with human immunodeficiency virus (HIV) or after chemotherapy.47,48 One case of small-fibre neuropathy responded favourably to thoracic and lumbar SCS.49 Furthermore, some researchers have published their experience with the application of SCS in patients with trigeminal neuropathy and neuropathy associated with herpes zoster virus infection.50,51
Phantom limb painThere have been reports on the successful use of SCS to treat phantom limb pain.52–54 However, there is no solid evidence to support its generalised use.55,56
Angina pectoris and peripheral vascular diseaseSCS has been applied successfully in patients with chronic angina due to coronary artery disease. A meta-analysis of 12 studies, including a total of 476 patients with refractory angina pectoris, found a reduction in angina attacks and nitroglycerin consumption after treatment with SCS.57 A similar analysis, including 518 participants from 14 studies, reached a similar conclusion.58 However, no significant differences were found with respect to such other methods as coronary artery bypass graft or percutaneous myocardial laser revascularisation.59
SCS provides some benefits in the management of peripheral vascular disease. SCS may induce a cell activation that results in the release of vasodilatory molecules, a decrease in vascular resistance, and relaxation of smooth muscle cells, as well as suppressing sympathetic vasoconstriction.60 This procedure should particularly be considered in patients in whom vascular reconstruction is not feasible.49 A meta-analysis of 6 studies (n = 450 patients) showed a higher probability of avoiding amputation (relative risk: 0.71) when applying SCS, in addition to reducing pain and disease severity.61
Neuropathic pain secondary to tumourThe increased survival of oncological patients is associated with an increase in chronic pain as a consequence of the tumour or as a sequela of oncological treatment. Approximately 7 million patients are estimated to present chronic pain associated with cancer, and 15% of this population may not achieve sufficient pain reduction.62,63 This pain has typically been treated with ablative surgery, which has clear disadvantages.64 Little research has been conducted into SCS in patients with neuropathic pain secondary to tumour.62,65 A literature review identified several series, but no randomised clinical trials. In total, 92 patients with neuropathic pain secondary to tumour were analysed, and showed a clear reduction in pain.66 Further studies are clearly needed before its routine use can be recommended.
Other emerging indications not associated with pain managementParkinson’s disease and other movement disordersParkinson’s disease is the second most frequent neurodegenerative disease, and is characterised by progressive and irreversible disability due to rigidity, bradykinesia, and cognitive and vegetative disorders. Symptoms manifest as a consequence of the reduction in dopaminergic neurons in the substantia nigra pars compacta.67 Pharmacological treatment is frequently insufficient to control axial motor symptoms (gait and posture disorders, gait freezing, and dysphagia). Deep brain stimulation of the subthalamic nucleus (STN) or internal globus pallidus (iGP) has become a viable treatment option.68–70 However, its effect is limited in patients with predominantly axial symptoms.
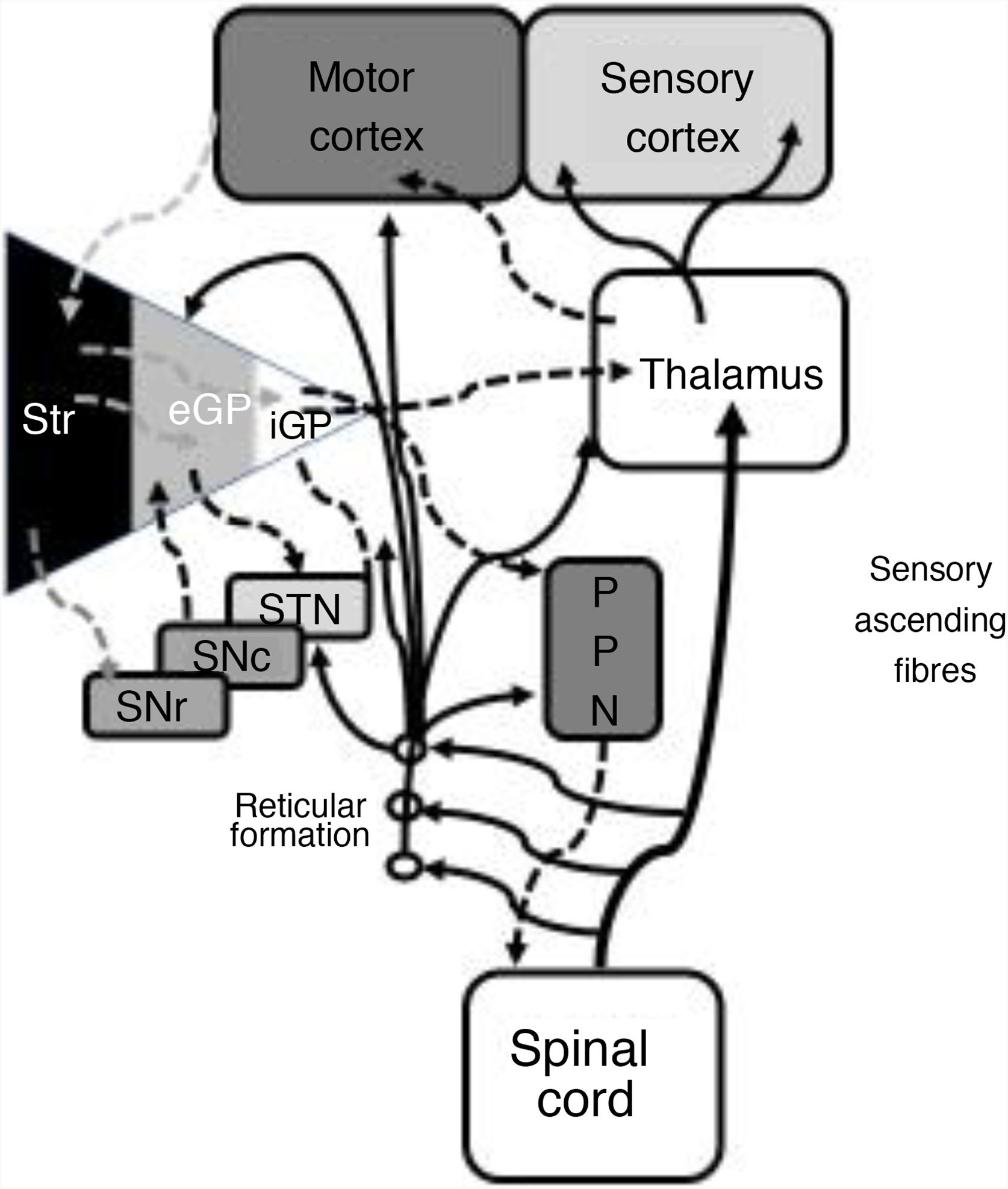
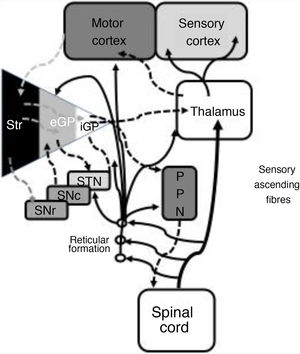
In 1978, Gildenberg71 applied SCS for the first time in patients with torticollis. In 2009, Fuentes et al.72 showed the benefits of dorsal column stimulation in an animal model. A systematic literature review by De Andrade et al.73 analysed 24 patients from 8 different studies. These researchers observed improvement of the central symptoms (gait and posture) in 75% of patients. From an electrophysiological point of view, pathological beta oscillations of corticobasal neurons are responsible for bradykinesia and tremor.74 The STN-external globus pallidus (eGP) complex works as a pacemaker within a feedback system that ultimately generates low-frequency bursts. Pallidal bursts modulate the system by inhibiting eGP neuron activity.73 Experimental studies have shown that SCS reduces the corticostriatal beta activity, in addition to ultimately acting on such structures as the mesencephalic locomotor region.72 This region includes such nuclei as the pedunculopontine nucleus (PPN) and the cuneate nucleus.75 The PPN uses cholinergic and glutamatergic fibres to project to neurons in the cortex, basal ganglia, thalamus, brainstem, and spinal cord (Fig. 2).76 Currently, routine use of SCS in movement disorders is not justified; further basic studies and early-phase clinical trials are needed.
Systematic representation of the direct and indirect motor networks between the basal ganglia, thalamus, and cortex, as well as gait and posture control pathways projected from the basal ganglia to the pedunculopontine nucleus and spinal cord. The sensory ascending fibres provide information on the musculoskeletal components and activate the reticular formation in the brainstem.
eGP: external globus pallidus; iGP: internal globus pallidus; PPN: pedunculopontine nucleus; SNc: substantia nigra pars compacta; SNr: substantia nigra pars reticulata; STN: subthalamic nucleus; Str: striate.
Modified from De Andrade et al.73
Spasticity results from an uncoupling of the central and peripheral nervous systems. Patients with spasticity develop pain and contractures that affect everyday activities and limit neurological recovery. Pharmacological or surgical treatment may improve symptoms, but is not curative or restorative, or close to selective. In this sense, SCS offers the opportunity to reactivate neuronal circuits.77
In humans, the stretch reflex is regulated by the dorsal (inhibition) and medial (excitation) reticulospinal tract. The vestibulospinal tract also facilitates this reflex, although it has more of a regulatory function; in simple terms, hyperactivity of gamma motor neurons contributes to the development of an exaggerated stretch reflex.78 However, its pathophysiology is much more complex, as spinal discharges depend not only on cortical afferents but also on their own neuromodulatory system regulated by metabotropic afferents of the brainstem originated as noradrenergic (NA) fibres and serotonergic fibres of the locus coeruleus and nucleus raphe magnus.79,80 Dysfunction of extrapyramidal systems contributes to the hyperexcitability of interneurons that mediate polysynaptic reflexes.80 Disruption of descending neuromodulatory systems has also been found to alter the pattern of sensory signals reaching the neurons of the ventral spinal horn.81 This way, the reciprocal inhibition that focuses on a given joint is lost. After a spinal injury, NA and 5-HT receptors gradually readjust to an activation state, even in the absence of these neurotransmitters.82
Furthermore, peripheral inputs, including sensory monosynaptic afferents and polysynaptic signals, are able to induce potent depolarisation in spinal neurons. Thus, muscles innervated by these neuronal groups may be inappropriately activated, exacerbating spasticity. Other molecular phenomena, such as reduced levels of the potassium-chloride co-transporter (KCC2), contribute to the persistence of spasticity.83 After a spinal injury, the signals generated in the cortex or basal ganglia are unrecognisable or incomplete, or are even not received. With time, motor neuron excitability returns with wider receptive fields; this increases aberrant activation and may explain the spread of spastic behaviour throughout an entire limb.84
Several case reports and case series have been published, as well as some pilot and prospective studies that support the use of SCS in more than 25 neurodegenerative and traumatic diseases.77,85 Several considerations must be made before applying SCS in the management of spasticity. The spinal cord located below the injured area must be anatomically and physiologically intact, and some degree of communication with supraspinal signals must exist. In general, the routine use of SCS in patients with spasticity is not yet justified.
Spinal trauma rehabilitationA traumatic lesion of the spinal cord causes severe neurological disorders, and although the mortality rate has decreased, functional recovery continues to be a challenge.86,87 The various cellular changes occurring during trauma, from the lesion itself to the recovery phase, have been widely studied.88 The use of neuroprotective measures has not obtained the desired results, and different therapies, including those based on the use of stem cells, are still being studied.88 Early surgical decompression has been established as a measure contributing to recovery.89 The current concept of management mainly focuses on prevention and complication management.86
The correlation between spinal cord segments and SCS was described early during the development of this technique.90 Although interneurons and motor neurons are not directly stimulated, a trans-synaptic effect may be achieved.91 The neuronal networks of the lumbar spinal cord are able to transfer coordinated discharges to the muscles of the lower limbs, independently of the supraspinal effect.90 Low-frequency SCS (2 Hz) may generate monosynaptic reflexes.91 Increasing the frequency may reduce motor discharges, although interneuronal circuits may simultaneously be activated, resulting in the generation of a stable discharge pattern.92 SCS at frequencies of 22-50 Hz causes electromyographic changes similar to those generated by locomotor activity, and may even lead to flexion or extension movements.93,94 There is clear evidence on the presence of residual functional white matter, even in patients whose clinical symptoms suggest complete spinal cord injury.95 It is also evident that the influence of supraspinal structures may be preserved.96
Clinical experience with SCS suggests improvement in motor function in patients with spinal lesions, including reports of patients with paraparesis.87–105 There is currently growing interest in developing implementation strategies that may facilitate the use of SCS to recover motor function.106 The use of SCS may even go beyond recovery of motor function and pain management; for example, the circuits involved in regulating bladder, bowel, and sexual function may also be reactivated, contributing to an improvement in quality of life.107,108
Future perspectivesTwo issues currently hinder the generalised use of SCS: the complications resulting from its use, and the cost of the treatment.109–113 In addition to the classical surgical complications, such as infection, this type of therapy involves such mechanical complications as electrode migration or breakage, as well as potential discomfort caused by the impulse generator (approximately 6%) and the need to replace the battery.112,114 However, the problem of regularly replacing the impulse generator has improved since the introduction of rechargeable devices. We should also underscore that technological improvements and the perfection of implantation techniques have helped to reduce the range of complications.115
The cost-benefit ratio for pain symptoms due to CRPS or failed back surgery syndrome justifies SCS use, despite its high cost.109,116 Although the first year of treatment is associated with high costs, this is compensated for by constant annual decreases, if we compare SCS with conventional therapy.117 The British Pain Society recommends that SCS should be considered early in management when first-line treatments have failed.118 In relation to this recommendation, it should be noted that delays between diagnosis of chronic pain and the placement of SCS directly result in an increase in total medical expenses and opioid prescriptions and payments, as well as in the number of medical consultations.119 In general, we may state that SCS seems to present a clear cost-benefit advantage in 80%-85% of cases, although delays in SCS implantation lead to greater use of healthcare system resources.120 Finally, high-frequency stimulation may be even more cost-effective in cases of failed spinal surgery.121
Moore’s law, formulated in the field of technology in 1965, states that the number of transistors in a dense integrated circuit doubles at regular intervals (approximately every 2 years). According to this theory, we may clearly expect the size of SCS generators to further decrease, while their power increases. In parallel to improvements in hardware, software will also improve, which will lead to the development of new stimulation paradigms or upgradeable impulse generators.122 There are 4 main currents in the development of paradigms: pseudo-randomness in time and/or place, more pleasurable stimulation, noise stimulation, and reconditioning stimulation.122 Closely linked to the last concept of reconditioning stimulation is closed-loop stimulation, which is modulated or adapted to physiological changes.123 An example of this technology is the RestoreSensor® system, which uses a 3-axis accelerometer and detects the patient’s position. Stimulation parameters are automatically adjusted in real time.124
Logically, advances will take place not only in impulse generators, but also in the electrodes used. Soft interfaces may be placed subdurally, which would enable greater selectivity with lower stimulation thresholds, and even chemical stimulation. These interfaces have been assessed in silico and in vivo.125 Another example of these interfaces is the so-called e-dura (electronic dura mater).126
Central nervous diseases, including chronic pain, are a significant problem. In the United States and Europe, expenditure on this type of patients amounts to 2 trillion dollars.127 One current problem in the development of the neurosciences resides in the fact that drugs under development have little likelihood of reaching the market, and involve higher development and marketing costs than, for example, cardiological drugs (30% higher). Therefore, the pharmaceutical industry may lose motivation to develop new drugs for central nervous system disorders.127 This critical point has given rise to speculation on the development of electroceuticals, which would exist in the form of nanoparticles capable of delivering action potentials.127
ConclusionFifty years after the introduction of SCS, several technological changes have occurred, with the most relevant taking place over the past few years. As hardware and software continue to improve, the effectiveness of the treatment will increase and the range of complications decrease. We may also expect that indication of SCS will extend to other conditions. Furthermore, if an affordable technology is developed, its application may expand to regions with less advantaged healthcare systems.
Conflicts of interestThe author presented a symposium for the company Medtronic™ during the 2018 annual meeting of the German Society of Neurosurgery in Münster.
Please cite this article as: Tapias Pérez JH. Estimulación de la médula espinal: más allá del manejo del dolor. Neurología. 2022;37:586–595.