Melatonin is the main hormone involved in the control of the sleep-wake cycle. It is easily synthesisable and can be administered orally, which has led to interest in its use as a treatment for insomnia. Moreover, as production of the hormone decreases with age, in inverse correlation with the frequency of poor sleep quality, it has been suggested that melatonin deficit is at least partly responsible for sleep disorders. Treating this age-related deficit would therefore appear to be a natural way of restoring sleep quality, which is lost as patients age. However, despite the undeniable theoretical appeal of this approach to insomnia, little scientific evidence is available that supports any benefit of this substitutive therapy. Furthermore, the most suitable dose ranges and pharmaceutical preparations for melatonin administration are yet to be clearly defined. This review addresses the physiology of melatonin, the different pharmaceutical preparations, and data on its clinical usefulness.
La melatonina es la principal hormona implicada en la regulación de la oscilación entre sueño y vigilia. Es fácilmente sintetizable y administrable por vía oral, lo que ha propiciado el interés para usarla en el tratamiento de una de las patologías humanas más prevalentes, el insomnio. Además, el hecho de que su producción se reduzca con la edad, en una relación inversamente proporcional a la frecuencia de mala calidad de sueño, ha reforzado la idea de que su déficit es, al menos en parte, responsable de estos trastornos. En esta línea de pensamiento, remontar el déficit que se va instaurando a medida que transcurre la vida sería un modo natural de restaurar la integridad del sueño, que se va perdiendo con la edad. Sin embargo, a pesar del innegable atractivo teórico de esta aproximación al problema del insomnio, la evidencia científica que sustenta el posible beneficio de esta terapia sustitutiva es escasa. Ni siquiera están bien definidos los rangos de dosis a los que administrarla o la formulación farmacológica más adecuada. En la presente revisión se repasa la fisiología de la melatonina, se revisan las características farmacológicas de su administración exógena y se analizan los datos existentes sobre su utilidad clínica.
Melatonin (N-acetyl-5-methoxytryptamine) is an indole compound. Until the mid-1960s, the hormone was thought to be secreted exclusively by the pineal gland. We now know that it is synthesised in multiple other, non-endocrine organs and tissues, including the retina, harderian glands (accessory to the lacrimal glands), bone marrow, skin, serotonin-producing cells in the gastrointestinal tract, cerebellum, and immune system.1 Therefore, melatonin is not a hormone in the classic sense, as it is synthesised in multiple organs and does not act on a specific target organ.
Melatonin synthesis and secretion is regulated by the suprachiasmatic nucleus (SCN). The hormone, in turn, modulates the SCN and peripheral clocks throughout the body, acting as a marker of circadian rhythm.
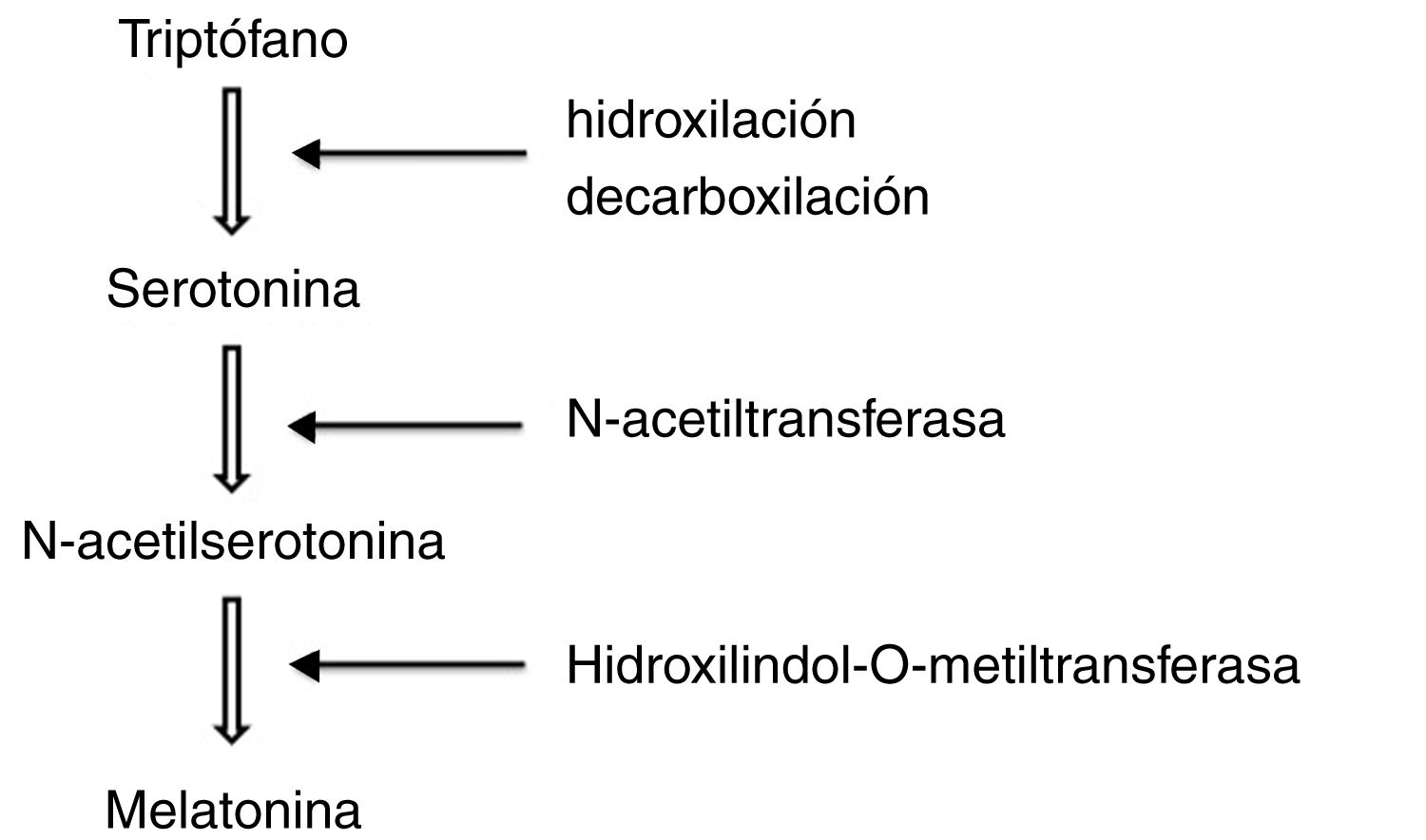
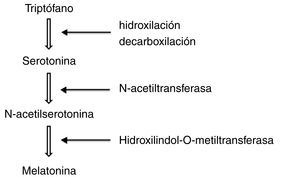
To synthesise melatonin, pineal cells first convert tryptophan in the blood into serotonin through hydroxylation and decarboxylation. N-acetyltransferase transforms serotonin into N-acetylserotonin, which is subsequently methylated by hydroxylindole-O-methyltransferase to form melatonin (Fig. 1).
Pineal melatonin concentrations do not exceed 1μmol/L, whereas concentrations of melatonin secreted by other tissues and organs range from 0.7 to 30μmol/L.
Once synthesised, melatonin is released into the blood stream and distributed throughout all body fluids, reaching the saliva, urine, antral follicles, semen, amniotic fluid, and breast milk. Melatonin is rapidly metabolised, mainly in the liver, and its metabolites are excreted in urine. Its main metabolite, 6-sulfatoxymelatonin, is present in the blood and urine. In the brain, melatonin is oxidised into N1-acetyl-N2-formyl-5-methoxykynuramine, which is demethylated to N1-acetyl-5-methoxykynuramine and excreted through the urine.
Changes in melatonin productionMelatonin levels change throughout life. In humans, melatonin production start at 3-4 months of age. Levels increase progressively during childhood, peaking between the ages of 8 and 10 years. Melatonin synthesis decreases dramatically during puberty. After the age of 40-45 years, melatonin levels decrease progressively, and by the age of 70 they represent barely 10% of prepubertal levels.2
In healthy individuals, melatonin is synthesised in response to darkness, between 20:00 and 22:00, peaking between 00:00 and 03:00, regardless of the sleep stage. After that, melatonin synthesis progressively decreases, remaining very low during the day. Melatonin levels peak when body temperature is lowest. At night, peak plasma melatonin levels range from 100 to 200pg/mL; concentrations range from 10 to 30pg/mL during the day.
Daylight exposure is the main factor in the regulation of melatonin secretion. Circadian synchronisation starts in the fetal period due to changes in maternal melatonin levels. The effects of daylight depend on timing and duration of exposure, sunlight intensity, and wavelength. The light spectrum is particularly important, since retinal ganglion cells contain melanopsin, a photoreceptor sensitive to blue light. Melanopsin plays an essential role in regulating the circadian rhythm.
Exposure to artificial light between 00:00 and 04:00 inhibits melatonin secretion. Morning light exposure causes a circadian phase advance, meaning that melatonin levels will peak earlier. Light exposure in the evening results in a phase delay. The phase response curve of melatonin concentrations in response to light exposure may be used in the treatment of circadian desynchrony.
Regulation of the circadian rhythmThe circadian rhythm is a biological cycle lasting approximately 25hours. This type of cycle is also observed in body temperature, feeding, motor activity, and sleep. Circadian rhythms are endogenous but adjust to the local environment. The light-dark cycle is the main factor for the synchronisation of endogenous rhythms, although other factors are also involved, including eating patterns, regular exercise, sleep habits, and regular social contact.
The circadian system has 3 main components: the central and peripheral circadian clocks (the central pacemaker and the peripheral oscillators), the input pathway, and the output pathway.
The SCN acts as the central pacemaker, coordinating circadian rhythms. These nuclei are located on either side of the third ventricle, directly above the optic chiasm. Neurons in the SCN mainly synthesise GABA, but also vasoactive intestinal peptide, gastrin-releasing peptide, and arginine vasopressin.3
The peripheral circadian clocks function much in the same way as the SCN and are located in different tissues and organs, including the cerebral cortex, liver, kidney, heart, skin, and retina. These clocks are autonomous, but need to be coordinated by the SCN.
The circadian rhythmicity of the neurons in the SCN and peripheral clocks depends on clock genes: Clock, Bmal1, Per1, Per2, Per3, Cry1, and Cry2.4
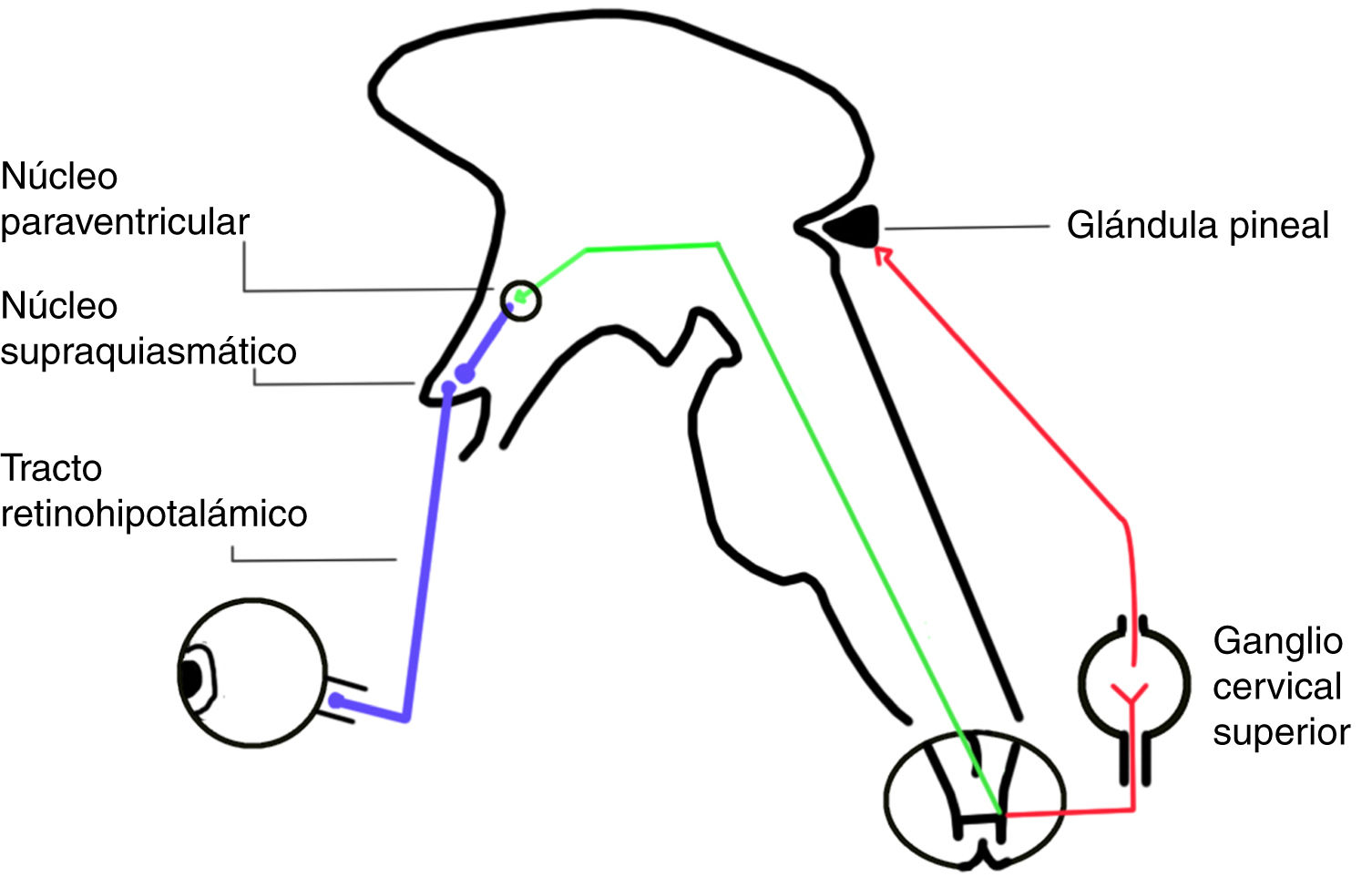
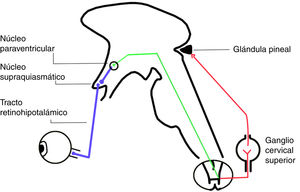
The circadian system involves 3 input pathways, which send information to the SCN. The retinohypothalamic tract is the main pathway, and is formed by axons of a subpopulation of retinal ganglion cells not involved in the formation of images, which express a pigment called melanopsin (Fig. 2). The neurotransmitters released in this tract are glutamate and pituitary adenylate cyclase-activating polypeptide.5
The second input pathway is the geniculohypothalamic tract, an indirect pathway that connects the retina to the SCN via the thalamus. This tract releases GABA and neuropeptide Y.6
The third pathway to the SCN arises from the median and dorsal raphe nuclei in the midbrain, and releases serotonin.7
Output pathways from the SCN reach the hypothalamus, preoptic area, forebrain, and thalamus. The main neurotransmitters of the output pathways are GABA, vasoactive intestinal peptide, and arginine vasopressin.8 The circadian system is also directly connected with neuroendocrine cells, such as neurons that express gonadotropin-releasing hormone, thereby modulating the reproductive cycle. The circadian system regulates the release of sex hormones, which in turn regulate the SCN.
One of the best known output pathways is the multisynaptic pathway, whose fibres reach the pineal gland, which secretes melatonin. Melatonin synthesis is activated by norepinephrine release from the SCN and is directly inhibited by light.
The SCN sends projections to the paraventricular nucleus in the hypothalamus. Sympathetic fibres of the paraventricular nucleus project to the spinal cord, passing through the superior cervical ganglion to reach the pineal gland (Fig. 2). This ganglion releases norepinephrine, which interacts with beta-1 receptors in the membranes of pinealocytes, triggering an intracellular cascade that increases the activity of aryl alkylamine N-acetyltransferase, which is essential for melatonin synthesis.9
The rhythmic profile of melatonin production is proportional to that of nocturnal noradrenergic stimulation, with the lowest levels during the day and peak values at night.
Reciprocal connections between the SCN and the arcuate nucleus are crucial to the proper functioning of the circadian system. The arcuate nucleus is essential for metabolic integration. This may explain why chronic circadian or metabolic alterations associated with eating habits or shift work may result in desynchronisation of hypothalamic oscillations and promote disease. The arcuate nucleus is a metabolic modulator of neuronal activity in the SCN.
Effects of melatoninMelatonin mainly promotes sleep through its chronobiotic effects on the SCN. The hormone also has an effect on thermoregulatory and cardiovascular centres.
The circadian cycle is regulated by the SCN and synchronised to the light-dark cycle, and synchronises other cycles in the body through melatonin synthesis. In turn, melatonin acts on the SCN, promoting resynchronisation when environmental conditions change. Elevated blood melatonin levels signal to tissues and organs that it is night-time, helping to regulate homeostasis.
Melatonin resynchronises the circadian rhythm and the sleep-wake cycle, and also regulates the reproductive cycle. In animals with seasonal patterns of reproduction, pinealectomy has been found to suppress seasonal changes and synchronisation with the annual cycle. However, these seasonal patterns reappear with the administration of exogenous melatonin.
Melatonin is also involved in sexual maturation in humans: puberty is associated with a marked decrease in plasma melatonin levels. Pineal gland dysfunction may accelerate puberty, whereas melatonin hyperproduction may delay it.
Other functions of melatoninIn addition to its role as a chronobiotic agent involved in the regulation of body temperature, sexual development, and the reproductive cycle, melatonin also plays a role in cell protection and has antioxidant, oncostatic, and immunostimulant properties.10–12
The main role of melatonin, both in health and disease, is associated with its antioxidant and anti-inflammatory effects. It presents a direct antioxidant effect, neutralising reactive oxygen and nitrogen species potentially harmful to cells. Due to its antioxidant effects, melatonin may interfere with bone resorption by inhibiting osteoclast activity, and in the formation of reactive substances by increasing superoxide dismutase activity.13 It also has an indirect antioxidant effect, stimulating antioxidant enzyme activity and suppressing pro-oxidant enzyme activity.
Epidemiological studies suggest that melatonin may have an oncostatic effect in patients with different types of tumours, although the results published are inconsistent due to methodological differences. Furthermore, melatonin has been shown to inhibit tumour growth in vitro and in experimental studies into MT1 and MT2 melatonin receptors. In the light of the above, melatonin may be useful for preventing and treating several types of cancer, such as breast, prostate, gastric, and colorectal cancer. Clinical trials have shown the efficacy of melatonin as adjuvant treatment for cancer, enhancing the effects of chemotherapy and radiotherapy while minimising their adverse effects, and improving sleep and quality of life in these patients.14
Melatonin is also an immunostimulator, antagonising the immunosuppressive effects of cortisol and stimulating lymphocyte activity.15 Active melatonin synthesis has been described in human lymphocytes; the hormone also regulates the production of interleukin-2, an essential protein for immune function.
Pharmacological properties of immediate- and prolonged-release exogenous melatoninPharmacokineticsMelatonin has a linear pharmacokinetic profile. It is rapidly absorbed and reaches its maximum concentration within approximately 40minutes of administration. Food intake delays absorption.
It presents low bioavailability (3%-33%) due to the hepatic first pass effect.
Over 90% of circulating melatonin is metabolised in the liver. Melatonin first undergoes 6-hydroxylation by cytochromes P450, mainly CYP1A2. This reaction is conjugated with sulphate and, to a lesser extent, with glucuronic acid. These conjugates are excreted in urine.
Melatonin is metabolised rapidly: immediate-release melatonin has a half-life of 45-65minutes. Increasing dosage delays peak concentrations and increases its half-life. Therefore, the larger the doses, the sooner melatonin should be administered before going to bed. Prolonged-release formulations show a significant delay in peak concentrations (90-210minutes, depending on the preparation). Half-life is also longer with prolonged-release melatonin, reaching 3.5-4hours. These formulations therefore resemble the physiological cycle of melatonin secretion. Circadin®, the only prolonged-release formulation approved by the European Medicines Agency for the treatment of primary insomnia in individuals older than 55 years, releases melatonin steadily over an extended period, maintaining plasma concentrations over 8-10hours.16
The pharmacokinetic differences between the 2 formulations suggest that immediate-release melatonin is more appropriate for inducing sleep, whereas prolonged-release formulations are more effective for maintaining sleep.
Melatonin absorption, distribution, and metabolism vary considerably between individuals. However, it is unclear whether this variability has clinical implications.
The half-life and area under the curve of melatonin are lower in children than in adults. No differences in pharmacokinetic parameters have been observed between sexes, or between pre- and postmenopausal women.17
Coadministration with caffeine increases melatonin bioavailability and decreases metabolism, increasing its half-life. Tobacco, however, decreases its bioavailability. This may be explained by the effects of caffeine (inhibitor) and the polycyclic aromatic hydrocarbons of tobacco (inductor) on CYP1A2.
Cytochrome P450 inhibitors, such as oral contraceptives and fluvoxamine, inhibit the metabolism of melatonin and increase its half-life.
Melatonin can freely cross the blood-brain barrier, without a transporter.
PharmacodynamicsMelatonin binds to 2 main receptors: MT1 and MT2.18 A third melatonin receptor (MT3) has been identified, but its function and relevance are yet to be determined.
The function of these receptors is unclear. It has been hypothesised that MT1 is linked to the hypnotic effects of melatonin, whereas MT2 is associated with regulation of the circadian rhythm. MT2 has also been linked to the modulation of pain, given that it is expressed in the reticular and ventromedial nuclei of the thalamus and the ventrolateral periaqueductal grey matter.19
The MT1 and MT2 receptors are dimers. Receptors form MT1/MT1 and MT2/MT2 homodimers but also MT1/MT2 heterodimers, with different responses to melatonin. MT1 also forms dimers with the GPR50 protein, which does not directly bind to melatonin but modifies MT1 response. Both MT1 and MT2 can form heterodimers with serotonin 5-HT2C receptors, which are involved in the regulation of mood.20
MT1 and MT2 are metabotropic receptors. Binding of melatonin to these G-protein-coupled receptors inhibits adenylate cyclase, leading to decreased synthesis of cyclic adenosine monophosphate. The MT1 and MT2 receptors ultimately regulate the expression of genes involved in circadian rhythm modulation, such as Bmal1, Clock1, mPer1, mCry1, and mCry2, as well as the expression of other genes and microRNA, and therefore also present anti-inflammatory, antioxidant, and antitumoural effects.21
Interaction between exogenous melatonin and the melatoninergic systemIn experimental models, acute exposure to high melatonin concentrations promotes desensitisation and internalisation of the MT1 and MT2 receptors. This does not occur when melatonin exposure follows the physiological cycle of melatonin secretion.22
Administration of immediate-release melatonin results in acute exposure of MT1 and MT2 receptors to elevated concentrations of the ligand. We may therefore expect these formulations to induce receptor desensitisation and internalisation. This should not be observed with prolonged-release formulations, which imitate the plasma concentration curve of endogenous melatonin. Receptor desensitisation and internalisation increases in parallel with melatonin concentration. As a result, higher melatonin doses may be less effective than those achieving melatonin concentrations similar to the physiological concentrations in the SCN.
The pharmacokinetics of the 2 formulations also have an impact on the plasma concentration curve. Oral administration of immediate-release melatonin achieves peak concentrations within minutes, inducing a hypnotic effect. Immediate-release melatonin is rapidly metabolised and eliminated in 3-4hours. Therefore, melatonin levels drop at a time when the concentration curve would describe a peak in physiological conditions, as endogenous secretion is inhibited. Absorption of prolonged-release melatonin is slower and more sustained, delaying and reducing the magnitude of peak concentration, and maintains stable melatonin levels for 8-10hours, replicating the physiological curve of melatonin secretion.
Melatonin for the treatment of insomniaPrimary insomniaChildren and adolescentsInsomnia is the most frequent sleep disorder in childhood and adolescence, especially in older adolescents. Prevalence ranges from 19% to 24%, depending on the diagnostic criteria used, and is slightly higher among girls.23
Treatment of sleep-onset insomnia in children and adolescents should be based on individualised cognitive behavioural therapy, with occasional pharmacological support. When pharmacological treatment is needed, melatonin should be the drug of first choice.24 The recommended dose is 1-3mg/night for infants and preschool children, 2.5-5mg/night for school-age children, and 1-5mg/night in adolescents. Treatment should be introduced progressively. Melatonin should be administered 30-60minutes before bedtime. Treatment with melatonin should not exceed 4 weeks. No data are available on the use of prolonged-release melatonin in children with normal psychomotor development.
Some children with chronic sleep-onset insomnia display circadian pacemaker dysfunction, which is reflected in delayed melatonin secretion under dim light conditions. As the diagnosis of delayed sleep phase syndrome is not clearly defined or recognised in children, the term “chronic sleep-onset insomnia with late melatonin onset” has been proposed.25 Exogenous melatonin, with appropriate timing and dosage, is effective for treating these symptoms,25,26 advancing the onset of melatonin secretion and regulating the sleep-wake cycle.27 Low doses (eg, 1mg) are recommended in these patients, since high doses are metabolised more slowly, resulting in reduced efficacy and increased wake time after sleep onset.26
Rather than primary insomnia, adolescents mainly present delayed sleep phase syndrome, with a relatively high prevalence (4%-6%).28,29
According to the clinical recommendations of the European Paediatric Neurology Society,26 melatonin is most effective for chronic sleep-onset insomnia and delayed sleep phase syndrome. Melatonin must be administered 3-5hours before the physiological dim light melatonin onset. There is currently no evidence that prolonged-release melatonin is superior to immediate-release formulations.
Adults (older than 18 years)This section is based on the 2017 European guideline for the diagnosis and treatment of insomnia,30 which reviews relevant meta-analyses published until June 2016.
Cognitive behavioural therapy is considered the first-line treatment for insomnia in adults without comorbidities. In the acute treatment phase, a combination of cognitive behavioural therapy and pharmacological treatment presents slight advantages over either treatment alone. During maintenance treatment, however, cognitive behavioural therapy alone appears to be more appropriate.
Most of the available evidence on the use of melatonin to treat insomnia in adults is from studies of immediate-release formulations, although the guideline also includes studies on prolonged-release melatonin and ramelteon, a melatonin receptor agonist. The meta-analyses reviewed do not provide uniform data on the efficacy of melatonin. According to Buscemi et al.31 and Ferracioli-Oda et al.,32 melatonin decreases sleep onset latency. Liu and Wang33 and Kuriyama34 also report significant, positive effects on sleep onset latency and sleep quality. However, these effects are small from a clinical viewpoint.
Some original studies into the adverse effects of melatonin support the safety of the drug.
The results of the meta-analyses suggest that the available evidence is insufficient to recommend the use of melatonin for treating insomnia in adults (weak recommendation, low quality of evidence).35
Elderly individualsIn 20 years’ time, elderly people will account for the largest population segment. The assessment, diagnosis, and treatment of insomnia will be essential in this population group, in whom the condition is most frequent; furthermore, elderly individuals are particularly vulnerable to adverse drug reactions.
Studies show that melatonin achieves moderate decreases in sleep onset latency, although its ability to reduce wake time after sleep onset is limited.
Immediate-release melatonin is recommended for sleep-onset insomnia.36 Prolonged-release melatonin and doxepin constitute the first-line treatments for sleep-maintenance insomnia, mixed insomnia (both sleep-onset and sleep-maintenance insomnia), and early awakening.37 The Spanish Society of Geriatrics and Gerontology and the Guidelines for the management and treatment of insomnia issued by the General Council of Official Medical Colleges of Spain recommend Circadin as the treatment of first choice for insomnia in adults older than 55.38,39
Comorbid insomniaAnxiety and depression are frequently associated with sleep disorders. Anxiety frequently causes sleep-onset insomnia, whereas depression is more frequently associated with sleep-maintenance insomnia and early awakening. Furthermore, reduced melatonin production has been observed in patients with depression. However, few studies into melatonin in patients with depression have been conducted. A recent meta-analysis found no evidence that melatonin improves mood. However, prolonged-release melatonin (2.5-10mg) as an adjunctive treatment to fluoxetine has been found to significantly improve mood disorders and the associated comorbid insomnia, compared to placebo.40 This finding is consistent with the results of experimental studies that suggest that melatonin may improve insomnia secondary to depression and bipolar disorder.41
In 40 patients with schizophrenia and comorbid insomnia, immediate-release melatonin dosed at 3mg decreased sleep onset latency, reduced the number of nocturnal awakenings, and increased sleep duration significantly more than placebo.42 In another study including 19 patients with schizophrenia and comorbid insomnia, 2mg Circadin achieved similar results.43
Sleep disorders are particularly frequent among individuals with intellectual disability. Furthermore, management of these patients is more complex since they usually take other medications that affect the central nervous system and may interact with hypnotic agents. Multiple disorders associated with intellectual dysfunction, including autistic spectrum disorders, Rett syndrome, and Angelman syndrome, have been associated with decreased melatonin production; therefore, melatonin supplementation may improve sleep and stabilise circadian rhythms.44 According to a meta-analysis of 9 double-blind placebo-controlled trials including a total of 183 patients with insomnia and intellectual disability, melatonin decreases sleep onset latency, increases total sleep duration, and decreases the number of nocturnal awakenings.45 These outcomes had considerable implications for caregiver quality of life, as caregivers found it easier to put these patients to bed, and the patients required less attention during the night. The dose administered varied between studies: 4 studies used a fixed dose of 5mg, 4 adjusted the dose to the patient's age and weight, and one study started with 3mg melatonin, and increased the dose by increments of 3mg up to a maximum dose of 9mg if no improvement was observed. The meta-analysis found no clear association between dosage and response to treatment in terms of sleep maintenance, although the drug may have a positive impact on sleep initiation. Only 4 of the studies included indicate the type of melatonin used: 3 used immediate-release formulations, and the other used a combination of 1mg immediate-release melatonin and 4mg prolonged-release melatonin. Administration time also varied between studies. In some studies, melatonin was administered at a fixed time, whereas in others it was administered 20-60minutes before the desired bedtime. No relevant adverse reactions to melatonin were reported. Other meta-analyses have concluded that melatonin is effective and safe for patients with autistic spectrum disorders.46,47 However, the level of evidence is low, as the studies published to date included small samples and are of poor methodological quality.
In such neurodegenerative conditions as Alzheimer disease or Parkinson's disease, insomnia is a symptom of cerebral dysfunction secondary to neuronal loss and the disintegration of brain networks. Experimental evidence from in vitro studies and studies with animal models suggests that melatonin has a neuroprotective effect, as it reduces oxidative stress, protects mitochondrial integrity, and minimises toxic protein aggregation.48 Patients with Alzheimer disease or Parkinson's disease display lower cerebrospinal fluid melatonin levels than age-matched controls, even in presymptomatic phases.49
Several double-blind placebo-controlled studies with small samples suggest that melatonin (3-5mg immediate-release or 2mg prolonged-release) has a positive impact on cognitive function and sleep disorders associated with Alzheimer disease. However, other studies report contradicting results. Data from some studies support the benefits of immediate-release melatonin at doses ranging from 3 to 24mg in patients with mild cognitive impairment, although these studies include small samples (n=6) or use retrospective designs.50
Other authors report that melatonin has a positive impact on sleep disorders associated with Parkinson's disease, improving actigraphy parameters and Pittsburgh Sleep Quality Index scores. These data are from double-blind, placebo-controlled studies including small numbers of patients. Another limitation of these studies is the heterogeneity in melatonin doses: 2 studies administered 3mg melatonin, whereas another study administered doses ranging from 5 to 50mg. Melatonin presented good tolerability, and no alterations in motor function were observed.51
Patients with Parkinson's disease frequently present REM sleep behaviour disorder, even several years before the onset of the typical motor symptoms of the disease. Melatonin may be beneficial in these patients, restoring muscle atonia during REM sleep. Its toxicity profile is far superior to that of clonazepam, the first line of treatment in these cases.52 A small double-blind study including 8 male patients reported improvements in REM sleep muscle atonia with 3mg melatonin.53 Small clinical case series have also shown the benefits of melatonin in these patients.54,55 Another advantage of melatonin over clonazepam is that it improves REM sleep behaviour disorder without worsening a possibly associated obstructive sleep apnoea syndrome.56
Fatigue is one of the most disabling symptoms of multiple sclerosis, and involves multiple factors. Poor sleep quality, which is reported by roughly half of patients, has been proposed as a partial explanation. In a case-control study including 102 patients with multiple sclerosis and 20 age- and sex-matched controls, 5mg melatonin improved sleep quality but had no effect on fatigue. Patients also displayed lower levels of inflammatory markers.57
Chronic pain, especially neuropathic pain, is frequently associated with insomnia. Preclinical studies suggest that melatonin may have an analgesic effect due to its action on MT2 receptors.58 However, very little clinical evidence is available. In randomised studies with small samples, patients taking melatonin the night before surgery felt less severe postsurgical pain than those receiving placebo, although other studies have found no clear analgesic effects.59–62 Furthermore, few data are available on the efficacy of melatonin for insomnia secondary to chronic pain. In a double-blind placebo-controlled study including 50 patients with cancer pain and insomnia, immediate-release melatonin dosed at 3mg significantly improved sleep quality.21 Doses of 5mg immediate-release melatonin also improved sleep quality and reduced pain intensity in 32 women with myofascial pain affecting the temporomandibular joint.63 In a double-blind placebo-controlled study of 101 patients with fibromyalgia, 3-5mg melatonin alone or in combination with 20mg fluoxetine improved sleep quality and reduced pain.64
Patients with restless legs syndrome frequently complain of difficulty falling and remaining asleep. In these patients, symptoms are most severe at the time of peak melatonin concentration, which suggests that administration of exogenous melatonin may accentuate the discomfort in their legs. The hypothesis that melatonin has a negative effect on restless legs syndrome was confirmed in a study including 8 patients who underwent the suggested immobilisation test.65
Therefore, the available data suggest that melatonin may improve sleep quality in patients with comorbid insomnia, as well as providing additional benefits for the underlying disease.
Diseases associated with insomniaLike many other sleep disorders, insomnia increases the risk of arterial hypertension.66,67 Furthermore, sleep fragmentation prevents the physiological decrease in blood pressure that occurs during nocturnal sleep; according to some studies, this is a more powerful predictor of cardiovascular risk than daytime blood pressure values.68 Some antihypertensive drugs, such as beta-blockers and calcium channel blockers, decrease melatonin production.
A meta-analysis of 7 randomised studies concluded that 2-3mg prolonged-release melatonin significantly decreased nocturnal systolic and diastolic blood pressure. The effect was not observed with immediate-release formulations. Melatonin did not alter daytime blood pressure values.69
An association has been suggested between sleep disorders, including insomnia, and risk of diabetes and metabolic syndrome. Patients with diabetes mellitus, particularly those with polyneuropathy, show reduced melatonin production, which makes them more likely to present insomnia. Furthermore, polymorphisms affecting the melatonin receptors, or low levels of the hormone, increase the risk of diabetes mellitus. Melatonin has a direct effect on pancreatic beta cells and modulates insulin sensitivity in hepatocytes.70
In an open-label study, prolonged-release melatonin (Circadin 2mg) improved insomnia in patients with diabetes mellitus type 2; in the long term, the drug also had a positive impact on glycosylated haemoglobin levels.71
Other studies report a decrease in the levels of inflammatory markers associated with metabolic syndrome.72
In conclusion, despite the small sample sizes and non-conclusive results of the studies published to date, the available evidence suggests that melatonin not only improves sleep quality but also provides additional benefits, improving comorbidities associated with chronic insomnia, such as nocturnal arterial hypertension, diabetes mellitus, or metabolic syndrome.
Circadian rhythm disordersSince the American Academy of Sleep Medicine published its clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders in 2015,73 no study has contributed significant modifications to the treatment of these disorders.
We summarise below the recommendations of the American Academy of Sleep Medicine on the use of melatonin in patients with circadian rhythm disorders.
- 1.
Advanced sleep phase syndrome. Patients with advanced sleep phase syndrome fall asleep, and wake up, several hours before the desired or necessary time. They present somnolence in the late evening and wake spontaneously in the early morning. No systematic studies have addressed the use of melatonin in patients with the disorder; melatonin and melatonin agonists are therefore not recommended in these patients. The risk-benefit balance is unclear in children and adolescents with advanced sleep phase syndrome.
- 2.
Delayed sleep phase syndrome. Patients with delayed sleep phase syndrome fall asleep 2 or more hours later than the socially accepted or conventional bedtime. Patients complain of difficulty falling asleep and waking at the desired times.
- 2.1.
Delayed sleep phase syndrome in adults. The guideline reviewed 2 open-label studies and a double-blind study. All 3 studies included small samples (≤ 20 patients) and used melatonin doses ranging from 0.3 to 5mg, administered at different times in the evening or night for a short period (≤ 29 days). Melatonin was found to improve sleep latency but did not increase total sleep time, nor did it improve alertness during the night. The level of recommendation of melatonin for adults with delayed sleep phase syndrome is low, given the limited evidence on its use.
- 2.2.
Delayed sleep phase syndrome in children and adolescents without comorbidities. The guideline only reviewed one study, which included 64 participants aged 6 to 12 years; 0.05-0.15mg/kg melatonin was administered 1.5-2hours before bedtime for 6 consecutive nights. Doses of 0.15mg/kg melatonin achieved the best results, improving sleep latency by −43minutes (CI, −24.06 to −63.54).
- 2.3.
Delayed sleep phase syndrome in children and adolescents with psychiatric disorders. The guideline analysed 2 studies in which patients received 3-5mg immediate-release melatonin between 18:00 and 19:00, for 4 weeks. The results suggest an advance in sleep onset time. In any case, the degree of recommendation for melatonin in adults, children, and adolescents with delayed sleep phase syndrome is low due to the low-to-moderate level of evidence currently available.
- 2.1.
- 3.
Free-running circadian rhythm. Free-running circadian rhythm is a disorder in which the sleep cycle is not entrained to the 24-hour cycle, usually lasting longer. This is due to lack of synchronisation between the SCN and the light-dark cycle; in these patients, the sleep cycle adjusts to the endogenous circadian rhythm, which runs to approximately 25hours. Most patients with the disorder are completely blind. The guideline reviewed 3 observational studies with small samples (24 patients in total), using melatonin doses ranging from 0.5 to 10mg; the drug was administered 1hour before bedtime or at a fixed time (21:00), for 26-81 days. The results provide evidence that melatonin is effective for the treatment of free-running circadian rhythm in blind individuals. However, the degree of recommendation is low due to the low level of evidence of the study.
- 4.
Irregular sleep-wake rhythm. Patients with the disorder display a chaotic, unpredictable sleep-wake pattern. These patients may also present insomnia and somnolence, depending on the time of the day. Napping is also frequent.
- 4.1
Irregular sleep-wake rhythm in elderly individuals with dementia. The American Academy of Sleep Medicine does not recommend melatonin in elderly individuals with dementia and irregular sleep-wake rhythm, based on a single study of 25 patients who received 6mg prolonged-release melatonin at bed time, which reported no increase in total sleep time. The experts also suggested that melatonin may have more risks than benefits in this patient group as it may affect mood and daytime activity.
- 4.2
Irregular sleep-wake rhythm in children and adolescents with neurological disorders. The guidelines review one study in which patients received 2-10mg melatonin 1hour before going to bed. The grade of recommendation for the treatment is low, and the level of evidence is moderate.
- 4.1
In conclusion, melatonin may be effective for treating delayed sleep phase syndrome, irregular sleep-wake rhythm in children and adolescents with neurological disorders, and free-running circadian rhythm in blind adults.
ConclusionsDue to its role in regulating the sleep-wake cycle, melatonin is potentially useful in the treatment of insomnia and sleep phase disorders. Reinforcing the physiological signal that induces sleep seems to be the most natural approach to the treatment of these sleep alterations, particularly at ages when melatonin production is reduced. The available data, mainly on prolonged-release formulations, support the drug's efficacy for both primary insomnia and insomnia associated with other neurological diseases, particularly in individuals older than 55 years. Furthermore, melatonin displays an excellent tolerability profile. Prolonged-release formulations seem to better reproduce the physiological curve of melatonin secretion and may therefore be more useful, especially in view of the fact that melatonin's action depends on the secretion cycle and may change according to whether peak concentrations coincide with certain phases of the circadian cycle. Finally, we should stress that melatonin is a pharmacological agent, not a nutritional supplement, and must therefore be indicated with caution to ensure patient safety.
Conflicts of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Poza JJ, Pujol M, Ortega-Albás JJ, Romero O, Melatonina en los trastornos de sueño. Neurología. 2022;37:575–585.